Abstract
Background
Gene-environment interactions (GEI) are involved in the pathogenesis of mental diseases. We evaluated interaction between mutant human Disrupted-In-Schizophrenia-1 (mhDISC1) and maternal immune activation implicated in schizophrenia and mood disorders.
Methods
Pregnant mice were treated with saline or polyinosinic:polycytidylic acid (Poly I:C) at gestation day 9. Levels of inflammatory cytokines were measured in fetal and adult brains, expression of mhDISC1, endogenous DISC1, LIS1, NDEL1, gp130, Grb2, and GSK-3β were assessed in cortical samples of newborn mice. Tissue content of monoamines, volumetric brain abnormalities, dendritic spine density in the hippocampus and various domains of the mouse behavior repertoire were evaluated in adult male mice.
Results
Prenatal interaction produced anxiety, depression-like responses, and altered pattern of social behavior. These behaviors were accompanied by decreased reactivity of the HPA axis, attenuated 5-HT neurotransmission in the hippocampus, reduced enlargement of lateral ventricles, decreased volumes of amygdala and periaqueductal gray matter and density of spines on dendrites of granule cells of the hippocampus. Prenatal interaction modulated secretion of inflammatory cytokines in fetal brains, levels of mhDISC1, endogenous mouse DISC1, and GSK-3β. The behavioral effects of GEI were observed only if mhDISC1 was expressed throughout the life span.
Conclusions
Prenatal immune activation interacted with mhDISC1 to produce the neurobehavioral phenotypes that were not seen in untreated mhDISC1 mice and that resemble aspects of major mental illnesses, including mood disorders. We propose that our DISC1 mouse model is a valuable system to study the molecular pathways underlying GEI relevant to mental illnesses.
Keywords: schizophrenia, mood disorders, DISC1, gene-environment interactions, mouse models, Tet-off system
The pathogenesis of major psychiatric diseases involves interactions between susceptibility genes and environmental factors (1–3). We believe that in vivo and in vitro systems based on identified genetic mutations and measurable environment factors can facilitate studies of the molecular mechanisms of gene-environment interaction (GEI) relevant to mental conditions (4). We set out to develop and characterize a mouse model of GEI based on our previously described system of inducible expression of mutant human Disrupted-In-Schizophrenia-1 (mhDISC1) in forebrain neurons (5, 6).
In a large Scottish family a balanced (1:11) (q42.1; q14.3) translocation disrupts two genes, named Disrupted In Schizophrenia 1 and 2 (DISC1 and DISC2) and co-segregates with schizophrenia, depression and bipolar disorder (7, 8). Accumulating evidence suggest that DISC1 may be a promising gene for mental health (9–13). The translocation may result in DISC1 haploinsufficiency or production of mutant DISC1 protein (14) that could act in a dominant-negative manner (15, 16). Both outcomes seem to lead to similar disturbance in DISC1-interacting proteins complexes and a loss of function of DISC1, a likely outcome of the translocation (17–19). Transgenic mice with inducible expression of dominant-negative mutant human DISC1 (mhDISC1) exhibit mild neurobehavioral abnormalities that allows us to evaluate if and how environment could modulate the pre-existing effects of mhDISC1 (4, 5).
Among non-genetic factors, in utero microbial infections have been implicated in the increased incidence of schizophrenia and mood disorders (20–24). It has been suggested that the maternal immune response to a microbe may account for the increased incidence of adult psychopathology (25, 26). For example, prenatal treatment with polyinosinic:polycytidylic acid (Poly I:C) or bacterial lipopolysaccharide (LPS) and prenatal influenza virus infection produce similar neurobehavioral abnormalities in adult mice or rats (27–30). As both influenza virus and poly I:C or LPS elicit a strong cytokine response in maternal serum, the amniotic fluid, placenta, and fetal brain (29, 31, 32), these inflammatory soluble factors produced in utero have been proposed to affect brain development (33–35).
In contrast to a previous study that injected Poly I:C into developing DISC1 mouse pups (36), we employed a widely used prenatal challenge with Poly I:C that is likely to be more relevant to the human situation, and to mimic maternal immune activation following microbial infections in humans (20–24). Another new development in the current study was a regulation of timing of expression of mhDISC1 to begin identifying time windows critical for GEI.
We chose to use treatment with Poly I:C at gestation day (GD) 9 because of the wealth of experimental data for this approach in mice (29, 37). This period roughly corresponds to the middle/end of the first trimester of human pregnancy with respect to developmental biology and percentage of gestation from mice to human (38). As our published findings have clearly indicated sex-dependent effects in mhDISC1 mice (6); and our pilot experiments revealed newly emerging behavioral phenotypes predominantly in male mice, this study was focused on male mhDISC1 mice. We found that prenatal interaction of immune activation with mhDISC1 produced elevated anxiety, depression-like responses, and an abnormal pattern of social behavior. The behavioral abnormalities were associated with decreased but prolonged reactivity of the HPA axis, attenuated 5-HT neurotransmission in the hippocampus, a decrease in enlargement of lateral ventricles, volumes of amygdale and periaqueductal gray matter, and linear density of spines on dendrites of granule cells of the dentate gyrus of the hippocampus. We also found that prenatal interaction modulated secretion of inflammatory cytokines in fetal brains and changed levels of mhDISC1, endogenous mouse DISC1 and GSK-3β in developing mouse pups. The behavioral effects of GEI were seen only if mhDISC1 was expressed throughout the life span.
Materials and Methods
Generation of experimental groups
Our mouse model of inducible expression of mutant hDISC1 is based on the Tet-off system (Figure S1A in Supplement 1) as described previously (5). The present study used lines 1001 and 1302B, which have high and low levels of expression of the mutant protein, respectively (5). Line 1001 was on the original mixed background (B6; SJL; CBA), and line 1302B was on C57BL6/J background. In addition, some experiments as indicated in the text were done on line 1001 backcrossed to C57BL6/J for 9 generations (1001N9) (Figure S1B in Supplement 1).
To study the effects of interactions between mhDISC1 and immune activation, pregnant single transgenic tTA mice were injected at gestation day 9 (GD9) with saline or Poly I:C (Sigma, 5 mg/kg). In contrast to previous studies that have utilized intravenous injections of Poly I:C (29), we performed intraperitoneal injections to minimize possible ceiling effects of Poly I:C itself. Thus, we generated the following experimental groups: single transgenic tTA mice (control mice) prenatally treated with saline or Poly I:C; double-transgenic mice (mutant mice) of all lines prenatally treated with saline or Poly I:C (Figure S1B in Supplement 1).
Expression of mhDISC1 can be regulated by Doxycycline (Dox)-containing food (200mg/kg of Dox, Bio-Serv, Frenchtown, NJ) (6). We evaluated the relative contributions of prenatal and postnatal expression of mhDISC1 to the behavioral effects. We generated tTA control and transgenic mice (line 1001N9) treated with Poly I:C at GD9 and raised on regular food until P21 followed by Dox-containing food until sacrifice. Mutant mice of this cohort, thus, had prenatal and early postnatal expression only (E0-P21). The other cohort of tTA and transgenic mice was raised on Dox food from the time of conception until weaning, P21 followed by regular food until sacrifice. As a result, mutant mice of this cohort had post-weaning expression that lasted until adulthood, (P21-AD) (Figure S1B in Supplement 1).
For all groups, pups were weaned on P21, genotyped and housed in sex-matched groups of five in standard mouse cages in accordance with Johns Hopkins University Animal Care and Use Committee guidelines.
Methods for cytokine assays, behavioral tests, monoamine assays, and western blotting were previously described (5, 6) and are presented in Supplemental Methods and Materials (see Supplement 1). Numbers of mice used in each test are indicated in the figure legends.
Acute stress challenge
Mice were acclimated to a quiet testing room for 60 min prior to testing. Animals were placed in plastic cylindrical restrainers and a blood sample (~20 μl) was collected (“baseline”) into a heparinized glass capillary tube within 2 min of restraint. Animals remained in the restrainers for 60 min and another blood sample (“stress”) was collected. Mice were released into their home cages to recover for 60 min at which time a final blood sample (“recovery”) was collected. Plasma was collected and stored at −80 °C until assay for corticosterone (CORT) concentration using commercially available radioimmunoassay kit (MP Biomedicals) according to manufacturer’s instructions.
Magnetic resonance imaging (MRI)
Upon completion of behavioral tests, live mice anesthetized by isoflurane were imaged with a 9.4 T nuclear magnetic resonance scanner (Bruker Biospin, Billerica, MA, USA). Fast-spin echo sequence was used for T2 weighted imaging with following parameters: TR=4.7 s and effective TE=22.4 ms, echo train length=4. Multiple slice 2D images were acquired with in-plane imaging matrix 192 168 and field of view 20 × 20 mm2. Slice thickness were 0.4 mm without gap between slices. Slice number was 60, covering the whole brain. The imaging resolution was 0.1 0.12 0.4 mm3. With six signal averaging, the scanning time was 40 min as described previously (5).
Golgi staining-based analysis
A modified, rapid Golgi staining was performed according to the manufacturer’s protocol (FD NeuroTechnologies, Germantown, MD) and as described before (6). We assessed the linear spine density on secondary and tertiary branches of basilar dendrites of pyramidal neurons of the CA1 region and granule cells of the dentate gyrus of the hippocampus. An Olympus microscope was used to trace each neuron using Neurozoom (San Diego, CA, USA). For spine density measurement, one terminal dendrite from the second and third order tip of each selected neuron was used to count spines using a 100× objective. Four neurons per section and 5 adjacent sections per each mouse (4 mice per group) were used to count the linear spine density. Images of Golgi staining were captured by Olympus microscope with a Nikon digital camera (DX M 1200) and processed with Nikon ACT-1 software.
Statistical analysis
The mouse behaviors, monoamine levels, volumetric brain measurements, linear spine density and levels of proteins or cytokines were evaluated with a mixed model ANOVA with the group and treatment as independent variables. Significant effects were explored further with lower levels ANOVAs and/or post hoc comparisons. P<0.05 was used for the significance level.
Results
Our prior reports have indicated expression of mhDISC1 as early as GD15 (5–6). Here, we demonstrate expression of mhDISC1 as early as GD9 (Figure S1C in Supplement 1), coinciding with the time of Poly I:C treatment.
Mutant human DISC1 modulated immune activation in fetal brains
There were significant alterations in expression of pro- and anti-inflammatory cytokines in control and mhDISC1 fetal brains after injection of Poly I:C. We found significant differences between saline-control and saline-mhDISC1 samples in basal levels of IL-1β [a group effect, F(1,19)=6.58, p<0.05] and IL-5 [the group by treatment interaction, F(1,19)=24.9, p<0.001] (Fig 1A). Consistent with previous reports (40), injections of poly I:C led to a significant up-regulation of cytokines in fetal brains: IL-1β [a treatment effect, F(1,19)=11.1, p=0.004]; IL-4 [a treatment effect, F(1,19)=23.9, p<0.001], and IL-5 [a treatment effect, F(1,19)=6.54, p=0.021]. In addition to modulating basal level, mhDISC1 altered poly I:C-induced secretion of cytokines. For example, while treatment with poly I:C led to the significant up-regulation of IL-4 and IL-5 in control mice, the levels of these factors remained unchanged in mutant hDISC1 mice; there were significant group × treatment interactions: IL-4 [F(1,19)=12.2, p=0.003] and IL-5 [F(1,19)=24.9, p<0.001] (Fig 1A). In contrast, poly I:C did not increase levels of IL-2 in control mice but augmented them in mhDISC1 mice, p<0.05 (Fig 1A). We observed a strong trend towards Poly I:C-induced up-regulation for IL-6 [a treatment effect, F(1,38)=3.4, p=0.075] but no significant changes in levels of TNF-α in mice (Fig 1B). Prenatal Poly I:C treatment had no significant effects on levels of cytokines in adult cortical samples (data not shown).
Figure 1.

Secretion of cytokines in fetal brains
(A) The levels of anti- and pro-inflammatory cytokines in fetal brains 6 hours after injection of Poly I:C, * denotes p<0.05 vs. the respective saline-treated group; # denotes p<0.05 vs. saline-treated control mice;
(B) The fold changes in the levels of IL-6 and TNF-α as calculated in relation to the averaged value for control saline group; the bars correspond to the minus and plus range of the fold changes, n=5–10 mice per group.
Immune activation produced phenotypes, resembling aspects of mood disorders
We evaluated general activity and fearfulness in mice using open field test (41). Treatments with poly I:C significantly increased peripheral activity in mutant DISC1 mice compared to control mice (Fig 2A), suggesting increased fearfulness. There was a significant effect of time for control mice [F(5,143)=8.7, p<0.001] and significant effects of time [F(5,131)=7.3, p<0.001] and treatment [F(1,131)=6.3, p=0.021] for mhDISC1 mice. No significant differences between groups were seen in central activity or rearing (Figure S2 in Supplement 1). Elevated plus maze is used to assess anxiety in mice (42). DISC1 mice treated with poly I:C spent significantly less time in open arms of elevated plus maze, p<0.05 (Fig 2B). FST is a popular test to examine depression-like behaviors in rodent (43, 44). We found that poly I:C-mhDISC1 interaction led to depression-like behaviors in mice in FST. Compared to mhDISC1 mice treated with saline, those given Poly I:C exhibited significantly increased immobility in FST (Fig 2C). There was the significant group by treatment interaction F(1,42)=8.2, p=0.007. No significant Poly I:C-induced changes were observed in control mice. Social interaction test assesses sociability and social recognition in mice (45, 46). Unlike other groups of mice, mutant DISC1 mice given Poly I:C showed an abnormal pattern of sociability manifested in comparably sniffing a live mouse and an inanimate object (Fig 2D and Figure S2D in Supplement 1). No differences between groups were found in numbers of entries to the chambers or social novelty preference (data not shown).
Figure 2.

The behavioral effects of interactions
(A) Elevated peripheral activity in open field in mhDISC1 mice treated with Poly I:C, * denotes p<0.05 vs. the saline-treated mhDISC1 group; n=10–14 male mice per group;
(B) Decreased time spent in open arms in mhDISC1 mice prenatally challenged with Poly I:C mice, * denotes p<0.05 vs. the saline-treated mhDISC1 group, n=10–14 male mice per group;
(C) Time of immobility in forced swim test (FST). Note increased time of immobility in mhDISC1 mice treated with Poly I:C compared to the saline-treated mhDISC1 group, * denotes p<0.05 vs. the saline-treated group, n=10–14 male mice per group;
(D) Sociability in mice in 3-chamber apparatus. The data reflect time of actively sniffing object or live mouse. Note an abnormal pattern of exploration in mhDISC1 given Poly I:C compared to all other groups, n=10–14 male mice per group, * denotes p<0.05 vs. object.
We also performed a series of cognitive tests to evaluate putative effects of GEI on learning and memory in mice. No effects of Poly I:C treatment were found in control or mhDISC1 mice in spontaneous alternation or spatial recognition in Y-maze, object recognition, Morris water maze tests or pre-pulse inhibition of the acoustic startle (Figure S3 in Supplement 1 and data not shown).
Interactions modulated stress reactivity in mhDISC1 mice
An acute stress test was used as an index of programming effects of GEI on the central nervous system functions. We found no differences between groups in basal levels of corticosterone (CORT). Although acute restraint stress produced a significant increase in CORT in all groups, mhDISC1 mice treated prenatally with Poly I:C demonstrated the smallest augmentation in CORT levels (Fig 3). There was a significant difference in CORT levels between saline-treated control mice and Poly I:C-treated mhDISC1 mice, p<0.05. The stress-induced rise in CORT levels was followed by a significant decrease and return to baseline levels 60 min after termination of the stressor in saline-treated control mice only, a significant effect of time, F(1,41)=44.58, p<0.01. Post-hoc test revealed a significant differences between the stress and recovery time points, p<0.05. In contrast, there was no significant decline in CORT levels in saline- or Poly I:C-treated-mhDISC1 or Poly I:C-treated control mice (Fig 3), all ps>0.05.
Figure 3.

Attenuated reactivity of the HPA axis
Quantitative analyses of levels of corticosterone (ng/ml) in blood sera in adult mice prenatally challenged with saline or Poly I:C; * denotes p<0.05 vs. stress; ** denotes p<0.05 vs. stress for saline-treated control mice only; # denotes p<0.05 vs. saline-treated control mice for stress conditions, n=5–7 in each group, line 1001B.
Immune activation altered brain abnormalities in DISC1 mice
Volumetric MRI analyses
Lateral ventricle enlargement is one the most consistent abnormalities of the brain of patients with schizophrenia (18, 47, 48). Prior studies with DISC1 mouse models, including our model, have found enlarged ventricles in adult mice (5, 49, 50). Consistent with prior observation, saline-treated mhDISC1 mice had larger lateral ventricles than saline-treated control mice (Fig 4A–D). Immune activation produced a significant increase in volumes of lateral ventricles in control mice and a modest decease in mhDISC1 mice, there was the significant group by treatment interaction, F(1,16)=5.77, p=0.032 (Fig 5E). Poly I:C treatment significantly and comparably decreased volumes of the right amygdala, the right and left hypothalamus and the right and left periaqueductal gray (PAG) in control and mhDISC1 mice (Fig 4F–G and Figure S4A in Supplement 1). Two-way ANOVA showed a significant effect of treatment for the right amygdala, F(1,23)=5.87, p<0.05; both right and left hypothalamus [right - F(1,23)=6.1, p<0.05, and left – F(1,23)=5.96, p<0.05]; and both right and left PAG [right - F(1,23)=7.51, p<0.05, and left – F(1,23)=7.13, p<0.05. No significant differences between saline-treated groups were found in the following brain regions: frontal cortex, hippocampus, dorsal and ventral striatum (data not shown).
Figure 4.
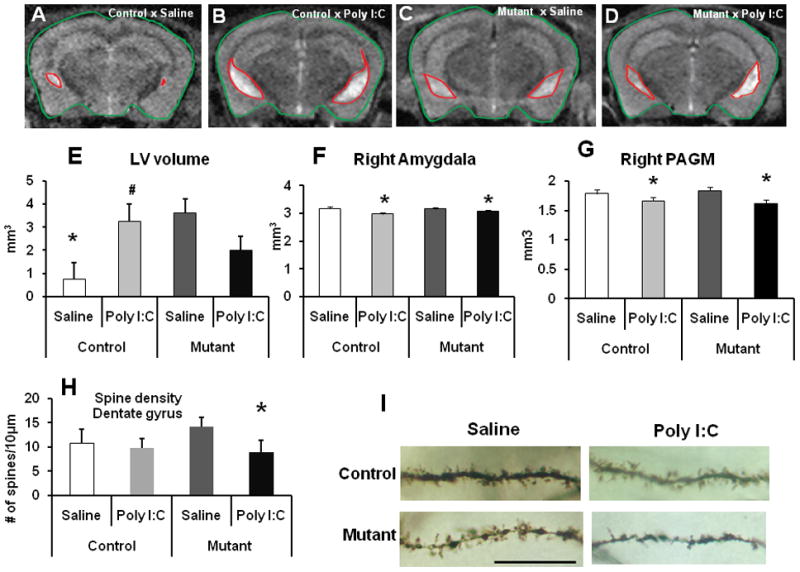
Brain effects of interaction
(A–D) Representative MRI coronal images for control-saline (a), control-Poly I:C (b), mutant-saline (c) and mutant-Poly I:C (d) groups. The boundaries of the brain and the lateral ventricles are outlined;
(E) Prenatal immune activation significantly increased volumes of the lateral ventricles in control mice and moderately decreased in mhDISC1 mice, n=3–5 mice per group, * denotes p<0.05 vs. saline-treated mhDISC1 mice; # denotes p<0.05 vs. saline-treated control mice;
(F) Prenatal immune activation significantly decreased volumes of the amygdala, n=4 mice per group, * denotes p<0.05 vs. the respective saline-treated group;
(G) Prenatal immune activation significantly decreased volumes of the periaqueductal gray matter (PAGM), n=4 mice per group, * denotes p<0.05 vs. the respective saline-treated group;
(H) Quantitative analysis of the linear spine density on dendrites of granule cells of the dentate gyrus; * denotes p<0.05 vs. saline-treated mhDISC1 mice; n=10–20 neurons per mouse, 4 mice per group;
(I) Representative images of dendritic spines from the groups of mice, scale bar - 20μm.
Figure 5.

Molecular markers of interactions
(A) Representative blots for mhDISC1, endogenous DISC1 (eDISC1) and GSK-3β from cortical samples collected at P2;
(B) Prenatal immune activation significantly increased levels of mhDISC1, n=6–7 samples per group, * denotes p<0.05 vs. saline-treated mhDISC1 mice;
(C) Prenatal immune activation significantly decreased levels of endogenous DISC1 in mhDISC1 mice, n=6–7 samples per group, # denotes p<0.05 vs. saline-treated mutant mice;
(D) Prenatal stimulation significantly decreased levels of GSK-3β in control mice but not in mhDISC1 mice, n=6–7 samples per group, * denotes p<0.05 vs. saline-treated control mice.
Dendritic spine density
Alterations in dendritic spine density have been demonstrated in several psychiatric disorders (51–53). In addition, there are reports of decreased spine density in hippocampal granule cells in the DISC1 mouse model (54). Prenatal immune activation significantly decreased linear spine density on dendrites of granule cells of the hippocampal dentate gyrus in mhDISC1 but not control mice. Two-way ANOVA found a significant effect of treatment, F(1,21)=13.6, and a significant effect of group, F(1,21)=8.5, p=0.009. There was a significant difference between saline-treated and Poly I:C-treated mhDISC1 mice, p=0.003 (Fig 4H–I). In pyramidal neurons of the CA1 area, Poly I:C treatment comparably decreased the linear density of spines in control and mutant mice (Figure S4B in Supplement 1). Two-way ANOVA showed a highly significant effect of treatment, F(1,10)=59.8, p<0.001. The effects on spine density were comparable in both mutant lines.
Regional monoamine levels
Alterations in monoamine neurotransmission have been associated with schizophrenia and mood disorders (55, 56). We evaluated tissue content of NE, DA, 5-HT and their metabolites, DOPAC and HVA, and 5-HIAA in the cortex, hippocampus and olfactory bulbs. In the cortex, NE levels were significantly increased by Poly I:C in control mice and were moderately decreased in mhDISC1 mice (Figure S5A in Supplement 1), there was the significant group × treatment interaction, F(1,22)=7.1, p=0.015. Poly I:C-treated control mice had significantly greater levels of NE in the cortex than saline-control animals, p<0.05. No other alterations in tissue content of monoamines, their metabolites or turnover were detected in cortical samples (Figure S5A–B in Supplement 1). In the hippocampus, treatment with Poly I:C significantly increased levels of 5-HT in the hippocampus of mhDISC1 (p=0.028) but not control mice (Figure S5C in Supplement 1). In addition, prenatal stimulation significantly decreased 5-HT turnover in the hippocampus of both groups, there was a significant effect of treatment, F(1,22)=13.7, p=0.002 (Figure S5D in Supplement 1). No alterations in tissue content of NE, DA, DOPAC, HVA or DOPAC/DA ratio were observed (Figure S5C–D in Supplement 1). No effects of Poly I:C on monoamine content were detected in olfactory bulbs (data not shown).
Molecular biomarkers of gene-environment interactions
Mutant human DISC1 has been proposed to exert its effects via dominant-negative mechanisms (15, 16), resulting in decreased levels of endogenous mouse DISC1 (5, 6). Here, we evaluated expression of mhDISC1, endogenous DISC1, its interacting partners, NDEL1, LIS1, GSK-3β and Grb2 as well as the cytokine receptor protein, gp130. We analyzed cortical samples from 2-day-old mice as significant alterations in levels of endogenous DISC1 and its interacting partners have been previously found at this time point (5, 6). Prenatal immune activation produced an up-regulation of mhDISC1 and decreased levels of endogenous mouse DISC1 (Fig 5A–C). While poly I:C stimulation was associated with decreased levels of phosphor-GSK-3β in control mice, no changes were found in mhDISC1 animals (Fig 5D). No alterations in levels of NDEL1, LIS1, gp130 or Grb2 were detected (Figure S6 in Supplement 1 and data not shown).
Level and timing of mhDISC1 expression modulated the outcomes of interactions
We analyzed dependence of the behavioral effects of interactions on mhDISC1 expression levels. To this effect, we assessed the behavioral phenotypes in a mouse line 1302B that expressed lower levels of mhDISC1 as described previously (5). We found that prenatal Poly I:C treatment of 1302B mice produced the behavioral abnormalities similar to ones found in 1001 mice in FST, TST and social interaction tests but not in anxiety test (Figure S7 in Supplement 1), suggesting that a certain level of expression could influence the pattern of behavioral effects of interactions. Similar to the line with strong expression of mhDISC1, we saw no effects of prenatal stimulation on cognitive functions in mice (data not shown).
It has been proposed that there are sensitive time windows for GEI to produce behavioral disease in adult offspring (1). We analyzed the effects of GEI in mice treated with poly I:C at GD9 but with selective expression during either prenatal and early postnatal (E0-P21) period or the period that lasted from post-weaning till adulthood (P21-AD). Despite immune activation, there were no significant alterations in FST, TST or social behaviors in either group of these mice (Fig 6 and Figure S8 in Supplement 1).
Figure 6.

Dependence of the behavioral effects on time of expression
(A) Time of immobility in forced swim test (FST) in mice with expression of mhDISC1 on E0-P21. Note the lack of differences between control and mutant mice, n=10–21 mice per group;
(B) Sociability in mice with expression of mhDISC1 on E0-P21. Note the lack of differences between control and mutant mice, n=10–21, * denotes p<0.05 vs. time spent in exploration of a live mouse;
(C) Time of immobility in forced swim test (FST) in mice with expression of mhDISC1 on P21-adulthood. Note the absence of differences between control and mutant mice; n=10–14 mice per group;
(D) Sociability in mice with expression of mhDISC1 on P21-adulthood. Note the lack of differences between control and mutant mice, * denotes p<0.05 vs. time spent in exploration of a live mouse; n=10–14 mice per group
Discussion
The study found that prenatal immune activation interacted with mhDISC1 in producing the behavioral abnormalities that were not present in unchallenged mhDISC1 mice: elevated anxiety, depression-like responses, an altered pattern of sociability and attenuated reactivity to stress. These behaviors were associated with decreased enlargement of lateral ventricles, reduced volumes of the amygdala and periaqueductal gray matter, and decreased density of dendritic spines on granule cells of the hippocampus. GEI in this model also gave rise to altered secretion of pro- and anti-inflammatory cytokines in fetal brains, attenuated 5-HT neurotransmission in the hippocampus and changed levels of mhDISC1, endogenous mouse DISC1, and GSK-3β in newborn mice. Table 1 summarizes the effects of GEI in this model. We also found that the behavioral changes were dependent on continuous expression of mhDISC1.
Table 1.
The summary of the effects of gene-environment interactions
| Test | Outcomes | Possible interpretations |
|---|---|---|
|
Affective behaviors Open field test - peripheral zone Elevated plus maze – open arms Forced swim test - immobility Tail suspension test - immobility Sociability test – sniffing time |
Increased Decreased Increased Increased Decreased |
The effects in these tests suggest increased anxiety and depression-like phenotypes, consistent with aspects of mood disorders |
|
Learning and memory Spontaneous alternation Spatial recognition in Y maze Morris water maze Object recognition test |
Unaffected Unaffected Unaffected Unaffected |
These tests evaluate cognitive changes in mice. The lack of the significant effects in the tests may indicate no effects on learning and memory |
|
Sensory-motor integration Pre-pulse inhibition of the acoustic startle |
Unaffected |
This test evaluates sensory--motor gating processing in mice |
|
Brain pathology Lateral ventricles enlargement Brain region volumes - Amygdala - Hypothalamus - PAGM - Cortex - Hippocampus - Striatum Spine density in the hippocampus |
Attenuated Decreased Decreased Decreased Unaffected Unaffected Unaffected Decreased |
The brain abnormalities may explain the affective phenotypes and seem to be consistent with brain pathological findings in patients with mood disorders |
|
Neurochemical changes - Dopamine - Norepinephrine - Serotonin - DA metabolism - 5-HT metabolism |
Unaffected Unaffected Increased Unaffected Decreased |
Attenuated 5-HT neurotransmission may contribute to the behavioral phenotypes in mice and appears in line with alterations found in affective disorders |
|
Molecular markers - Mutant DISC1 - Endogenous DISC1 - GSK-3β |
Increased Decreased Unaffected by poly I:C stimulation in mutant DISC1 mice |
Altered expression of DISC1 and its interactors may underlie the brain abnormalities in mice GSK-3β is a key DISC1 partner and a main factor of the immune signaling. Abnormal interaction between GSK-3β and DISC1 could contribute to altered cytokine production in mhDISC1 mice |
Our study is the first multi-disciplinary work to use a relevant genetic mutation in a combination with an environmental event implicated in mental diseases in humans. Prior gene-environment studies either used genetic mouse models without clear relevance to the human data or utilized environmental challenges that have not been really associated with mental conditions (4). One such study evaluated the effects of postnatal injections of poly I:C in mouse pups with constitutive expression of mutant DISC1 (36) and found that neonatal poly I:C treatment in DISC1 mice resulted in the deficits of object recognition and fear conditioning. By contrast, we used a more relevant approach to induce immune activation in pregnant mice, modeling the human situation in which prenatal infections produce maternal immune responses (22, 26, 38), and we observed the phenotypes that may resemble aspects of mood disorders.
Our findings suggest that the same mutation can lead to different phenotypes, depending on interactions with environmental factors. The Scottish chromosomal translocation is associated with a range of major mental diseases, including schizophrenia, recurrent depression and bipolar disorder (9, 11, 57). It is tempting to speculate that the diversity of clinical phenotypes in the pedigree could be in part explained by exposures to environmental factors. In addition to the behavioral changes, GEI also modulated the pre-existing brain pathology. Previous studies have demonstrated enlargement of lateral ventricles in unchallenged DISC1 mice (5, 6, 58). Consistently, saline-treated mhDISC1 mice had significantly enlarged lateral ventricles compared to saline-treated control mice. Increased volumes of brain ventricles were somewhat attenuated by immune activation in mhDISC1 mice. In contrast, prenatal immune challenge itself produced significant enlargement of ventricles in control mice, in line with previous reports (59, 60). Prenatal immune activation also decreased volumes of the amygdala, hypothalamus, and periaqueductal gray matter. The observed affective behaviors could be partly related to decreased volumes of those brain regions since they are involved in the neuronal circuitry of emotional responses in mice (61, 62). Intriguingly, similar volumetric abnormalities were reported for patients with major depression and bipolar disorder (63–67), although several conflicting reports have been published as well (63, 68, 69). We also found that prenatal immune challenge significantly decreased spine density on granule cells in mhDISC1 mice. The decreased spine density in the hippocampal granule cells seems to be in line with data for human mental diseases (52, 53, 70), and the results obtained with some rodent models of maternal immune challenge (71, 72).
A number of investigations have highlighted the importance of abnormal fetal expression of cytokines in neurodevelopmental abnormalities (25, 26, 73). The cytokines detected in fetal samples were unlikely from dam’s blood sera as the same dam had mutant and control fetuses that displayed differences in cytokines levels. The differential outcomes of prenatal challenge in mhDISC1 and control mice might be due to mhDISC1-related modulation of basal and/or Poly I:C-induced cytokine production. One possible mechanism to explain these effects might involve mhDISC1-produced perturbations in the GSK-3β-dependnet signaling that plays a critical in the innate immune response (74). Given direct interaction between DISC1 and GSK-3β, it is tempting to speculate that dominant-negative effects of mhDISC1 on endogenous DISC1 might affect its interaction with GSK-3β (75–76), resulting in altered cytokines production, derailed neurodevelopment and associated behavioral changes, consistent with aspects of major mental diseases, including mood disorders.
There are also other mechanisms whereby prenatal immune activation can affect brain maturation. Secreted cytokines can activate the HPA axis, stimulate the release of corticotropin-releasing factor from the hypothalamus and adrenocorticotropic hormone from the pituitary gland and, thus, interfere with fetal neurodevelopment and/or the functions of the HPA axis in adulthood (33, 77, 78). Prenatal immune activation and its interaction with mhDISC1 were associated with attenuated and prolonged CORT response to acute stress in adult mice, suggesting impaired glucocorticoid negative feedback (79), consistent with attenuated HPA axis reactivity in mood disorders (80). In addition, elevated levels and decreased turnover of 5-HT in the hippocampus in mhDISC1 mice treated with Poly I:C are reminiscent of serotonin abnormalities associated with affective states (64, 81, 82).
An interesting finding of this work is that the behavioral effects of GEI were dependent on continuous expression of mhDISC1. When expression of mhDISC1 was present either only before or only after P21, we no longer observed the behavioral abnormalities, suggesting that the effects of GEI can modulate the effects of mhDISC1 but that continuous expression of the mutant gene seems to be required for manifestation of the phenotypes. Elimination of the behavioral phenotypes after turning off expression during late postnatal period may indicate that some neurobehavioral abnormalities produced by GEI could be amenable to postnatal treatments even though GEI took place during embryogenesis. More studies are clearly needed to better evaluate the time windows of GEI in this model.
In conclusion, the findings suggest that prenatal interaction between immune activation and mhDISC1 modulated the existing neurobehavioral phenotypes, recapitulating aspects of mood disorders. The data indicate that our DISC1 mouse model is a valuable system to study the molecular pathways underlying gene-environment interplay relevant to major mental illnesses, and may suggest design and timing of treatment trials.
Supplementary Material
Acknowledgments
The study was supported by NIMH, Autism Speaks, NARSAD and the Mortimer W Sackler Foundation (MVP), and The Cell Science Research Foundation Japan (JN) and by NIH/NIDA-IRP (BL, INK, JLC). Author contributions: MVP designed research. BA, JN, GK, KT, KI, FN, VP, CY, BL and IK performed research. SM analyzed MRI data, JLK contributed to HLPC analysis; AK contributed to biochemical analysis; MV analyzed spine density data; CPV contributed to cytokine analysis; AS and CAR contributed to behavioral and biochemical analysis; BA, JN and MVP wrote the paper.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- 3.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayhan Y, Sawa A, Ross CA, Pletnikov MV. Animal models of gene-environment interactions in schizophrenia. Behav Brain Res. 2009;204:274–281. doi: 10.1016/j.bbr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. 115. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 6.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 Jan 5; doi: 10.1038/mp.2009.144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar JK, Christie S, Semple CA, Porteous DJ. Chromosomal location and genomic structure of the human translin-associated factor X gene (TRAX; TSNAX) revealed by intergenic splicing to DISC1, a gene disrupted by a translocation segregating with schizophrenia. Genomics. 2000;67:69–77. doi: 10.1006/geno.2000.6239. [DOI] [PubMed] [Google Scholar]
- 8.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 9.Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 10.Hennah W, Porteous D. The DISC1 pathway modulates expression of neurodevelopmental, synaptogenic and sensory perception genes. PLoS One. 2009;4:e4906. doi: 10.1371/journal.pone.0004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14:865–873. doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- 12.Hennah W, Tuulio-Henriksson A, Paunio T, Ekelund J, Varilo T, Partonen T, et al. A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol Psychiatry. 2005;10:1097–1103. doi: 10.1038/sj.mp.4001731. [DOI] [PubMed] [Google Scholar]
- 13.Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL. A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry. 2005;10:758–764. doi: 10.1038/sj.mp.4001667. [DOI] [PubMed] [Google Scholar]
- 14.Millar JK, Christie S, Anderson S, Lawson D, Hsiao-Wei Loh D, Devon RS, et al. Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry. 2001;6:173–178. doi: 10.1038/sj.mp.4000784. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 16.Pletnikov MV, Xu Y, Ovanesov MV, Kamiya A, Sawa A, Ross CA. PC12 cell model of inducible expression of mutant DISC1: new evidence for a dominant-negative mechanism of abnormal neuronal differentiation. Neurosci Res. 2007;58:234–244. doi: 10.1016/j.neures.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1--an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 22.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–637. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 25.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson PH. NEUROSCIENCE: Maternal Effects on Schizophrenia Risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 29.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 33.Leonard BE, Myint A. The psychoneuroimmunology of depression. Hum Psychopharmacol. 2009;24:165–175. doi: 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- 34.Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M, et al. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 206:32–37. doi: 10.1016/j.bbr.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 39.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 40.Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 41.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 42.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 43.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 44.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 46.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 52.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001;58:203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- 54.Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remington G. Alterations of dopamine and serotonin transmission in schizophrenia. Prog Brain Res. 2008;172:117–140. doi: 10.1016/S0079-6123(08)00906-0. [DOI] [PubMed] [Google Scholar]
- 56.Wood MD, Wren PB. Serotonin-dopamine interactions: implications for the design of novel therapeutic agents for psychiatric disorders. Prog Brain Res. 2008;172:213–230. doi: 10.1016/S0079-6123(08)00911-4. [DOI] [PubMed] [Google Scholar]
- 57.Porteous D. Genetic causality in schizophrenia and bipolar disorder: out with the old and in with the new. Curr Opin Genet Dev. 2008;18:229–234. doi: 10.1016/j.gde.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009:108135. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58:123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 63.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 64.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrett A, Chang K. The role of the amygdala in bipolar disorder development. Dev Psychopathol. 2008;20:1285–1296. doi: 10.1017/S0954579408000618. [DOI] [PubMed] [Google Scholar]
- 66.Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 67.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 68.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 69.McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biological Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 71.Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Lowe GC, Luheshi GN, Williams S. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1563–1571. doi: 10.1152/ajpregu.90350.2008. [DOI] [PubMed] [Google Scholar]
- 73.Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- 74.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Kloet ER, Karst H, Joels M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 79.Jansen LM, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden JA, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr Res. 1998;33:87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- 80.Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, et al. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biol Psychiatry. 2006;60:867–873. doi: 10.1016/j.biopsych.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 81.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 82.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


