Abstract
Background
Dietary patterns have been linked to chronic diseases such as cardiovascular disease, but sparse data are currently available on associations between dietary patterns and microalbuminuria or kidney function decline.
Study Design
Subgroup analysis from a prospective observational cohort study
Setting and Participants
Female participants in the Nurses’ Health Study who had dietary patterns data from food frequency questionnaires returned in 1984, 1986, 1990, 1994, and 1998 and urinary albumin-creatinine ratios (ACR) from the year 2000 (n=3121); estimated glomerular filtration rate (eGFR) change between 1989 and 2000 was available in 3071.
Predictor
Prudent (higher intake of fruits, vegetables, legumes, fish, poultry, and whole grains), Western (higher intake of red and processed meats, saturated fats, and sweets), and Dietary Approach to Stop Hypertension (DASH) style dietary patterns (also greater intake of vegetables, fruits, and whole grains).
Outcomes and Measurements
Microalbuminuria (albumin-to-creatinine ratio 25 to 354 mcg/mg) in 2000 and change in kidney function by eGFR between 1989 and 2000.
Results
After multivariable adjustment, the highest quartile of Western pattern score compared to the lowest quartile was directly associated with microalbuminuria (OR, 2.17; 95% CI, 1.18 to 3.66; p-for trend=0.01) and rapid eGFR decline of ≥3 ml/min/1.73 m2 per year (OR, 1.77; 95% CI, 1.03 to 3.03). Women in the top quartile of the DASH score had decreased risk for rapid eGFR decline (OR, 0.55; 95% CI, 0.38 to 0.80), but had no association with microalbuminuria. These associations did not vary with diabetes status. The prudent dietary pattern was not associated with microalbuminuria or eGFR decline.
Limitations
Study cohort included primarily older white women and generalizability of results would benefit from validation in non-whites and men.
Conclusions
A Western dietary pattern is associated with a significantly elevated risk for microalbuminuria and rapid kidney function decline whereas a DASH-style dietary pattern may be protective against rapid eGFR decline.
The presence of microalbuminuria and moderately decreased kidney filtration function are powerful predictors of cardiovascular disease1–3 and mortality,3, 4 but there are limited data on how diet, an important modifiable risk factor, might be associated with microalbuminuria or kidney function decline. In particular, the influence of dietary patterns over time on the kidney is not well defined. Whereas traditional nutritional epidemiology has focused on individual nutrients or foods, their additive or interactive influence perhaps may be better observed when overall diet patterns are considered for incident chronic diseases. In addition to the ability to capture potential synergy between foods and nutrients, dietary patterns also may allow for easier translation into practical dietary advice as people eat many different foods in combination.5 Furthermore, classifying individuals according to their eating pattern can yield a larger contrast between exposure groups than analyses based on multiple single nutrients or foods, which can be influenced by collinearity.
One previously published study analyzed dietary patterns and albuminuria. The Multiethnic Study of Atherosclerosis (MESA) study reported a diet pattern rich in whole grains, fruit, and low-fat dairy foods was associated with lower albumin-creatinine ratio (ACR).6 We therefore investigated the associations between dietary patterns and the presence of microalbuminuria or eGFR decline in 3,121 women participating in the Nurses’ Health Study (NHS). We hypothesized that healthier eating patterns as measured by the prudent or DASH (Dietary Approach to Hypertension)-style dietary patterns would be inversely associated, while the Western dietary pattern would be directly associated with microalbuminuria and eGFR decline.
METHODS
Study Design
The NHS was initiated in 1976 with the enrollment of 121,700 U.S. female nurses aged 30–55 years. This cohort is followed through mailed biennial questionnaires related to lifestyle factors and health outcomes. Between 1989 and 1990, 32,826 participants provided blood samples that were shipped on ice by overnight delivery and stored at −130 degrees Celsius as previously described.7 In the year 2000, 18,720 of these participants submitted a second blood and spot urine specimens. Participants who did and did not return blood samples were similar in terms of demographics and lifestyle characteristics.
The NHS women in this investigation were participants in sub-studies of analgesic use and kidney function8 or type 2 diabetes and kidney function. The women in the analgesic study had submitted plasma in both 1989 and 2000 and were sent supplemental questionnaires to obtain detailed information regarding lifetime analgesic use. In total, 3876 women returned the analgesic questionnaires. There were 2712 women selected, with oversampling of those from the highest levels of lifetime analgesic consumption. For the type 2 diabetes sub-study, we included 674 women who had submitted biological samples and who had reported a diagnosis of diabetes that was confirmed by a diabetes supplemental questionnaire. The total number of diabetics was 694.
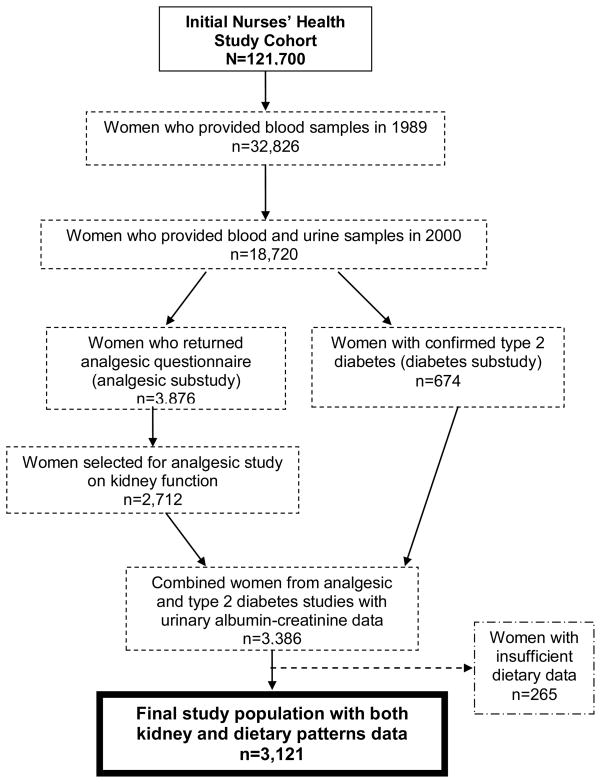
We included those women who had cumulative average dietary patterns data available and who submitted a urine in the year 2000 (n=3121) (Figure 1). The majority of these women (n=3071) also had plasma creatinine measured in samples collected in 1989 and 2000, which allowed us to examine eGFR change over 11 years.
Figure 1.
Inclusion and exclusion criteria for Nurses’ Health Study women in this analysis of dietary patterns with albuminuria and kidney function decline
Dietary Assessment
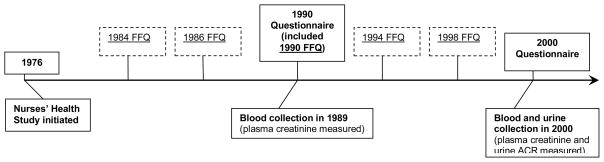
We used information collected from the 1984, 1986, 1990, 1994, and 1998 semi-quantitative food frequency questionnaires (FFQ) (Figure 2). The FFQs were designed to assess average food intake over the preceding year and each has approximately 116 items. A standard portion size and 9 possible frequency-of-consumption responses, ranging from “never or less than once per month” to “6 or more times per day” was given for each food item. Total energy and nutrient intake was calculated by summing up energy or nutrients from all foods. Previous validation studies among members of the NHS revealed a correlation coefficient of 0.66 between assessment by the FFQ and multiple weeks of food records completed over the preceding year.9
Figure 2.
Time-line of questionnaire and biological sample data collection in Nurses’ Health Study for these analyses. Questionnaires are administered every two years beginning in 1976 but only questionnaire data for non-dietary covariates from years used for this study (1990 and 2000) are shown. Food frequency questionnaires (FFQ’s) asking about diet over the previous 12 months were administered in 1984, 1986, 1990, 1994, and 1998. ACR = albumin-creatinine ratio. Reproduced from inted from Lin et al^28 with permisision of the American Society of Nephrology.
Diet Pattern Indices
We have previously identified two major diet patterns by the statistical procedure factor analysis (principal components).10 Briefly, foods from the FFQ were first classified into 38 food groups based on similar nutrient profiles or culinary usage. The principle components procedure identifies diet patterns based on the correlations between these food groups. We also used an orthogonal rotation procedure which results in uncorrelated factors or patterns.11 The factor score for each pattern was calculated by summing intakes of food groups weighted by their factor loadings.12 Factor scores were standardized to have a mean of 0 and standard deviation of 1. The scores reflect how closely a participant’s diet resembles each identified pattern, with higher scores representing closer resemblance.
Each woman received a factor score for each identified pattern. The first identified factor, which we called the prudent pattern, is characterized by higher intakes of fruits, vegetables, whole grains, fish and poultry. The second factor, which we called the Western pattern, is characterized by a higher intake of processed and red meats, refined grains, sweets and desserts. Factor scores generated by this approach are not correlated with each other. Factor analysis was conducted using SAS PROC FACTOR.13
We also constructed the DASH score as previously detailed14,15 (ranging from 8 to 40 points) based on food and nutrients emphasized or minimized in the DASH diet16 focusing on 8 components: high intake of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains and low intake of sodium, sweetened beverages, and red and processed meats.
Of note, three independent dietary patterns were derived based on a large number of correlated food items, which were aggregated into a small number of conceptually meaningful food patterns. Each individual receives a score for each pattern. There is only minor overlap between the Western and prudent patterns because we used “orthogonal rotation” algorithm to derive the patterns (e.g., the correlations between them are close to zero).
Measurement of Urinary Albumin-Creatinine Ratio
Urinary assays were performed on spot collections. Urinary creatinine concentration was measured by a modified Jaffe method (CV, 1.6%). Urinary albumin was measured using solid-phase fluorescence immunoassay using the Hitachi 911 analyzer and Roche diagnostics reagents (www.roche.com) with a lower limit of detection of 0.1 mg/L (CV, 8.0%). A urinary albumin-creatinine ratio (ACR) of ≥25 mcg/mg was used to define the microalbuminuria threshold; this sex-specific cutpoint for women has been reported to approximate a urinary albumin excretion rate of ~30 mg/24 hours,17 which is classically considered clinically relevant microalbuminuria. In this study, 177 women (5.7%) met the criterion for microalbuminuria (ACR 25 to 355 mcg/mg). There were 30 women with macroalbuminuria (ACR > 355 mcg/mg ranging from 393 to 6234 mcg/mg) who were excluded from the microalbuminuria analyses.
Measurement of Kidney Function Decline
Plasma creatinine was analyzed using a modified kinetic Jaffe reaction (CV 10%). In 2007, repeat creatinine assays of 20 NHS plasma samples (with a range of 0.6 to 1.4 mg/dL) initially measured in the year 2000 revealed a mean re-calibration coefficient (new value/original value) of 0.97 and confirmed that plasma creatinine is stable for many years under our storage conditions.
Glomerular filtration was estimated by the 4-variable MDRD Study equation where GFR (ml/min/1.73 m2) =186 × [PCr (mg/dl)]−1.154 × [Age]−0.203 × [0.742 if female] × [1.21 if black]18 An eGFR decline of ≥30% between 1989 and 2000 was determined a priori as a clinically significant change in kidney function and has been used in a previous analysis of kidney function decline in NHS participants.19 We also examined “rapid” eGFR decline, defined as ≥3 ml/min/1.73 m2 per year, which has been previously used as a cutoff that reflects three times more rapid decline than expected by normal aging.20
Measurement of Covariates
Race and height were initially reported on the 1992 questionnaire. Other self-reported clinical and lifestyle variables including weight, hypertension, smoking status, physical activity as calculated by a weekly metabolic score, CVD (angina, myocardial infarction, coronary artery bypass surgery, percutaneous coronary revascularization, or stroke), and blood pressure medication use were reported on the biennial questionnaires. Self-reported hypertension has been previously validated in a subset of this cohort through direct medical records review.21 In addition, we obtained self-reported blood pressure from the 1990 questionnaire.
Systolic blood pressure was reported in 9 categories (<105, 105 to 114, 115 to 124, 125 to 134, 135 to 144, 145 to 154, 155 to 164, 165 to 174, and >=175 mm Hg), and diastolic blood pressure was reported in 7 categories (<65, 65 to 74, 75 to 84, 85 to 89, 90 to 94, 95 to 104, and >=105 mm Hg). A participant’s blood pressure was defined as the middle systolic and middle diastolic value of the reported category. Many of these variables have been previously validated through direct medical records review21, 22.
Body mass index (BMI) was calculated by [weight (kg)/(height (m)2]. Questionnaire data collected closest to the year when kidney function was measured (the 1988 questionnaire for eGFR decline and the 2000 questionnaire for urinary ACR) were used (Figure 2). Many of these variables have been previously validated through direct medical records review.21, 22
Participants report newly diagnosed diabetes by physicians on the biennial questionnaires. We mailed a diabetes supplementary questionnaire to all women reporting diabetes to obtain further information about the date of diagnosis, symptoms, diagnostic tests, and treatment. We used the National Diabetes Data Group criteria to define diabetes self-reported up to the 1996 biennial questionnaire;23 the American Diabetes Association diagnostic criteria for diabetes released in 1997 were used for incident cases of diabetes reported in 1998 and after.24 Self-reported diagnosis of type 2 diabetes using the diabetes supplementary questionnaire has been established as 98% accurate in a separate validation study through medical records review.25 We considered a participant who was diagnosed with diabetes up through the year 2000 as having diabetes.
Statistical analyses
For albuminuria analyses, cumulative averaging for each dietary pattern using available data for five FFQs (1984, 1986, 1990, 1994, and 1998) was performed for each participant and divided into quartiles as the primary exposure of interest. Similarly, for analyses of eGFR decline between 1989 and 2000, cumulative average dietary patterns from 1984, 1986 and 1990 were divided into quartiles. This modeling approach used the dietary patterns exposures as measured up to the time of each outcome assessment (Figure 2). A cumulative average approach was chosen because it generally reflects long-term diet and also likely reduces measurement error from intra-individual variation over time.26 Covariates included in adjusted models were determined from questionnaire data up to the nearest time of measurement of albuminuria or first eGFR
Wilcoxon sum rank and chi-square tests were used to test for differences between groups as appropriate. Primary analysis was performed with outcomes of microalbuminuria using a sex-specific category of ACR 25 to 355 mcg/mg or presence of eGFR decline ≥ 30% or ≥ 3 ml/min/1.73 m2 per year decline. Exposures of interest were quartiles of each dietary pattern with the lowest quartile as the referent category. Logistic regression was used to model associations between quartiles of diet scores and presence of microalbuminuria, eGFR ≥ 30% decline or ≥ 3 ml/min/1.73 m2 per year decline between 1989 and 2000. In all analyses of microalbuminuria and eGFR decline, adjustment for alcohol intake and eGFR in 1989 did not influence the results so these covariates were not included in the multivariable adjusted models.
All analyses were performed with SAS software, version 9.1 (SAS Institute, Inc., www.sas.com). This study was approved by the Partners’ Healthcare Brigham and Women’s Hospital Human Research Committee Institutional Review Board.
RESULTS
Study Participants and Dietary Patterns Assessment
Characteristics of these 3121 women in the year 2000 are given in Tables 1–3. Median age was 67 years, 97% were Caucasian, 54% had hypertension, and 23% had diabetes. The Western and prudent dietary patterns had a weak but statistically significant inverse correlation (r=−0.07, p<0.001). The DASH score was directly correlated with prudent pattern (r=0.76, p<0.001) and inversely correlated with the Western pattern (r= −0.30, p<0.001). Cumulative average dietary pattern scores were highly correlated when comparing 1990 to 1998 values (all r>0.94, p<0.001), suggesting that dietary patterns remained relatively unchanged in these women over time. Participant characteristics stratified by quartiles of dietary pattern scores are shown in Tables 1–3.
Table 1.
Demographic, Clinical, and Nutrient Characteristics of NHS Participants in the Year 2000 Stratified by Western Pattern Diet Score
| Cumulative Averaged Western Pattern Diet Score | ||||||
|---|---|---|---|---|---|---|
| All NHS (N= 3121) | Q1 n=780 | Q2 n=780 | Q3 n=781 | Q4 n=780 | p-for-trend | |
| Age (years) | 67 (62, 73) | 69 (63, 74) | 67 (62, 73) | 66 (61, 72) | 65 (59, 71) | <.001 |
| Caucasian (%) | 97.4 | 96.1 | 97.1 | 98.3 | 98.2 | 0.02 |
| Hypertension (%) | 54.1 | 53.1 | 44.9 | 43.4 | 42.2 | <.001 |
| SBP (mm/hg) in 1990 | 130 (120, 140) | 120 (110, 130) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | <.001 |
| DBP (mm/hg) in 1990 | 105 (70, 80) | 80 (70, 80) | 80 (70, 80) | 80 (70, 88) | 80 (70, 88) | <.001 |
| Diabetes (%) | 23.1 | 17.1 | 22.8 | 23.8 | 28.6 | <.001 |
| High cholesterol (%) | 65.0 | 66.3 | 66.3 | 65.4 | 61.8 | 0.2 |
| CVD (%) | 6.0 | 6.4 | 6.0 | 5.4 | 6.0 | 0.9 |
| Current smoker (%) | 5.8 | 3.0 | 4.6 | 5.8 | 9.7 | <.001 |
| Ever smoker (%) | 52.8 | 52.8 | 54.6 | 51.5 | 52.2 | 0.6 |
| Alcohol intake (g/day) | 1.7 (0.18, 7.0) | 1.9 (0.2, 7.1) | 2.1 (0.4, 8.0) | 1.9 (0.2, 7.4) | 1.2 (0, 5.7) | 0.2 |
| Calorie intake (kcal/day) | 1726 (1468, 2020) | 1413 (1230, 1626) | 1607 (1408,1805) | 1800 (1605, 1996) | 2153 (1923, 2392) | <.001 |
| Activity level (METs/week) | 11.4 (3.6, 25.2) | 14.0 (4.3, 27.9) | 12.7 (4.1, 26) | 11.5 (4.0, 24.6) | 8.1 (2.4, 20.8) | <.001 |
| BMI (kg/m2) | 26.4 (23, 30.2) | 25.0 (22.3, 28.7) | 26.4 (23.3, 30) | 26.6 (23.2, 30.1) | 27.5 (23.5, 32.0) | <.001 |
| Aspirin use lifetime (g/day) | 748 (98, 3169) | 414 (33, 2438) | 748 (82, 3006) | 813 (98, 3656) | 975 (98, 3656) | 0.01 |
| NSAID use lifetime (g/day) | 60 (10, 1000) | 20 (10, 500) | 50 (10, 900) | 100 (20, 1200) | 100 (20, 1500) | 0.06 |
| Acetominophen use through 1999 (g/day) | 98 (33, 1138) | 81 (33, 650) | 98 (33, 98) | 98 (33, 1138) | 260 (33, 2438) | 0.001 |
| ACEi or ARB medication use (%) | 21.3 | 18.7 | 20.3 | 23.9 | 22.2 | 0.06 |
| Cholesterol lowering medication (ever used by year 2000) | 28.8 | 32.2 | 27.3 | 28.6 | 27.2 | 0.1 |
| Plasma Cr in 2000 (mg/dl) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.9 |
| eGFR in 2000 (ml/min/1.73 m2) | 76 (65, 88) | 76 (65, 88) | 76 (65, 87) | 76 (65, 88) | 76 (66, 89) | 0.9 |
| Urinary ACR (mcg/mg) | 3.4 (2.0, 6.2) | 3.3 (2.1, 6.2) | 3.3 (1.9, 6.0) | 3.4 (1.9, 6.2) | 3.4 (2.0, 6.1) | 0.9 |
| Total protein (g/day) | 74 (68, 80) | 76 (69, 83) | 75 (69, 81) | 73 (68, 79) | 72 (66, 77) | <.001 |
| Animal protein (g/day) | 53 (46, 59) | 54 (47, 61) | 53 (47, 59) | 52 (46, 58) | 51 (46, 58) | <.001 |
| Vegetable protein (g/day) | 21 (19, 23) | 22 (20, 25) | 21 (19, 23) | 21 (19, 23) | 20 (18, 22) | <.001 |
| Total fat (g/day) | 56 (50, 61) | 49 (44, 55) | 54 (50, 59) | 57 (53, 62) | 60 (56, 65) | <.001 |
| Animal fat (g/day) | 30 (25, 34) | 26 (22, 30) | 29 (25, 33) | 31 (27, 35) | 33(29, 37) | <.001 |
| Sodium (mg/d) | 2001 (1801, 2227) | 1907 (1718, 2116) | 1981 (1781, 2181) | 2033 (1827, 2262) | 2085 (1888, 2325) | <.001 |
| Beta-carotene (mg/day) | 4406 (3281, 5930) | 5763 (4198,7522) | 4651 (3499,5851) | 4031 (3216,5344) | 3620 (2732,4727) | <.001 |
| Median Western Diet pattern score | −0.1 (−0.6, 0.4) | −0.9 (−1.1, −0.7) | −0.3 (−0.5, −0.2) | 0.1 (0.0, 0.3) | 0.9 (0.7, 1.3) | NA |
Results expressed as median (25th, 75th percentile), percentage, or median (percentage). Conversion factors for units: Cr in mg/dL to μmol/L, x88.4; eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, x0.01667.
p<0.05 or
p<0.01 when compared to Q1 as referent group
ACR= albumin-creatinine ratio; eGFR=estimated glomerular filtration rate; Cr, creatinine; NA, Not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ACR, albumin-creatinine ratio; CVD, cardiovascular disease; BMI, body mass index; NSAID, nonsteroidal antiiinflamatory drugs; Q, quartile; MET, metabolic equivalent; NHS, Nurses’ Health Study
Table 3.
Demographic, Clinical, and Nutrient Characteristics of Participants in the NHS in the Year 2000 Stratified by DASH-Style Pattern Score
| Cumulative Averaged DASH-Style Pattern Score | ||||||
|---|---|---|---|---|---|---|
| All NHS (N= 3121) | Q 1 n=780 | Q 2 n=780 | Q3 n=781 | Q 4 n=780 | p-for-trend | |
| Age (years) | 67 (62,73) | 64 (61, 73) | 66 (61, 73) | 68 (63, 73) | 70 (64, 74) | <.001 |
| Caucasian (%) | 97.4 | 96.3 | 98.0 | 98.0 | 97.1 | 0.08 |
| Hypertension (%) | 54.1 | 56.5 | 56.3 | 56.7 | 48.3 | 0.001 |
| SBP (mm/hg) in 1990 | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 120 (120, 140) | 0.05 |
| DBP (mm/hg) in 1990 | 105 (70, 80) | 80 (70, 88) | 80 (70, 88) | 80 (70, 80) | 80 (70, 80) | <.001 |
| Diabetes (%) | 23.1 | 24.6 | 24.1 | 26.1 | 20.3 | 0.04 |
| High cholesterol (%) | 65.0 | 65.0 | 63.3 | 66.1 | 66.4 | 0.6 |
| CVD (%) | 6.0 | 6.8 | 6.3 | 6.6 | 5.3 | 0.6 |
| Current smoker (%) | 5.8 | 11.6 | 6.0 | 3.3 | 2.2 | <.001 |
| Ever smoker (%) | 52.8 | 56.3 | 57.7 | 50.3 | 48.4 | <.001 |
| Alcohol intake (g/day) | 1.7 (0.18, 7.0) | 1.4 (0,6.7) | 2.0 (0.3,8.0) | 1.6 (0.2,6.5) | 1.8 (0.2,6.5) | 0.03 |
| Calorie intake (kcal/day) | 1726 (1468, 2019) | 1560 (1301, 1902) | 1685 (1447, 1978) | 1745 (1496, 2019) | 1876 (1628, 2157) | <.001 |
| Activity level (METs/wk) | 11.4 (3.6, 25.2) | 7.7 (1.9, 17.7) | 9.8 (3.1, 22.3) | 12.7 (4.4, 27.0) | 17.7 (7.7, 32.7) | <.001 |
| BMI (kg/m2) | 26.4 (23, 30.2) | 27.1 (23.5, 31.4) | 27.1 (23.5, 31.4) | 26.3 (23.0, 30.5) | 24.9 (22.3, 28.4) | <.001 |
| Aspirin use lifetime (g) | 748 (98, 3169) | 813 (98, 3169) | 748 (98, 3169) | 569 (98, 2844) | 748 (81, 3169) | 0.4 |
| NSAID use (lifetime, grams) | 60 (10, 1000) | 60 (10, 1200) | 60 (10, 1200) | 60 (10, 900) | 50 (10, 760) | 0.2 |
| Acetominophen use (lifetime, grams) | 98 (33, 1138) | 163 (33,1463) | 98 (33, 1235) | 98 (33, 975) | 98 (33, 650) | 0.001 |
| ACEi or ARB medication use (%) | 21.3 | 22.9 | 22.3 | 23.9 | 17.2 | 0.004 |
| Cholesterol lowering medication (ever used by year 2000) | 28.8 | 30.9 | 27.9 | 30.4 | 28.0 | 0.4 |
| Plasma Cr in 2000 (mg/dl) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.03 |
| eGFR in 2000 (ml/min/1.73 m2) | 76 (65, 88) | 77 (65, 89) | 75 (64, 88) | 75 (64, 86) | 77 (66, 89) | 0.2 |
| Urinary ACR (mcg/mg) | 3.4 (2.0, 6.2) | 3.4 (1.9, 6.2) | 3.6 (1.9, 5.6) | 3.4 (2.1, 6.8) | 3.6 (2.1, 6.5) | 0.7 |
| Total protein (g/day) | 74 (68, 80) | 71 (65, 77) | 73 (67, 79) | 75 (69, 81) | 77 (70, 83) | <.001 |
| Animal protein (g/day) | 53 (46, 59) | 52 (46, 58) | 53 (47, 59) | 53 (47, 59) | 53 (46, 60) | 0.1 |
| Vegetable protein (g/day) | 21 (19, 23) | 19 (17, 21) | 20 (19, 22) | 22 (20, 23) | 23 (21, 25) | <.001 |
| Total fat (g/day) | 56 (50, 61) | 61( 56, 65) | 57 (53, 61) | 54 (50, 59) | 49 (45, 54) | <.001 |
| Animal fat (g/day) | 30 (25, 34) | 34 (30, 39) | 31 (27, 35) | 29 (25, 33) | 25 (22, 29) | <.001 |
| Sodium (mg/d) | 2001 (1801, 2227) | 2084 (1893, 3276) | 2031 (1831, 2274) | 1964 (1788, 2165) | 1926 (1730, 2111) | <.001 |
| Beta-carotene (mg/day) | 4406 (3281, 5930) | 3109 (2327,4087) | 4037 (3225,5226) | 4619 (3730,6011) | 5979 (4833,7531) | <.001 |
| Median DASH-style pattern score | 25 (22, 27) | 20 (18, 21) | 23 (22, 24) | 26 (25, 27) | 29 (28, 31) | NA |
Results expressed as median (25th, 75th percentile), percentage, or median (percentage). Conversion factors for units: Cr in mg/dL to μmol/L, x88.4; eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, x0.01667.
p<0.05 or
p<0.01 when compared to Q1 as referent group
ACR= albumin-creatinine ratio; eGFR=estimated glomerular filtration rate; Cr, creatinine; NA, Not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ACR, albumin-creatinine ratio; CVD, cardiovascular disease; BMI, body mass index; NSAID, nonsteroidal antiiinflamatory drugs; Q, quartile; MET, metabolic equivalent; NHS, Nurses’ Health Study; DASH, Dietary Approach to Hypertension
Microalbuminuria
The 177 women (5.7%) who met the criterion for microalbuminuria (ACR of 25 to 355 mcg/mg) were more likely to be older, have hypertension, diabetes, cardiovascular disease, higher BMI and lower activity levels (Tables 1–3). In age and energy-adjusted models, the Western pattern diet was directly associated with microalbuminuria (OR of 3.55 [95% CI, 2.03 to 6.20] for 4th vs. 1st quartile) while the DASH score was inversely associated with microalbuminuria (OR of 0.53 [95% CI 0.33 to 0.84] for 4th vs. 1st quartile). There was no association between the prudent diet pattern and microalbuminuria. After multivariable adjustment, the association between Western diet and microalbuminuria remained significant (OR of 2.17 [95% CI 1.18 to 3.96] for 4th vs. 1st quartile) but not the DASH-style diet (Table 4). BMI, diabetes, and physical activity level appeared to be the major confounders in the association between dietary patterns and microalbuminuria.
TABLE 4.
Odds Ratios for Microalbuminuiria by Quartiles of Diet Pattern Scores
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Western | ||||
| Age and energy intake adjusted | 1.00 (reference) | 1.29 (0.81–2.05) | 1.51 (0.91–2.51) | 3.55 (2.03–6.20) |
| Multivariable* | 1.00 (reference) | 1.11 (0.68–1.81) | 1.12 (0.66–1.92) | 2.17 (1.18–3.96) |
| Prudent | ||||
| Age and energy intake adjusted | 1.00 (reference) | 0.98 (0.63–1.53) | 1.11 (0.71–1.74) | 0.94 (0.58–1.52) |
| Multivariable* | 1.00 (reference) | 0.89 (0.57–1.42) | 1.05 (0.66–1.67) | 0.97 (0.58–1.61) |
| DASH-style | ||||
| Age and energy intake adjusted | 1.00 (reference) | 0.82 (0.54–1.23) | 0.72 (0.47–1.10) | 0.53 (0.33–0.84) |
| Multivariable* | 1.00 (reference) | 0.80 (0.52–1.23) | 0.77 (0.49–1.21) | 0.71 (0.44–1.14) |
Microalbuminuria defined as an albumin-creatinine ratio of 25–355 mcg/mg. 95% CI shown in parentheses.
Adjusted for age, hypertension, body mass index, physical activity (metabolic equivalents per week), energy intake, cigarette smoking, diabetes, cardiovascular disease, and ACE-inhibitor/ARB medication use (note: alcohol intake and eGFR did not influence results and were removed).
Abbreviations: DASH, Dietary Approach to Hypertension; Q, quartile
Stratifying analyses by diabetes status also did not meaningfully change the results. When the outcome of ACR ≥ 25 mcg/mg was examined, the results also were not meaningfully changed.
Estimated GFR decline
There were 346 (11.3%) women who experienced an eGFR decline ≥ 30% between 1989 and 2000; this reflected a median increase in plasma creatinine of 0.33 mg/dL. There were 230 (7.5%) women with eGFR decline ≥ 3 ml/min/1.73 m2 per year, representing a median eGFR decline rate of 3.8 ml/min/1.73 m2 per year and a median increase in plasma creatinine of 0.34 mg/dL.
There were no significant associations between the prudent pattern and eGFR decline. After multivariable adjustment, the Western pattern was not significantly associated with an eGFR decline ≥ 30% (Table 5). The DASH score maintained a significant inverse association with eGFR decline ≥ 30% after adjustment (OR of 0.55 [95% CI 0.38 to 0.80] comparing top to bottom quartiles). The results were not meaningfully different when adjusted for anagelsic medication use, high cholesterol or lipid lowering medication use, or diabetes duration (Table 3). These associations for Western and DASH score did not vary by baseline eGFR<80 ml/min/1.73 m2 or diabetes status as p-values for interaction terms were all non-significant. Results using ≥ 3 ml/min/1.73 m2 per year eGFR decline were virtually identical except that the highest quartile of the Western pattern remained statistically significantly associated after multivariable adjustment (OR, 1.77; 95% CI 1.03 to 3.03).
TABLE 5.
Odds Ratios for eGFR Decline ≥ 30% by Quartiles of Diet Pattern Scores
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Western | ||||
| Age and energy intake adjusted | 1.00 (reference) | 1.37 (0.98,1.93) | 1.84 (1.29–2.64) | 1.95 (1.27–2.97) |
| Multivariable * | 1.00 (reference) | 1.22 (0.87,1.73) | 1.57 (1.08–2.28) | 1.48 (0.95–2.30) |
| Multivariable + analgesic medication use┼ | 1.00 (reference) | 1.22 (0.86,1.72) | 1.52 (1.04,2.20) | 1.40 (0.90,2.19) |
| Multivariable + high cholesterol or lipid lowering drug | 1.00 (reference) | 1.23 (0.87,1.73) | 1.57 (1.08–2.26) | 1.46 (0.94–2.28) |
| Multivariable + diabetes duration | 1.00 (reference) | 1.22 (0.86,1.72) | 1.58 (1.09–2.29) | 1.46 (0.94–2.28) |
| Prudent | ||||
| Age and energy intake adjusted | 1.00 (reference) | 1.44 (1.05–1.97) | 1.06 (0.76–1.48) | 0.78 (0.53–1.13) |
| Multivariable* | 1.00 (reference) | 1.43 (1.04–1.98) | 1.07 (0.76–1.51) | 0.81 (0.55–1.19) |
| Multivariable + analgesic medication use┼ | 1.00 (reference) | 1.44 (1.04,1.98) | 1.10 (0.78,1.56) | 0.82 (0.56,1.21) |
| Multivariable + high cholesterol or lipid lowering drug | 1.00 (reference) | 1.45 (1.05–2.00) | 1.09 (0.77–1.54) | 0.84 (0.57–1.23) |
| Multivariable + diabetes duration | 1.00 (reference) | 1.44 (1.04–1.98) | 1.07 (0.76–1.51) | 0.81 (0.55–1.19) |
| DASH-style | ||||
| Age and energy intake adjusted | 1.00 (reference) | 0.87 (0.64–1.18) | 0.79 (0.58–1.09) | 0.51 (0.36–0.72) |
| Multivariable* | 1.00 (reference) | 0.86 (0.63–1.17) | 0.79 (0.57–1.09) | 0.55 (0.38–0.80) |
| Multivariable + analgesic medication use┼ | 1.00 (reference) | 0.88 ((0.65–1.21) | 0.82 (0.60–1.13) | 0.57 (0.39–0.83) |
| Multivariable + high cholesterol or lipid lowering drug | 1.00 (reference) | 0.86 (0.63–1.18) | 0.79 (0.58–1.09) | 0.55 (0.38–0.79) |
| Multivariable + diabetes duration | 1.00 (reference) | 0.87 (0.64–1.18) | 0.79 (0.58–1.09) | 0.55 (0.38–0.80) |
Abbreviations: eGFR, estimated glomerular filtration rate; DASH, Dietary Approach to Hypertension; Q, quartile.
Adjusted for age, hypertension, body mass index, physical activity (metabolic equivalents per week), energy intake, cigarette smoking, diabetes, cardiovascular disease, and ACE-inhibitor/ARB medication use. (Note: alcohol intake and eGFR did not influence results and were removed)
We mailed a supplementary questionnaire in 1999 to collect detailed information on the current use of each of the 3 analgesic medication classes (aspirin, non-steroidal anti-inflammatory drugs (NSAIDS), and acetaminophen), including frequency in days per months, tablets per day, tablet dosage, brand, and indication for current use. The questionnaire also asked about total consumption in 2 periods: the past 10 years and before 1990. The total number of tablets taken in those 2 periods was collected in 11 categories: none, 1 to 100, 101 to 500, 501 to 1000, 1001 to 1500, 1501 to 3000, 3001 to 5000, 5001 to 10000, 10001 to 15000, 15001 to 20000, and 20001 or more. We used the combined total from the 2 periods by adding the midpoints of the categories. We converted number of tablets to lifetime intake, in grams, by multiplying the total number of tablets the midpoint of each category) by the most common dosage of each analgesic (aspirin and acetaminophen, 325 mg; and NSAIDs, 200 mg).
Our results for kidney function decline were unchanged when the CKD-EPI equation was used to estimate GFR,27 or if change in weight or BMI were included as covariates in the adjusted models.
DISCUSSION
Our data suggest that dietary patterns are associated with microalbuminuria and kidney function decline in middle-aged and older women. Women who were in the highest quartile of the Western pattern had a significant two-fold increased odds of having microalbuminuria and experiencing more rapid eGFR decline of ≥ 3 ml/min/1.73 m2 per year. Moreover, a DASH-style pattern appears to decrease risk by more than 40% for eGFR decline ≥ 30% over 11 years.
We previously reported that higher dietary intake of animal fat was associated with the presence of microalbuminuria whereas higher sodium intake was directly associated and higher beta-carotene intake was inversely associated with faster eGFR decline over 11 years.28 This current study provides further information regarding reductions in kidney function and dietary patterns that may be more easily interpreted by the general public.
The lack of association with the prudent pattern with the renal outcomes despite its correlation with the DASH pattern may be due to the different weights given to food groups used to derive each score. Our results suggest that the DASH score may better reflect food groups most relevant to microalbuminuria and eGFR decline. Of note, we do not think that the DASH-style diet is merely a surrogate for low sodium intake because we did not find significant associations DASH scores with 24-hour urinary sodium excretion in a subset of ~1200 NHS 1 women with these data (Taylor et al., CJASN 2010, in press). While dietary protein (particularly red meat) intake may potentially affect plasma creatinine concentrations, we have previously ascertained that all nutrient intake, including total and subtypes of protein, varied by <= 16% over time in this cohort of women,28 so changes in dietary protein intake are unlikely to explain change in eGFR.
We hypothesize that inflammation might be one possible pathophysiologic link between dietary patterns and microalbuminuria. A number of studies have reported significant direct associations between markers of inflammation and higher albuminuria. For example, in a recent publication from the National Health and Nutrition Examination Surveys 1999 to 2004, each one mg/dl unit increase in CRP was independently associated with a 1.02 (95% CI, 1.01 to 1.02; p=0.0003) odds ratio for presence of microalbuminuria in this large, nationally representative cohort.29 Furthermore, the highest tertile of ICAM-1 (inter-Cellular Adhesion Molecule 1), a vascular endothelial transmembrane glycoprotein upregulated by inflammation, also has been previously reported to be independently associated with development of incident sustained microalbuminuria (OR, 1.67; p-for-trend=0.03) in type 1 diabetics.30
Previous work on dietary patterns and inflammation reported that the Western diet pattern showed significant direct correlations between CRP, ICAM-1, and VCAM-1 (vascular cell adhesion molecule 1) in multivariable models that included adjustment for BMI in NHS women31 as well as Health Professionals Follow-Up Study (HPFS) men 32. The strong associations between Western pattern and inflammatory markers may explain the significant direct association of the Western dietary pattern with microalbuminuria. In these previous studies, the prudent pattern was not associated with inflammatory markers after multivariable adjustment, which may be consistent with the lack of association between the prudent pattern and microalbuminuria. No data appear to be currently available about the DASH score and markers of inflammation.
An investigation in the Multiethnic Study of Atherosclerosis (MESA) that included almost 5000 ethnically diverse men and women has similarly reported that a dietary pattern rich in whole grains, fruit, and low-fat dairy foods was associated with lower urinary ACR (20% lower ACR across quintiles, p-for-trend=0.004) whereas non-dairy animal-based food intake was directly associated (11% higher ACR across quintiles, p-for-trend 0.03).6 The MESA cohort has also reported that diets high in whole grains, fruits or vegetables, and fish are inversely associated with markers of inflammation including CRP and soluble ICAM-1 (sICAM-1) whereas a diet pattern high in fats and processed meats was directly associated with markers of inflammation including CRP.33 data in other cohorts provide external validation for our findings regarding diet patterns, inflammation, and albuminuria in the NHS cohort.
There are no published data on dietary patterns and eGFR decline, but recent investigations have suggested that markers of inflammation,34 including CRP,35 are associated with faster eGFR decline. Therefore, as inflammatory biomarkers have been proposed to be potential mediators for associations observed between diet and cardiovascular disease,36 we propose that inflammation may also be a factor in associations between diet and eGFR decline.
Notable strengths of this investigation include the relatively large number of women with data on both albuminuria and eGFR decline. Change in eGFR was assessed over an 11-year period, and repeated measures of diet intake over 14 years were performed. The substantial numbers of covariates, most which have been extensively validated in this large and well established longitudinal cohort are additional assets in these analyses.
Limitations of this study include the predominant Caucasian population of older women; therefore, results may not necessarily be generalizable to men or non-Caucasian populations. The similar results in the analysis of dietary patterns and albuminuria in the ethnically diverse MESA cohort, however, would suggest that the associations may not vary substantially by race or ethnicity.6 In addition, no data on change in urinary ACR was available in our participants, and albuminuria analyses are cross-sectional. Markers of inflammation are not available in this sub-cohort of women. The presence of residual confounding is also possible as in any observational study. Measurements of glycemic control to define glucose intolerance or pre-diabetes were not available in most of these women, although we conservatively considered a participant diagnosed with diabetes up to 10 years after the initial blood draw to address this issue. The possibility of survival bias is present because those women who died before 2000 would not have been included in this study; however, we would expect this to bias the results towards the null whereas statistically significant associations between dietary patterns and microalbuminuria and eGFR decline were observed.
In conclusion, a Western pattern diet was associated with a two-folds higher odds ratio for microalbuminuria and an increased risk for rapid eGFR decline (≥3 ml/min/1.73 m2 per year). A DASH-style diet was associated with an almost 50% decreased risk for eGFR decline. Therefore, diets higher in fruits, vegetables, and whole grains but lower in meat and sweets may be protective against eGFR decline. Future directions of interest include validation of these findings in other cohorts as well as examining how individual foods might influence microalbuminuria and eGFR decline.
Table 2.
Demographic, Clinical, and Nutrient Characteristics of NHS Participants in the Year 2000 Stratified by Prudent Pattern Diet Score
| Cumulative Averaged Prudent Pattern Diet Score | ||||||
|---|---|---|---|---|---|---|
| All NHS (N= 3121) | Q1 n=780 | Q2 n=780 | Q3 n=781 | Q4 n=780 | p-for-trend | |
| Age (years) | 67 (62, 73) | 65 (60, 71) | 66 (61, 72) | 68 (63, 73) | 69 (63, 74) | <.001 |
| Caucasian (%) | 97.4 | 96.4 | 97.9 | 98.4 | 96.9 | 0.05 |
| Hypertension (%) | 54.1 | 51.4 | 56.3 | 55.3 | 53.5 | 0.2 |
| SBP (mm/hg) in 1990 | 130 (120, 120) | 120 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 0.3 |
| DBP (mm/hg) in 1990 | 105 (70, 80) | 80 (70, 80) | 80 (70, 88) | 80 (70, 80) | 80 (70, 80) | 0.6 |
| Diabetes (%) | 23.1 | 20.1 | 22.1 | 26.5 | 23.6 | 0.02 |
| High cholesterol (%) | 65.0 | 61.2 | 64.2 | 65.7 | 68.7 | 0.02 |
| CVD (%) | 6.0 | 4.7 | 6.8 | 5.8 | 6.5 | 0.3 |
| Current smoker (%) | 5.8 | 11.3 | 6.0 | 2.3 | 3.5 | <.001 |
| Ever smoker (%) | 52.8 | 54.1 | 55.4 | 52.2 | 49.4 | 0.09 |
| Alcohol intake (g/day) | 1.7 (0.18, 7.0) | 1.6 (0, 7.6) | 1.8(0.2, 7.4) | 2.0 (0.2, 7.4) | 1.6 (0.2, 5.9) | 0.01 |
| Calorie intake (kcal/day) | 1726 (1468, 2020) | 1474 (1256, 1798) | 1661 (1458, 1929) | 1750 (1555, 2005) | 1989 (1708, 2273) | <.001 |
| Activity level (METs/week) | 11.4 (3.6, 25.2) | 8.2 (2.3, 18.5) | 10.2 (3.4, 22.1) | 13.2 (4.0, 26.1) | 16.2 (6.7, 32.4)) | <.001 |
| BMI (kg/m2) | 26.4 (23, 30.2) | 26.1 (23.0, 30.1) | 26.1 (22.9, 30.1) | 26.6 (23.0, 30.3) | 26.3 (23.0, 30.4)) | 0.9 |
| Aspirin use lifetime (g/day) | 748 (98, 3169) | 650 (98, 2454) | 748 (98, 3169) | 731 (98, 3656) | 975 (98, 3169) | 0.9 |
| NSAID use lifetime (g/day) | 60 (10, 1000) | 60 (10, 900) | 60 (10, 1200) | 60 (10, 900) | 60 (10, 900) | 0.9 |
| Acetominophen use through 1999 (g/day) | 98 (33, 1138) | 98 (33, 1138) | 98 (33, 1138) | 98 (33, 1138) | 98 (33, 975) | 0.4 |
| ACEi or ARB medication use (%) | 21.3 | 19.2 | 22.4 | 22..0 | 21.4 | 0.4 |
| Cholesterol lowering medication (ever used by year 2000) | 28.8 | 28.2 | 28.3 | 28.3 | 30.4 | 0.7 |
| Plasma Cr in 2000 (mg/dl) | 0.8 (0.7, 0.9) | 0.8 (0.7,0.9) | 0.8 (0.7,0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.02 |
| eGFR in 2000 (ml/min/1.73 m2) | 76 (65, 88) | 76 (66, 89) | 75 (63, 86) | 75 (64, 87) | 77 (66, 90) | 0.01 |
| Urinary ACR (mcg/mg) | 3.4 (2.0, 6.2) | 3.2 (1.9, 5.7) | 3.3 (2.0, 5.8) | 3.4 (2.1, 6.4) | 3.6 (2.1, 6.7) | 0.9 |
| Total protein (g/day) | 74 (68, 80) | 70 (63, 75) | 73(68, 78) | 75 (69, 81) | 78 (72, 84) | <.001 |
| Animal protein (g/day) | 53 (46, 59) | 51 (44, 57) | 53 (47,59) | 54 (47,60) | 54 (48, 61) | <.001 |
| Vegetable protein (g/day) | 21 (19, 23) | 19 (17, 21) | 20(19, 22) | 22 (20, 23) | 23 (21, 25) | <.001 |
| Total fat (g/day) | 56 (50, 61) | 60 (54, 65) | 57 (52, 61) | 55 (50, 59) | 51 (46, 57) | <.001 |
| Animal fat (g/day) | 30 (25, 34) | 33 (29, 38) | 31(27, 35) | 29 (25, 33) | 27 (22, 30) | <.001 |
| Sodium (mg/d) | 2001 (1801, 2227) | 1994 (1782, 2230) | 1988 (1790, 2204) | 2013 (1826, 2214) | 2018 (1806, 2253) | 0.4 |
| Beta-carotene (mg/day) | 4406 (3281, 5930) | 2944 (2215,3840) | 3888 (3190,4840) | 4856 (4054,5991) | 6239 (5073,7822) | <.001 |
| Median Prudent diet pattern score | −0.004 (−0.5,0.5) | −0.8 (−0.3, −0.1) | −0.2 (−0.3, −0.1) | 0.2 (0.1, 0.4) | 0.9 (0.7, 1.3) | NA |
Results expressed as median (25th, 75th percentile), percentage, or median (percentage). Conversion factors for units: Cr in mg/dL to μmol/L, x88.4; eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, x0.01667.
p<0.05 or
p<0.01 when compared to Q1 as referent group
ACR= albumin-creatinine ratio; eGFR=estimated glomerular filtration rate; Cr, creatinine; NA, Not applicable; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ACR, albumin-creatinine ratio; CVD, cardiovascular disease; BMI, body mass index; NSAID, nonsteroidal antiiinflamatory drugs; Q, quartile; MET, metabolic equivalent; NHS, Nurses’ Health Study
Acknowledgments
We would like to thank Elaine Coughlan-Gifford and Gideon Aweh for statistical programming support and Manyee To for assistance in manuscript preparation.
Support: This work was supported by NIH grants K08DK066246 and R03DK078551 (to Dr Lin), R01DK066574 (to Dr Curhan), R01HL065582 (to Dr Hu), and R01CA087969.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004 Sep 23;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003 Dec 2;139(11):901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Lin J, Solomon CG, et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007 Dec 4;116(23):2687–2693. doi: 10.1161/CIRCULATIONAHA.107.723270. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002 Feb;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR., Jr Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr. 2008 Jun;87(6):1825–1836. doi: 10.1093/ajcn/87.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005 Sep;82(3):675–684. doi: 10.1093/ajcn.82.3.675. quiz 714–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003 Mar 18;138(6):460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989 Dec;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 10.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001 Aug 13–27;161(15):1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 11.Kim JO, Mueller CW. Factor analysis: statistical methods and practical issues. California: Sage Publications, Inc; 1978. [Google Scholar]
- 12.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. Duxbury Press; 1988. Variable reduction and factor analysis; p. 718. [Google Scholar]
- 13.Institute IS. SAS/STAT User’s Guide. 6. Cary, NC: SAS Institute; 1989. [Google Scholar]
- 14.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008 Apr 14;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EN, Fung TT, Curhan GC. DASH-style diet and risk of incident kidney stones. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2009030276. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Your guide to lowering your blood pressure with DASH. 1. National Institutes of Health, National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 17.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996 Jun;7(6):930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006 Jun 8;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 19.Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004 Jul 26;164(14):1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- 20.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008 Nov 10;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999 Oct 15;150(8):806–816. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 22.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009 Mar 3;119(8):1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979 Dec;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 24.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 25.Shai I, Schulze MB, Manson JE, et al. A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care. 2005 Jun;28(6):1376–1382. doi: 10.2337/diacare.28.6.1376. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999 Mar 15;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Hu FB, Curhan GC. Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol. 2010;5:836–843. doi: 10.2215/CJN.08001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kshirsagar AV, Bomback AS, Bang H, et al. Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Surveys, 1999 to 2004) Am J Cardiol. 2008 Feb 1;101(3):401–406. doi: 10.1016/j.amjcard.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Glynn RJ, Rifai N, et al. Inflammation and progressive nephropathy in type 1 diabetes in the diabetes control and complications trial. Diabetes Care. 2008 Dec;31(12):2338–2343. doi: 10.2337/dc08-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004 Oct;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 32.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001 Jan;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006 Jun;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2009 Apr;53(4):596–605. doi: 10.1053/j.ajkd.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005 Jul;68(1):237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Garcia E, Hu FB. Nutrition and the endothelium. Curr Diab Rep. 2004 Aug;4(4):253–259. doi: 10.1007/s11892-004-0076-7. [DOI] [PubMed] [Google Scholar]