Abstract
We previously reported that granulocytes are able to produce superoxide (O2-), a highly reactive compound formed by the one-electron reduction of oxygen. The demonstration of O2- production was based on the observation that the reduction of extra-cellular cytochrome c by granulocytes was greatly diminished by superoxide dismutase, an enzyme catalyzing the conversion of O2- to hydrogen peroxide and oxygen. In the present report, studies concerning the effect of bacteria and serum on O2--dependent cytochrome c reduction by granulocytes are described.
In the absence of bacteria, the O2--dependent reduction of extracellular cytochrome c by granulocytes under optimal assay conditions amounted to 9.2±2.8 SD nmol/3 × 106 cells/20 min. When bacteria (100 organisms/cell) were present, the O2--dependent cytochrome c reduction under otherwise similar conditions increased by a factor of nearly four (34.5±9.4). There was no effect of albumin or catalase on cytochrome c reduction, and boiled dismutase had only a small effect. Omission of granulocytes or substitution of live cells by cells by cells killed by heat abolished O2--dependent cytochrome c reduction. Bacteria killed by autoclaving were almost as effective as live bacteria in stimulating granulocyte O2- production. Measurements of particle uptake and O2 uptake by granulocytes indicated that superoxide dismutase did not affect granulocyte metabolism nonspecifically, supporting the conclusion that the diminution of cytochrome c reduction in the presence of dismutase was due to the destruction of O2- by this enzyme.
Stimulation of O2- production by bacteria was strongly dependent on the presence of serum in the incubation mixture. Serum heated to 56°C for 45 min was as effective as unheated serum in stimulating O2- production in the presence of bacteria, but boiled serum had no effect. Other experiments suggested that incubation of bacteria with serum resulted in the release of a nonparticulate heat-labile substance capable of stimulating O2- production in the absence of bacteria.
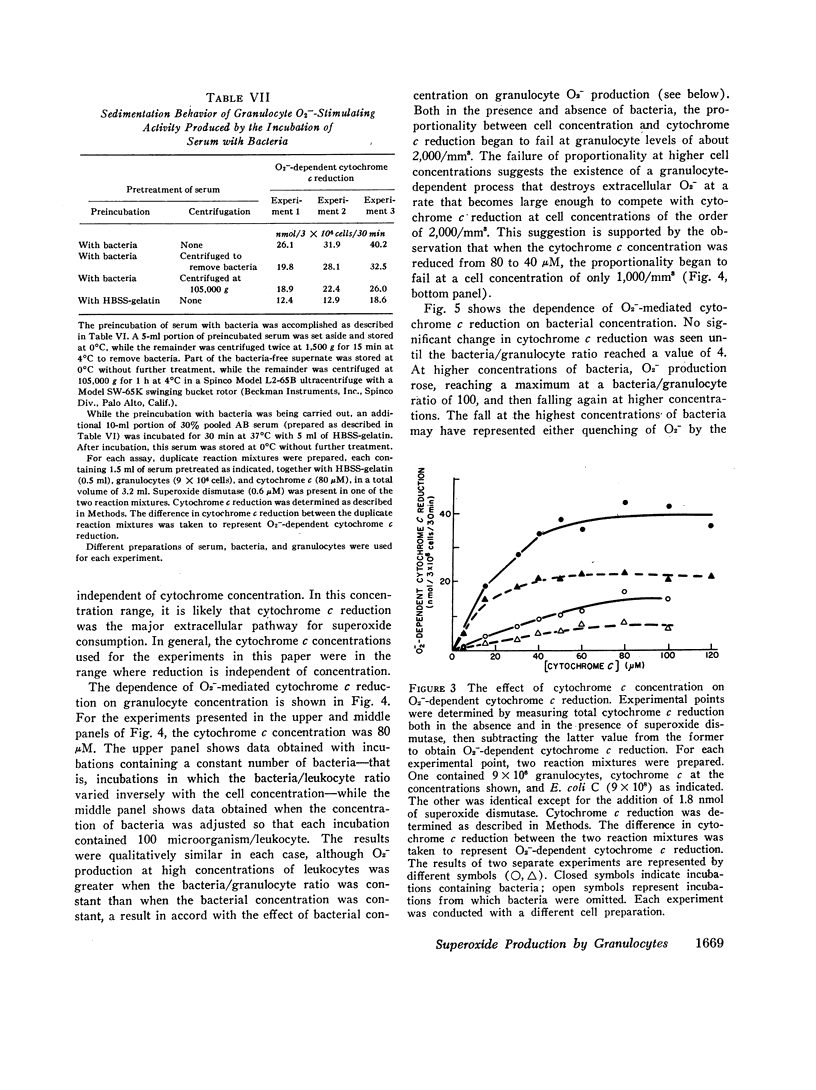
Certain characteristics of the O2--dependent cytochrome c reduction by granulocytes were studied, including the dependence of this process on granulocyte, cytochrome c, and bacterial concentrations. In addition, O2--dependent cytochrome c reduction was followed as a function of time. A constant rate was found with resting granulocytes. With bacteria the time course was more complex. A well-defined lag was followed by a fairly brief period of extremely vigorous cytochrome c reduction. During this period, the maximum rate of cytochrome c reduction exceeded the rate observed in the absence of bacteria by a factor of 12. The rate then decreased until by 40 min, it had slowed to the rate observed in the absence of bacteria.
From the above results, it was concluded that the exposure of the granulocyte to bacteria plus serum initiates a process in which a defined quantity of O2- is formed in a rapid burst lasting 20-30 min. It is conceivable that the O2- generated by this process may be involved in the killing of bacteria by the granulocytes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., LaVia M. F., Spurr C. L., Baehner R. L. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. 1972 Apr;51(4):769–778. doi: 10.1172/JCI106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar V. A., Nishioka K. "Tuftsin": a natural phagocytosis stimulating peptide. Nature. 1970 Nov 14;228(5272):672–673. doi: 10.1038/228672a0. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L. Disorders of phagocytic cell function. Prog Hematol. 1971;7(0):235–254. [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Role of the phagocyte in host-parasite interactions. 38. Metabolic activities of the phagocyte as related to antimicrobial action. J Reticuloendothel Soc. 1972 Aug;12(2):109–126. [PubMed] [Google Scholar]
- Smith M. R., Wood W. B., Jr Heat labile opsonins to pneumococcus. I. Participation of complement. J Exp Med. 1969 Dec 1;130(6):1209–1227. doi: 10.1084/jem.130.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Evaluation of opsonic and leukocyte function with a spectrophotometric test in patients with infection and with phagocytic disorders. Blood. 1973 Jul;42(1):121–130. [PubMed] [Google Scholar]
- Woeber K. A., Doherty G. F., Ingbar S. H. Stimulation by phagocytosis of the deiodination of L-thyroxine in human leukocytes. Science. 1972 Jun 2;176(4038):1039–1041. doi: 10.1126/science.176.4038.1039. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]



