Abstract
Aims
Late phase ischemic preconditioning (LPC) protects the heart against ischemia reperfusion (I/R) injury. However, its effect on myocardial tissue oxygenation and related mechanism(s) are unknown. The aim of the current study is to determine whether LPC attenuates post-ischemic myocardial tissue hyperoxygenation through preserving mitochondrial oxygen metabolism.
Main methods
C57BL/6 mice were subjected to 30 min coronary ligation followed by 60 min or 24 hr reperfusion w/o LPC (3 cycles of 5 min I/5 min R): Sham, LPC, I/R, and LPC+I/R group. Myocardial tissue Po2 and redox status were measured with electron paramagnetic resonance (EPR) spectroscopy.
Key findings
Upon reperfusion, tissue Po2 rose significantly above the pre-ischemic level in the I/R mice (23.1 ± 2.2 vs. 12.6 ± 1.3 mmHg, p<0.01). This hyperoxygenation was attenuated by LPC in the LPC+I/R mice (11.9 ± 2.0 mmHg, p<0.01). Activities of NADH dehydrogenase (NADH-DH), succinate-cytochrome c reductase (SCR) and cytochrome c oxidase (CcO) were preserved or increased in the LPC group, significantly reduced in the I/R group, and conserved in the LPC+I/R group. Manganese superoxide dismutase (Mn-SOD) protein expression was increased by LPC in the LPC and LPC+I/R mice compared to that in the sham control (1.24 ± 0.01 and 1.23 ± 0.01, p<0.05). Tissue redox status was shifted to the oxidizing state with I/R (0.0268 ± 0.0016/min) and was corrected by LPC in the LPC+I/R mice (0.0379 ± 0.0023/min). Finally, LPC reduced the infarct size in the LPC+I/R mice (10.5 ± 0.4% vs. 33.3 ± 0.6%, p<0.05).
Significance
Thus, LPC preserved mitochondrial oxygen metabolism, attenuated post-ischemic myocardial tissue hyperoxygenation, and reduced I/R injury.
Keywords: ischemia reperfusion injury, redox status, mitochondrial electron transport chain, EPR oximetry, reactive oxygen/nitrogen species
Introduction
Myocardial ischemia is characterized by insufficient supply of oxygen and nutrient to the risk area, resulting in tissue infarction. Reperfusion restores blood flow to the ischemic myocardium and reduces the extent of damage to the heart. However, reperfusion causes additional damage to the affected myocardium. (Braunwald and Kloner 1985) Among many of the causative effectors, reactive oxygen/nitrogen species (ROS/RNS) has been recognized as a key mediator in the progression of ischemia reperfusion (I/R) injury. (Yasmin et al. 1997, Zweier 1988)
Despite advances in the treatment of ischemic heart disease, morbidity and mortality remains significant. (Balakumar et al. 2008) Therefore, new protective interventions are needed. Ischemic preconditioning (IPC), with several cycles of short ischemia and reperfusion, triggers protective signaling pathways, suppresses ROS/RNS generation, and reduces subsequent I/R injury. (Baxter and Ferdinandy 2001, Bolli et al. 1998, Murry et al. 1991) Later on, IPC was found to be biphasic, with an early phase of protection that develops within minutes and lasts for 2-3 hours, and a late phase of protection that becomes apparent after 12-24 hours and lasts for 3-4 days. (Bolli 2000, Kuzuya et al. 1993, Marber et al. 1993) Because of its sustained duration of protection, late phase ischemic preconditioning (LPC) should have greater clinical relevance. While the underlying signaling pathways for both acute and late protection have been extensively studied, there remains incomplete understanding of the whole protective mechanisms. Especially, there is no prior study on the effect of LPC on myocardial tissue oxygenation which reflects a delicate balance between oxygen supply and demand in the post-ischemic heart.
Our previous study has demonstrated that there is a hyperoxygenation status after I/R in mouse heart. (Zhao et al. 2005) Endothelium-derived NO and its derivative peroxynitrite (ONOO-) has been implicated in the suppression of myocardial oxygen consumption by irreversible modifications to the subcomponents of mitochondrial respiratory chain, such as NADH dehydrogenase (NADH-DH) and cytochrome c oxidase (CcO). (Zhao et al. 2005) Therefore, enhancing tissue antioxidant capability should be protective to the ischemic heart. Indeed, over-expression of manganese (Mn-SOD), copper/zinc (CuZn-SOD) or extracellular superoxide dismutase (EC-SOD) rendered the heart resistant to I/R injury. (EP Chen et al. 1998, Z Chen et al. 2000, Z Chen et al. 1998) In vitro, it was shown that Mn-SOD activity was increased in preconditioned myocytes. (Pan et al. 2007, Rui et al. 2003) In vivo, LPC was reported to increase Mn-SOD expression and to reduce the production of ROS/RNS after I/R in canine and rat models. (Csonka et al. 2001, Hoshida et al. 1993, Yamashita et al. 1998) However, in vivo tissue oxygenation status and its relationship to the expression of SODs in the LPC mouse hearts are not clear. We hypothesize that LPC attenuates post-ischemic myocardial hyperoxygenation by protecting mitochondrial oxygen consumption through increasing the expression of superoxide dismutase and reducing the formation of ROS/RNS.
To measure in vivo tissue oxygenation (Po2), electron paramagnetic resonance (EPR) oximetry with oxygen-sensitive probe lithium phthalocyanine (LiPc) was employed. (Swartz et al. 1994, Zhu and Liu et al. 2007) Regional tissue blood flow was measured with laser Doppler technique, in conjunction with in vivo EPR oximetry, to enable in vivo assessment of myocardial oxygen consumption. (Zhu and Liu et al. 2007) With these techniques, the current study demonstrated that LPC attenuated myocardial reperfusion hyperoxygenation and preserved tissue oxygen consumption by up-regulating Mn-SOD expression and preventing the impairment of mitochondrial enzyme activities. This protection was associated with reduction of infarct size in the post-ischemic mouse heart.
Methods and material
Animals
Male C57BL/6 mice from Jackson Laboratory (Bar Harbor, ME) were housed under a 12:12-hr light-dark cycle and were provided with water and food ad libitum. All procedures were performed with the approval of the Institutional Animal Care and Use Committee at the Ohio State University, Columbus, Ohio, and conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No.86-23, revised 1996).
Myocardial I/R model and LPC protocol
The I/R model and surgical procedures were similar to methods described previously. (Hoffmeyer et al. 2000, Jones et al. 2000, Zhao et al. 2005) Mice were anesthetized with ketamine (55 mg/kg) and xylazine (15 mg/kg). Atropine (0.05 mg s.c.) was administered to reduce airway secretions. Isofluorane was used to maintain anesthesia in all animals and due to its cardioprotective effect, (Cesarovic et al.) controls were included with the same isofluorance dosage for all experimental groups for comparison. Animals were orally intubated with PE-90 tubing and connected to a mouse mini-ventilator (model 845; Harvard Apparatus). Core body temperature was maintained at 37 °C with a thermo heating pad and monitored with a rectal thermometer.
A median thoracotomy was performed, and the left anterior descending coronary artery (LAD) was visualized and completely ligated for 30 min in the I/R mice by tightening a 7-0 silk suture after passing it over a length of PE-10 tubing above the LAD at points 1 to 2 mm inferior to the left auricle. The 7-0 silk suture was similarly placed in the sham group but without LAD occlusion. The PE-10 tubing was used to achieve easier ligature release and better reperfusion. Ischemia was confirmed visually by the appearance of pale and bulging myocardium in the area at risk (AAR). The ligature was removed, and reperfusion was visually confirmed after 30 min LAD occlusion. Reperfusion was maintained for 60 min for oximetry measurements and 24 hr for the measurement of infarct size.
LPC was introduced by three cycles of 5 min ischemia, followed by 5 min reperfusion. Then, the chest was closed and the mouse was allowed to recover. All the surgeries were performed with sterilized tools and pain-killer ibuprofen was used as needed. Twenty four hours later, the LPC+I/R mice were subjected to 30 min LAD occlusion followed by 60 min reperfusion (for EPR oximetry measurements) or 24 hr reperfusion (for measurements of infarct size).
EPR oximetry
For measurement of in vivo myocardial tissue Po2, approximately 10 μg of LiPc, an oxygen-sensitive probe, was loaded in a 27-gauge needle and implanted in the midmyocardium of the AAR after the heart was exposed. The location of the LiPc was confirmed by histology to be in the mid-myocardium in the area at risk. Then the mouse was transferred to the L-band EPR spectrometer (Magnettech GmbH, Germany), and after 30 min equilibration of the probe with the surrounding tissue, EPR spectra were collected before, during, and after ischemia and reperfusion with the following parameters: frequency, 1.1 GHz; microwave power, 16 mW; and modulation amplitude, 0.045 G. The sensitivity of the probe to tissue Po2 is 5.8 mG/mm Hg. (Xu et al. 2008)
Regional blood flow measurement
For measuring regional blood flow, after thoracotomy and exposure of the heart, an optical surface suction probe with a diameter of 2 mm (P10d; Moor Instruments, Devon, UK) was placed on the surface of the heart at the AAR to be able to move with the beating heart. The probe was connected to a laser Doppler tissue perfusion monitor (Moor Instruments) and the blood flow under the probe was monitored during preischemia, 30 min ischemia, and 60 min reperfusion as described previously. (Xu et al. 2008)
Activities of NADH-DH, Succinate-cytochrome c reductase (SCR), and CcO
Heart tissue from the risk area was excised, washed, and frozen immediately at the end of 60 min reperfusion. Then tissue samples were homogenized in ice-cold HEPES buffer (3mmol/l, pH 7.2) containing sucrose (0.25 mol/l), EGTA (0.5 mmol/l) and protease-inhibitor cocktail (1:40; Roche). NADH-DH activity was measured in the presence of Tris·HCl buffer (20 mmol/l, pH 8.0), NADH (150 μmol/l; Sigma) and coenzyme Q1 (100 μmol/l; Sigma). The activity of SCR (or the super complex containing complex II and complex III) was measured in the presence of phosphate buffer (50 mmol/l, pH 7.4), EDTA (0.3 mmol/l, pH7.93), KCN (100 μmol/l), succinate (19.8 mmol/l, pH8.0; Sigma) and ferricytochrome c (50 μmol/l; Sigma). CcO activity was measured in the presence of phosphate buffer (50 mmol/l, pH 7.4) and reduced cytochrome c (60 μmol/l; Sigma). (Xu et al. 2008) The extinction coefficients, ε550 nm = 18.5 mmol/l·cm for cytochrome c and ε340 nm = 6.22 mmol/l·cm for NADH, were used for activity calculation. (YR Chen et al. 2004) Protein concentration of the tissue homogenate was measured by BCA assay (PIERCE Biotechnology).
Western blot analysis
Proteins of heart tissue homogenate were subjected to electrophoresis on 4-20% Trisglycine polyacrylamide gradient gels and electrophoretically transferred onto nitrocellulose membranes (Amersham Biosciences). After blocking with 5% dry milk in TTBS for 1 hr at room temperature, the membranes were incubated with monoclonal antibodies specific for oxidative phosphorylation: anti-OxPhos Complex I subunit 39 kDa (42.5 kD), anti-OxPhos Complex III subunit FeS (29.6 kD), Anti-Oxphos Complex IV subunit VIb (10.0 kD) (Invitrogen, Carlsbad, CA). GAPDH were blotted to confirm equal loading (anti-GAPDH, Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies were conjugated with horseradish peroxidase, and the protein was detected with enhanced chemiluminescence (Denville Scientific). The exposure time was chosen such that the weakest bands were clearly shown. The band intensity was quantified in an AlphaImager high-performance gel documentation and image analysis system (model 3300; Alpha Innotech, San Leandro, CA).
In vivo myocardial tissue redox status
In vivo myocardial tissue redox status was measured with EPR spectroscopy using nitroxide 2,2,5,5-tetramethyl-3-carboxylpyrrolidine-N-oxyl (PCA) (Sigma Chemical Co., Milwaukee, WI) as the redox probe as described previously. (Zhu and Liu et al. 2007, Zhu and Zuo et al. 2007) About 10 μl of 10 mM PCA solution (in PBS) was injected intramuscularly as a bolus into the area at risk and EPR spectra were recorded immediately after. The lower field peak-height was monitored with time to determine the reduction rate constant. As previously validated, this reduction rate constant indicates the global tissue reducing capability.
Infarct size
At the end of 24 hr reperfusion, hearts were excised and cannulated through the ascending aorta with a 23-gauge needle for perfusion with 3-4 ml of 1.0% triphenyl tetrazolium chloride (TTC) in phosphate buffer (pH 7.4, 37 °C). The LAD was re-occluded by tightening the suture left in the myocardium after I/R and TTC staining. The hearts were then perfused with 2-3 ml of 2.5% Evans Blue (Sigma-Aldrich) to mark the non-ischemic myocardium. The hearts were weighed, frozen, and cut into five transverse slices, each about 1-mm thick. Each slice was photographed from both sides with a high-resolution digital camera on a dissecting microscope. The sections were photographed and contoured with a planimeter (MetaVue Version 6.2r6) to delineate the borders of the entire heart, the non-ischemic area, and the infarct area. The sizes of the AAR (total left ventricle (LV) area – non-ischemic area) and the infarct size were calculated as percentages of the total LV area and the AAR multiplied by the weight of that slice.
Statistic analysis
A two-way ANOVA was used for data analysis of Po2 and blood flow. A one-way ANOVA was used for enzyme activity and protein expression; these were followed by Student Newman-Keuls multiple-comparison test among the groups. A t-test was used for data analysis of AAR and infarct size. Generally, the number of animals were 5/group, except that in the Po2 measurement, where N = 6/group. Data were represented as means ± SE. A value of p<0.05 was considered significant.
Results
Myocardial tissue Po2 in the risk area
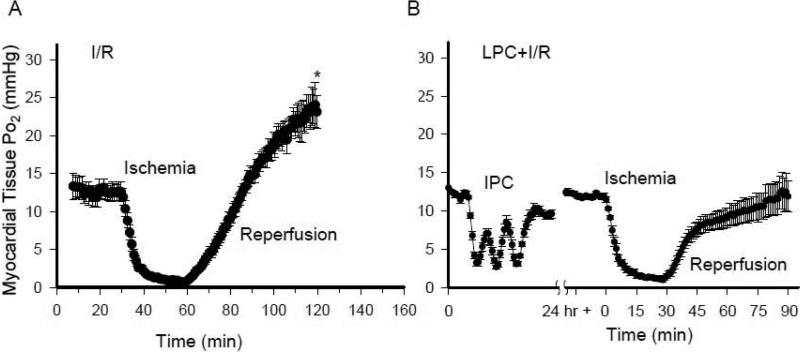
As tissue oxygenation is a delicate balance between oxygen supply and consumption, we measured tissue Po2 with EPR oximetry. As shown in Fig. 1, in the pre-ischemic state, the baseline values of myocardial tissue Po2 were 12.6 ± 1.3 mmHg and 12.4 ± 0.3 mmHg in the I/R and LPC+I/R group. Marked decreases and increases of Po2 value were observed during each 5-min ischemia and reperfusion in the LPC+I/R mice. Twenty four hours after the ischemic stimuli and at the beginning of the index ischemia, tissue Po2 reached a steady state value of 11.5 ± 0.4 mmHg in the LPC+I/R group. In both the I/R and LPC+I/R mice, tissue Po2 value decreased rapidly to 0.7 ± 0.1 mmHg and 1.3 ± 0.3 mmHg and remained hypoxic during the 30 min ischemic period. Upon reperfusion, there was a rapid increase of tissue Po2 in both groups. In the I/R mice, Po2 level increased and overshot the baseline with a value of 23.1 ± 2.2 mmHg at the end of 60 min reperfusion (*, p<0.05 versus pre-ischemia). In contrast, Po2 value in the LPC+I/R mice increased to 11.9 ± 2.0 mmHg at the end of 60 min reperfusion which was not significantly different from the pre-ischemic state. These data suggested that LPC attenuated tissue hyperoxygenation that usually occurred during reperfusion in the I/R mice. However, LPC alone did not alter the basal tissue oxygenation status.
Fig. 1.
In vivo measurements of myocardial tissue oxygenation with EPR oximetry. A, tissue Po2 before and during ischemia and after reperfusion in the I/R mouse hearts; B, issue Po2 before, during and after the ischemic stimuli, during the index ischemia and after reperfusion in the LPC+I/R mouse hearts. There was an overshoot of tissue Po2 after reperfusion in the I/R mouse hearts and the hyperoxygenation status was attenuated in the LPC+I/R group. *, p<0.01, I/R vs. LPC+I/R at 60 min reperfusion; N = 6/group.
Regional myocardial tissue blood flow
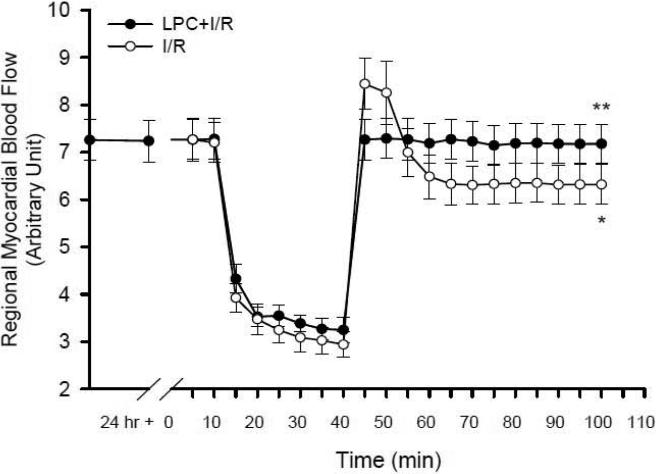
In order to understand how tissue oxygen consumption was affected by LPC, regional myocardial tissue blood flow was measured in both I/R and LPC+I/R mice to determine whether the reperfusion hyperoxygenation in the I/R group and the attenuation of this hyperoxygenation status in the LPC+I/R mice were due to regulations on mitochondrial oxygen consumption. As shown in Fig. 2, the basal blood flow in the I/R group and the values before and 24 hr after the preconditioning stimuli in the LPC+I/R mice showed no significant differences (7.20 ± 0.42, 7.24 ± 0.44 and 7.28 ± 0.44). After LAD occlusion, blood flow decreased markedly in both groups to 2.94 ± 0.26 and 3.25 ± 0.27 at the end of 30 min ischemia. Upon reperfusion, blood flow was restored rapidly within 5 min in both groups when LAD ligation was released. In the I/R mice, after the appearance of a transient peak, blood flow reached a lower value of 6.32 ± 0.42 at the end of 60 min reperfusion (*, p<0.05 at the 100-min time point vs. pre-ischemic baseline at the 10-min time point). However, in the LPC+I/R mice, blood flow reached a plateau of 7.18 ± 0.41 at the end of 60 min reperfusion, which is significantly higher than that in the I/R group (**, p<0.05 at the 100-min time point). These data indicated that LPC protected the regional blood flow in the I/R group. The blood flow data further suggested that LPC attenuated the reperfusion hyperoxygenation in the /R group (shown in the previous section) probably through regulations on mitochondrial oxygen consumption.
Fig. 2.
Measurements of regional blood flow with Doppler flow meter. Blood flow in the area at risk was measured before, during and after the index ischemia in the I/R and the LPC+I/R mice (N = 5/group). As shown with the open circles, regional blood flow was decreased significantly in the I/R mice at 60 min reperfusion compared to that in the pre-ischemic state (*, p < 0.05). Late phase preconditioning significantly improved the blood flow at 60 min reperfusion (the solid circles) compared to that in the I/R mice (**, p < 0.05).
Mitochondrial NADH-DH, SCR, and CcO activities
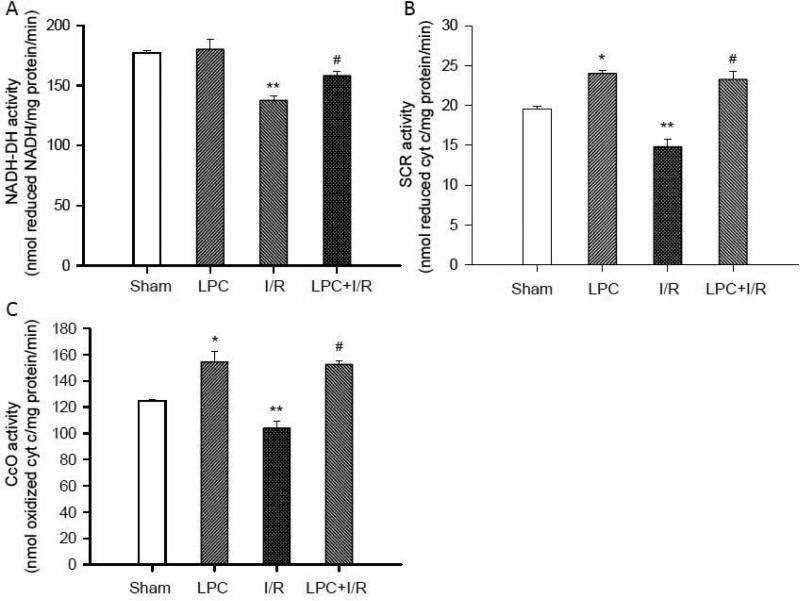
To test how mitochondrial oxygen consumption was affected by LPC, enzyme activities on the mitochondrial respiratory chain (NADH-DH, SCR, and CcO) were measured. As shown in Fig. 3, LPC alone significantly increased the activities of SCR (B) and CcO (C) (24.00±0.36 and 154.43±7.87 nmol/mg protein/min, *, p<0.05), but not NADH-DH (A) (180.04 ± 8.21 nmol/mg protein/min) in comparison to that in the Sham groups respectively (Sham SCR 19.53±0.35, Sham CcO 124.78±1.34 and Sham NADH-DH 177.16 ± 2.01 nmol/mg protein/min). I/R significantly decreased the activities of NADH-DH, SCR and CcO (137.59±3.73, 14.81±0.89 and 103.96±5.38 nmol/mg protein/min, **, p<0.05) in comparison to that in the Sham controls. However, the activities of these enzymes in the LPC+I/R group were protected (158.12±3.45, 23.28±1.05 and 152.55±2.79nmol/mg protein/min, #, p<0.05 versus I/R groups). These data suggested that LPC protected mitochondrial oxygen consumption by restoring activities of these enzymes, which is consistent with the suppression of reperfusion hyperoxygenation in the LPC+I/R mice.
Fig. 3.
Measurements of mitochondrial enzyme activities. The activity of NADH-DH (A), SCR (B), and CcO (C) were measured with the extinction coefficients set as ε550 nm = 18.5 mmol/l·cm for cytochrome c and ε340 nm = 6.22 mmol/l·cm for NADH. As shown, the preconditioning stimuli enhanced the activities of mitochondrial enzymes except that of NADH-DH. I/R significantly reduced all the enzyme activities. After reperfusion with LPC, mitochondrial enzyme activities were significantly improved in comparison to that in the I/R groups. *, p<0.05, LPC vs. Sham control; **, p<0.05, I/R vs. Sham control; #, p<0.05, LPC+I/R vs. I/R; N = 5/group.
Protein expression of Complex I subunit 39 kD, Complex III subunit FeS and Complex IV subunit VIb
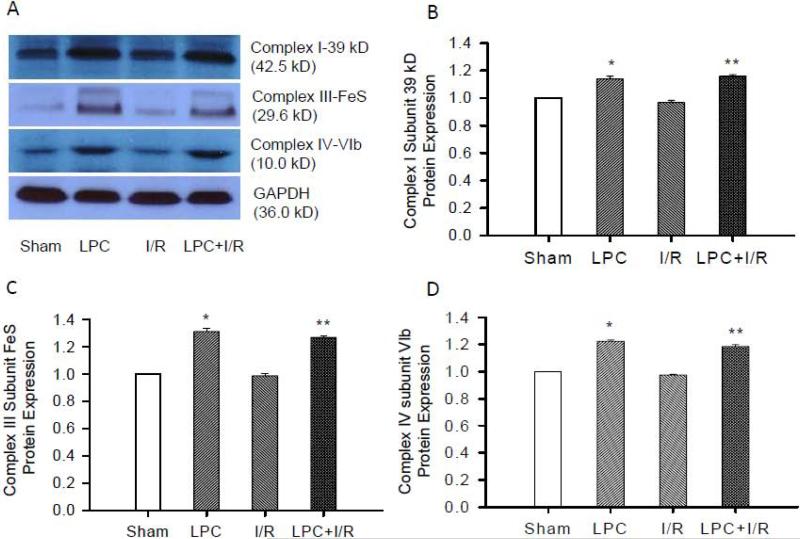
Changes in enzyme activities could be due to either protein synthesis or post-translational modifications. To determine whether the effect of LPC on mitochondrial enzyme activities (NAHD-DH, SCR, and CcO) is associated with changes in respiratory protein synthesis, protein expressions of selected Complex I subunit 39 kD, Complex III subunit FeS and Complex IV subunit VIb were determined with Western blot. Fig. 4A shows a representative blot depicting the change in band intensity in different groups. As shown in Fig. 4B, C and D, LPC significantly increased protein expression of Complex I subunit 39 kD, Complex III subunit FeS and Complex IV subunit VIb compared to that in the Sham control mice (1.14±0.02, 1.31±0.02, and 1.22±0.01, *, p<0.05). I/R did not induce any changes in these proteins (0.97±0.01, 0.99±0.01, and 0.98±0.01 vs. sham controls) probably due to the relative short time scale. The protein expressions in the LPC+I/R group (1.16±0.01, 1.27±0.01, 1.19±0.01, **, p<0.05) were also significantly higher than that in the I/R groups. These data demonstrated that LPC increased mitochondrial respiratory chain protein expression, which was critical to the protection of enzyme activities and mitochondrial oxygen consumption by LPC.
Fig. 4.
Mitochondrial protein expression with Western blot. A, representative Western blots of Complex I subunit 39 kD, Complex III subunit FeS, and Complex IV subunit VIb. B, C, and D are statistic analysis of protein expressions. As shown, there was no significant difference in protein expressions between sham and I/R groups. However, protein expression of Complex I subunit 39 kD (B), Complex III subunit FeS (C) and Complex IV subunit VIb (D) were increased in the LPC and LPC+I/R groups. *, p<0.05, LPC vs. Sham; **, p<0.05, LPC+I/R vs. I/R; N = 5/group.
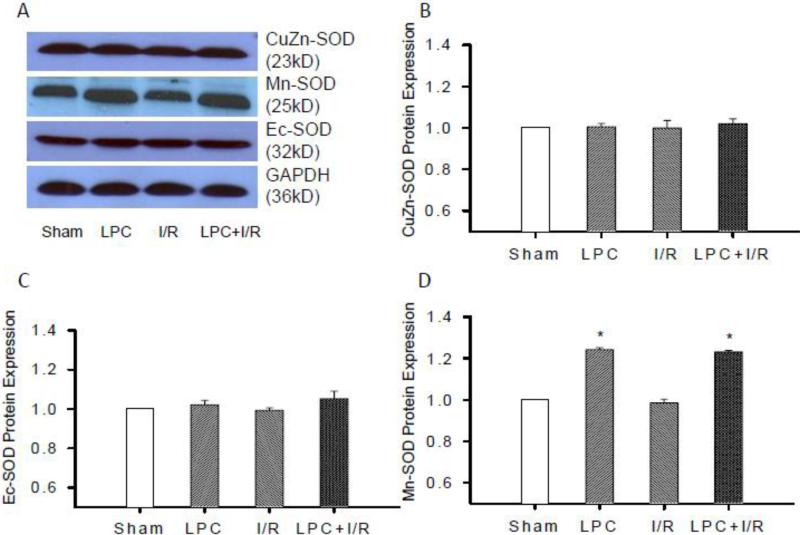
SOD protein expression
NADH-DH has been reported as a prominent target of ONOO- at reperfusion. (Murray et al. 2003) Since LPC did not increase the activity of NADH-DH, induction of SODs and attenuation of ROS/RNS at reperfusion by LPC might be the reason for the protection of NADH-DH activity in the post-ischemic heart. To determine which isoform of SODs was induced by LPC, protein expressions of three different isoforms of SODs (Mn-SOD, CuZn-SOD, and EC-SOD) were measured with Western blot. Fig. 5A shows a representative blot of three SOD isoforms depicting the band intensity. As shown in Fig. 5B and C, CuZn-SOD and EC-SOD expressions were not significantly altered in the LPC, I/R, and LPC+I/R groups. However, the expression of Mn-SOD (Fig. 5D) in the LPC and LPC+I/R groups were significantly increased (1.24 ± 0.01 and 1.23 ± 0.01, *, p<0.05) compared to that in the Sham control, though there was no significant change in the I/R group. These data suggested that LPC specifically induced Mn-SOD expression in the mitochondrial matrix.
Fig. 5.
Protein expressions of SODs with Western blot. Tissue lysate of sham, LPC, I/R and LPC+IR groups were subjected to SDS-PAGE and immunoblot analysis using Mn-SOD, CuZn-SOD and EC-SOD antibodies, respectively. A, representative blots showing the changes in band intensity in different groups. Protein expressions of CuZn-SOD (B), EC-SOD (C) and Mn-SOD (D) were measured relative to the intensity of the sham groups. Mn-SOD protein expression was increased significantly in the LPC and LPC+I/R groups with no differences between the sham and I/R groups. However, CuZn-SOD and EC-SOD were not altered in LPC, I/R and LPC+I/R groups; *, p<0.05, LPC and LPC+I/R vs. sham group, N = 5/group.
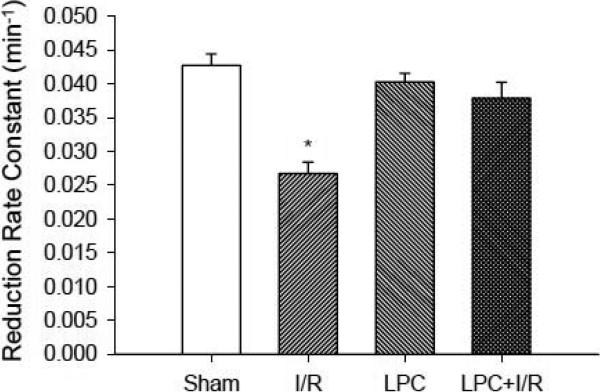
Myocardial tissue redox status
In order to understand how the global redox status at tissue level was affected by LPC, myocardial tissue redox state was determined with EPR redox spectroscopy. (Zhu and Zuo et al. 2007) As shown in Fig. 6, I/R shifted the redox balance in the post-ischemic heart to the oxidizing state with a significantly lower PCA reduction rate constant in comparison to that in the Sham control group (0.0268 ± 0.0016 vs. 0.0427 ± 0.0017/min, *, p<0.001). LPC alone did not change the redox state (0.0403 ± 0.0013/min). However, the redox status in the LPC+I/R mice was protected by LPC (0.0379 ± 0.0023/min). These data suggested that LPC corrected the redox shift in the post-ischemic heart even though LPC alone did not alter the global redox status.
Fig. 6.
In vivo EPR measurements of myocardial tissue redox status. After thoracotomy and exposure of the heart or after 30 min ischemia, 5 μl of 10 mM PCA PBS solution was injected into the area at risk and EPR measurements were followed. Reduction rate constant of PCA was measured in the myocardial tissue in Sham, I/R, LPC and LPC+I/R mice. I/R reduced the rate constant 37 % (0.0427 ± 0.0017/min in Sham vs. 0.0268 ± 0.0016/min in I/R group) and LPC restored the redox status in the LPC+I/R group (0.0379 ± 0.0023/min). LPC alone did not cause significant change to the redox status (0.0403 ± 0.0013/min). *, p<0.001, I/R vs. Sham; N = 5/group.
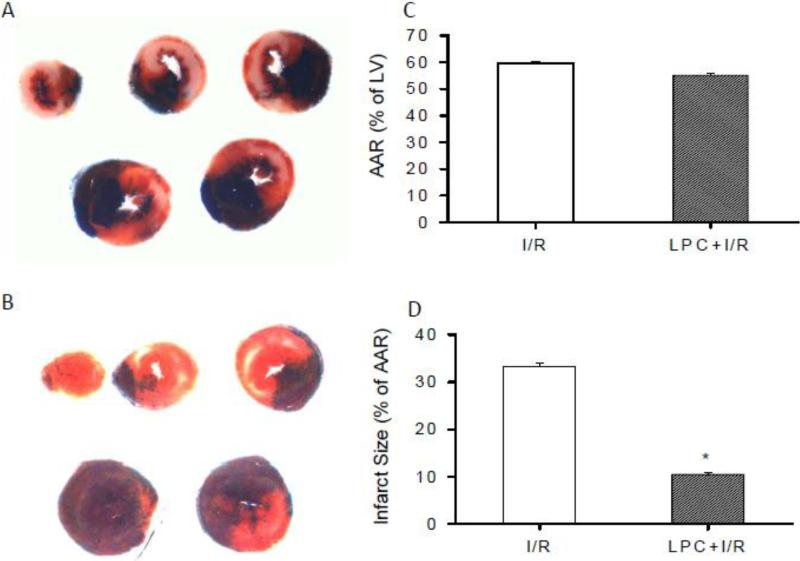
Infarct size
In order to correlate the preservation of mitochondrial oxygen consumption with cardiac protection by LPC, the ratio of AAR over LV and infarct area over AAR were measured in the I/R and LPC+I/R mice. As shown in Fig. 7, there is no significant difference in the ratio of AAR over LV between the I/R and LPC+I/R mice (59.5 ± 0.9% vs. 55.1 ± 0.8%). The ratio of infarct size over AAR in the I/R and LPC+I/R mice were 33.3 ± 0.6% and 10.5 ± 0.4%, with the infarct size of the LPC+I/R mice significantly lower than that in the I/R mice (*P < 0.05). These results confirmed the myocardial protection of LPC against I/R injury.
Fig. 7.
Measurement of AAR and infarct size with Evans blue and TTC. A and B, Representative heart slices from I/R and LPC+I/R mice. C and D, Statistic analysis of the ratio of AAR/LV and infarct size/AAR after 30 min ischemia and 24 hr reperfusion. AAR/LV in the I/R and LPC+I/R groups were 59.5±0.9% and 55.1±0.8% and there was no significant difference between the two groups. Infarct size in the I/R and LPC+I/R groups were 33.3±0.6% and 10.5±0.4%. LPC significantly reduced the infarct size/AAR ratio in the LPC+I/R group compared to that in the I/R group. *, p<0.05, LPC+I/R vs. I/R group; N = 5/group.
Discussion
IPC is a potent endogenous protective mechanism activated by a mild ischemic stress that enhances the ability of the heart to resist I/R injury. (Dekker 1998, Yellon et al. 1998) Because of its robust and persistent protection, many studies have been performed to investigate this adaptive response, including alterations of myocardial oxygen metabolism. (Rudzinski et al. 2002, Zhu and Liu et al. 2007) It was reported that reduction of mitochondrial oxygen radical generation and prevention of respiratory dysfunction were involved in the cardioprotection of IPC against I/R injury. (Park et al. 1997, Yabe et al. 1997) We have previously demonstrated that there was a reperfusion hyperoxygenation state in the post-ischemic mouse heart, which was due to the impaired mitochondrial oxygen consumption. Further, we have shown that acute phase IPC attenuated myocardial post-ischemic tissue hyperoxygenation via preserving mitochondrial enzyme activities from post-translational protein modifications. (Zhao et al. 2005, Zhu and Liu et al. 2007)
In the current study, we demonstrated that LPC suppressed the post-ischemic tissue hyperoxygenation. Laser Doppler blood flow measurement indicated that LPC restored the regional blood flow in the risk area which may be due to the preserved NO production, adenosine-mediated vasodilation (Bolli et al. 1997, Baxter et al. 1994, Dana et al. 1998), or simply the reduced necrosis in the risk area. Since LPC actually protected the regional blood flow in the post-ischemic heart, (Bolli et al. 1997) the suppression of the post-ischemic tissue hyperoxygenation strongly suggested that LPC protected mitochondrial oxygen consumption/function. Indeed, the activities of NADH-DH, SCR, and CcO were decreased in the I/R mice and conserved in the LPC+I/R mice. The LPC-induced increase in mitochondrial enzyme activities (except NADH-DH) and protein expressions suggested that LPC up-regulated mitochondrial protein synthesis and protected the enzyme activities against I/R injury. This is consistent with previous studies that LPC induced mitochondrial biogenesis through PGC1α transcriptional signaling. (McLeod et al. 2004) However, the discrepancy between the increase of protein expression and unchanged enzyme activity of NADH-DH in this study could be due to limitations in the selection of a specific complex I subunit 39 kD in representing the whole complex I protein expression.
ROS/RNS were reported to increase after I/R and IPC was demonstrated to diminish the subsequent ROS/RNS formation. (Zweier 1988, Zweier et al. 1989, Baxter and Ferdinandy 2001, Bolli et al. 1998) The LPC-induced up-regulation of Mn-SOD, which was similarly reported in other studies, (Hoshida et al. 1993, Jin et al. 2005) strongly suggested that the LPC-induced protection of mitochondrial enzyme activities could also be due to the prevention of these enzymes from post-translational modification by ROS/RNS, as occurred in the acute phase protection. (Zhu and Liu et al. 2007, Zhao et al. 2005) Our in vivo tissue redox measurement indicated that LPC corrected the I/R-induced redox oxidizing shift in the post-ischemic heart as occurred in the acute IPC (Zhu and Liu et al. 2007). These result further suggested that LPC-induced Mn-SOD protein up-regulation may contribute to the suppression of ROS/RNS and amelioration of oxidative stress in the post-ischemic heart. The lower reducing state in the non-conditioned reperfused myocardium was consistent with the higher tissue Po2 and higher levels of ROS. Ischemic preconditioning restored the reducing state in the preconditioned reperfused myocardium which was consistent with the attenuated tissue Po2, higher level of Mn-SOD and lower levels of ROS. The preconditioning-restored redox status and the preconditioning-attenuated ROS/RNS formation may eventually contribute to the protection of the postischemic myocardial injury.
Finally, attenuation of ROS/RNS formation and up-regulation of mitochondrial protein synthesis and function may work in concert with other protective mechanisms leading to a decrease of infarct size illustrated by TTC staining, which is consistent with a number of previous studies. (Guo et al. 1998, Liu et al. 1991)
Conclusions
In conclusion, our study demonstrated that LPC attenuated the post-ischemic myocardial tissue hyperoxygenation status induced by I/R via improving mitochondrial oxygen consumption. The LPC-induced up-regulation of mitochondrial proteins may contribute to the protection on mitochondrial enzyme activities. In addition, the LPC-induced up-regulation of Mn-SOD expression may also have protected the mitochondrial respiratory enzymes from post-translational modifications. These protective mechanisms eventually resulted in a decrease of infarct size and preservation of cardiac function.
Acknowledgements
This study was supported by NIH HL081630, HL081630-04S1, and AHA 0435299N to GH. The LiPc was made with the support of PO1 EB2180 (Swartz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balakumar P, Rohilla A, Singh M. Pre-conditioning and postconditioning to limit ischemia-reperfusion-induced myocardial injury: what could be the next footstep? Pharmacological Research. 2008;57:403–412. doi: 10.1016/j.phrs.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Baxter GF, Ferdinandy P. Delayed preconditioning of myocardium: current perspectives. Basic Research in Cardiology. 2001;96:329–344. doi: 10.1007/s003950170041. [DOI] [PubMed] [Google Scholar]
- Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- Bolli R. The late phase of preconditioning. Circulation Research. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Research in Cardiology. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon AK. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circulation Research. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? The Journal of Clinical Investigation. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Laboratory Animals. 2010;44:329–336. doi: 10.1258/la.2010.009085. [DOI] [PubMed] [Google Scholar]
- Chen EP, Bittner HB, Davis RD, Van Trigt P, Folz RJ. Physiologic effects of extracellular superoxide dismutase transgene overexpression on myocardial function after ischemia and reperfusion injury. The Journal of Thoracic Cardiovascular Surgery. 1998;115:450–458. doi: 10.1016/s0022-5223(98)70289-2. discussion 458-459. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Chen W, Zweier JL, Augusto O, Radi R, Mason RP. Formation of protein tyrosine ortho-semiquinone radical and nitrotyrosine from cytochrome c-derived tyrosyl radical. The Journal of Biological Chemistry. 2004;279:18054–18062. doi: 10.1074/jbc.M307706200. [DOI] [PubMed] [Google Scholar]
- Chen Z, Oberley TD, Ho Y, Chua CC, Siu B, Hamdy RC, Epstein CJ, Chua BH. Overexpression of CuZnSOD in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radical Biology and Medicine. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. Journal of Molecular and Cellular Cardiology. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- Csonka C, Csont T, Onody A, Ferdinandy P. Preconditioning decreases ischemia/reperfusion-induced peroxynitrite formation. Biochemical and Biophysical Research Communications. 2001;285:1217–1219. doi: 10.1006/bbrc.2001.5308. [DOI] [PubMed] [Google Scholar]
- Dana A, Baxter GF, Walker JM, Yellon DM. Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. Journal of the American College of Cardiology. 1998;31:1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Dekker LR. Toward the heart of ischemic preconditioning. Cardiovascular Research. 1998;37:14–20. doi: 10.1016/s0008-6363(97)00241-1. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. The American Journal of Physiology. 1998;275:H1375–1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circulation Research. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- Hoshida S, Kuzuya T, Fuji H, Yamashita N, Oe H, Hori M, Suzuki K, Taniguchi N, Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. The American Journal of Physiology. 1993;264:H33–39. doi: 10.1152/ajpheart.1993.264.1.H33. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Zhou HZ, Cecchini G, Gray MO, Karliner JS. MnSOD in mouse heart: acute responses to ischemic preconditioning and ischemia-reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology. 2005;288:H2986–2994. doi: 10.1152/ajpheart.01144.2004. [DOI] [PubMed] [Google Scholar]
- Jones SP, Trocha SD, Strange MB, Granger DN, Kevil CG, Bullard DC, Lefer DJ. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology. 2000;279:H2196–2201. doi: 10.1152/ajpheart.2000.279.5.H2196. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circulation Research. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Jeyabalan AP, Minners JO, Clevenger R, Hoyt RF, Jr., Sack MN. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation. 2004;110:534–539. doi: 10.1161/01.CIR.0000136997.53612.6C. [DOI] [PubMed] [Google Scholar]
- Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. The Journal of Biological Chemistry. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. New insights into potential mechanisms of ischemic preconditioning. Circulation. 1991;84:442–445. doi: 10.1161/01.cir.84.1.442. [DOI] [PubMed] [Google Scholar]
- Pan YX, Ren AJ, Zheng J, Rong WF, Chen H, Yan XH, Wu C, Yuan WJ, Lin L. Delayed cytoprotection induced by hypoxic preconditioning in cultured neonatal rat cardiomyocytes: role of GRP78. Life Sciences. 2007;81:1042–1049. doi: 10.1016/j.lfs.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sciences. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- Rudzinski T, Mussur M, Wawrzycki M, Gwiazda Z, Zaslonka J, Mussur M. Ischemic preconditioning diminishes oxygen demand and increases coronary flow in the early phase of reperfusion in rat heart. Medical Science Monitor. 2002;8:BR362–368. [PubMed] [Google Scholar]
- Rui T, Cepinskas G, Feng Q, Kvietys PR. Delayed preconditioning in cardiac myocytes with respect to development of a proinflammatory phenotype: role of SOD and NOS. Cardiovascular Research. 2003;59:901–911. doi: 10.1016/s0008-6363(03)00502-9. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Bacic G, Friedman B, Goda F, Grinberg O, Hoopes PJ, Jiang J, Liu KJ, Nakashima T, O'Hara J, et al. Measurements of pO2 in vivo, including human subjects, by electron paramagnetic resonance. Advances in Experimental Medicine and Biology. 1994;361:119–128. doi: 10.1007/978-1-4615-1875-4_16. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu B, Zweier JL, He G. Formation of hydrogen peroxide and reduction of peroxynitrite via dismutation of superoxide at reperfusion enhances myocardial blood flow and oxygen consumption in postischemic mouse heart. The Journal of Pharmacology and Experimental Therapeutics. 2008;327:402–410. doi: 10.1124/jpet.108.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K, Nasa Y, Sato M, Iijima R, Takeo S. Preconditioning preserves mitochondrial function and glycolytic flux during an early period of reperfusion in perfused rat hearts. Cardiovascular Research. 1997;33:677–685. doi: 10.1016/s0008-6363(96)00269-6. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. A “second window of protection” occurs 24 h after ischemic preconditioning in the rat heart. Journal of Molecular and Cellular Cardiology. 1998;30:1181–1189. doi: 10.1006/jmcc.1998.0682. [DOI] [PubMed] [Google Scholar]
- Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovascular Research. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Baxter GF, Garcia-Dorado D, Heusch G, Sumeray MS. Ischaemic preconditioning: present position and future directions. Cardiovascular Research. 1998;37:21–33. doi: 10.1016/s0008-6363(97)00214-9. [DOI] [PubMed] [Google Scholar]
- Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–2972. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, He G. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. American Journal of Physiology. Heart and Circulatory Physiology. 2007;293:H1442–1450. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zuo L, Cardounel AJ, Zweier JL, He G. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxidants & Redox Signaling. 2007;9:447–455. doi: 10.1089/ars.2006.1389. [DOI] [PubMed] [Google Scholar]
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. The Journal of Biological Chemistry. 1988;263:1353–1357. [PubMed] [Google Scholar]
- Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. The Journal of Biological Chemistry. 1989;264:18890–18895. [PubMed] [Google Scholar]