Abstract
Objectives
In this study, we have investigated the effects of cannabidiol (CBD) on myocardial dysfunction, inflammation, oxidative/nitrosative stress, cell death and interrelated signaling pathways, using a mouse model of type I diabetic cardiomyopathy and primary human cardiomyocytes exposed to high glucose.
Background
CBD, the most abundant nonpsychoactive constituent of Cannabis sativa (marijuana) plant, exerts antiinflammatory effects in various disease models and alleviates pain and spasticity associated with multiple sclerosis in humans.
Methods
Left ventricular function was measured by pressure-volume system. Oxidative stress, cell death and fibrosis markers were evaluated by molecular biology/biochemical techniques, electron spin resonance spectroscopy and flow cytometry.
Results
Diabetic cardiomyopathy was characterized by declined diastolic and systolic myocardial performance associated with increased oxidative-nitrosative stress, NF-κB and MAPK (JNK and p-38, p38α) activation, enhanced expression of adhesion molecules (ICAM-1, VCAM-1), TNF-α, markers of fibrosis (TGF-β, CTGF, fibronectin, collagen-1, MMP-2 and MMP-9), enhanced cell death (caspase 3/7 and PARP activity, chromatin fragmentation and TUNEL) and diminished Akt phosphorylation. Remarkably, CBD attenuated myocardial dysfunction, cardiac fibrosis, oxidative/nitrosative stress, inflammation, cell death, and interrelated signaling pathways. Furthermore, CBD also attenuated the high glucose-induced increased reactive oxygen species generation, NF-κB activation and cell death in primary human cardiomyocytes.
Conclusions
Collectively, these results coupled with the excellent safety and tolerability profile of cannabidiol in humans, strongly suggest that it may have great therapeutic potential in the treatment of diabetic complications, and perhaps other cardiovascular disorders, by attenuating oxidative/nitrosative stress, inflammation, cell death and fibrosis.
Keywords: diabetic complications, cannabinoids, oxidative stress, inflammation
Introduction
Cardiovascular complications are the leading cause of morbidity and mortality in diabetic patients. Diabetic cardiomyopathy characterized by myocardial left ventricular dysfunction (both diastolic and later systolic one), independent of atherosclerosis and coronary artery disease, has been well documented in both humans and animals (1-3). The mechanism of diabetic cardiac dysfunction is complex and involves increased oxidative/nitrosative stress (4-7), activation of various downstream transcription factors, pro-inflammatory and cell death pathways such as NF-κB (8,9), poly(ADP-ribose) polymerase (PARP) (10), and MAPKs (11,12), inactivation of pro-survival pathways such as Akt (13), eventually culminating in cell death (14), changes in the composition of extracellular matrix with enhanced cardiac fibrosis and increased inflammation (15).
Various components of Cannabis sativa (marijuana) plant, termed cannabinoids (e.g. the most characterized active ingredient, the delta 9-tetrahydrocannabinol (THC)), exert potent analgesic effects through the activation of classic CB1 receptors located in the central nervous system and anti-inflammatory properties through the activation of CB2 cannabinoid receptors on immune cells (16). However, the major limitation of the therapeutic utility of THC is the development of centrally mediated CB1-depend psychoactive effects (16). Furthermore, CB1 receptor activation in the cardiovascular system by endocannabinoids may also contribute to the pathophysiology of multiple cardiovascular diseases including heart failure and atherosclerosis (17). In contrast to THC, cannabidiol (CBD), the most abundant cannabinoid of Cannabis sativa, which has been approved for the treatment of inflammation, pain and spasticity associated with multiple sclerosis in humans since 2005 in Canada (18), does not bind to these receptors (19), therefore it is devoid of psychoactive properties and has no potential to cause adverse cardiac toxicity (20). Importantly, CBD is well tolerated without side effects when chronically administered to humans (21,22). A previous study has demonstrated cardiac protection by CBD in myocardial ischemic reperfusion injury (23); therefore, we have investigated the potential protective effects of CBD in diabetic hearts and in primary human cardiomyocytes exposed to high glucose. Our findings underscore the potential of CBD for the prevention/treatment of diabetic complications.
Materials and Methods
Animals and treatment
All the animal protocols conformed to the National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and use Committee of NIAAA/NIH. Diabetes was induced in 8-12 weeks C57/BL6J mice weighing 23-25g (male, Jackson Laboratories, Bar Harbor, ME) by intraperitoneal (I.P.) injection of streptozotocin (STZ, Sigma, St. Louis, MO) at the dose of 50 mg/kg dissolved in 100 mM citrate buffer pH 4.5 for 5 consecutive days. After one week, blood glucose levels were measured using Ascensia Coutour Glucometer (Bayer HealthCare, NY) by mandibular puncture blood sampling. Mice which had blood sugar values >250 mg/dl were used for the study. In the first set of experiments 1 week diabetic mice were treated with cannabidiol (CBD; 1, 10 or 20 mg/kg I.P) or vehicle for 11 weeks (Suppl.Fig.1). In another set of experiments, 8 weeks diabetic mice were treated with CBD or vehicle for 4 weeks (Suppl.Fig.2). CBD was isolated as described earlier (24). The corresponding control groups were treated with either vehicle or CBD alone for the same duration. All the animals were provided with food and water ad libitum.
Hemodynamic measurements in mice
Left ventricular performance was measured in mice anesthetized with 2% isoflurane as previously described (25) (26).
Determination of SOD activity, MDA, GSH, GSSG, 4-HNE, protein carbonyl content
SOD activities and reduced/oxidized glutathione (GSH, GSSG), malondialdehyde (MDA), 4-HNE, protein carbonyl levels in the myocardial tissues were determined as described in the appendix.
Determination of myocardial ROS by electron paramagnetic resonance spectrometer (EPR) is described in the appendix.
Reverse transcription and real time PCR
Preparation of samples and reverse transcription and real time PCR experiments from heart tissues and the primers are described in the appendix and Suppl. Table1.
Determination of PARP, caspase 3/7 activities, chromatin fragmentation, TUNEL and 3-nitrotyrosine (3-NT) content
PARP and caspase 3/7 activities, chromatin fragmentation, TUNEL, and 3-nitrotyrosine (3-NT) content in the heart homogenates and/or human cardiomyocyte extracts are described in the appendix.
Western immunoblot analysis
Sample preparations, Western immunoblot analysis and sources of antibodies are described in the appendix.
Immunohistochemistry
The immunohistochemistry/staining from frozen or formalin-fixed myocardial tissues (nitrotyrosine, TUNEL, Sirius red) is described in the appendix.
Cell culture studies
Human cardiomyocytes (HCM) along with the culture medium were purchased from ScienCell Research Laboratories (Carlsbad, CA) and were maintained and treated as described in the appendix.
Simultaneous determination of cytosolic and mitochondrial ROS generation and apoptosis by flow cytometry
Mitochondrial superoxide/ROS generation and cell death were determined as described (27) and detailed in the appendix.
Statistical analysis
Results are expressed as mean±SEM. Statistical comparisons were made by one way ANOVA followed by Newman-Keuls post-hoc analysis using GraphPad Prism 5 software (San Diego, CA). When heterogeneity of variance was present, ANOVA was performed after logarithmic transformation of the data. Probability values of P<0.05 were considered significant.
Results
Blood glucose levels, pancreas insulin content and body weights
Diabetic animals exhibited increased blood glucose levels (Suppl. Figs.1A&2A) with the decrease in the body weight (Suppl. Fig.1D). Diabetic animals also had increased glycosylated hemoglobin (HbA1c) levels with concomitant decline in the pancreas insulin content (Suppl. Fig. 1B/2B). CBD or vehicle treatment (1, 10 or 20 mg/kg I.P) for 11 or 4 weeks, did not significantly alter the body weight/blood glucose level/pancreas insulin content neither in control nor in diabetic animals (Suppl. Figs.1& 2).
CBD treatment attenuates diabetes-induced hemodynamic alterations
12 weeks of established diabetes was associated with impaired diastolic and systolic left ventricular function, which was largely attenuated by the treatment with CBD for 11 weeks (starting 1 week after the establishment of diabetes)(Fig.1). CBD treatment also improved the diabetes-induced myocardial dysfunction when it was given for 4 weeks in 8 weeks diabetic mice (Suppl.Fig.3.).
Fig.1. Cannabidiol attenuates diabetes-induced left ventricular dysfunction.
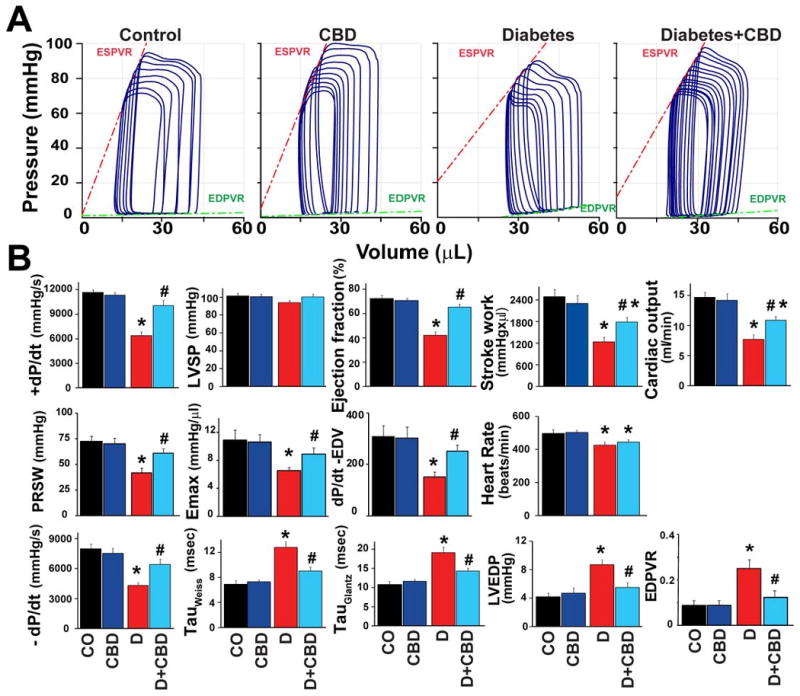
A) Representative pressure–volume (P–V) loops at different preloads after inferior vena cava occlusion, showing differences in the end-systolic and end-diastolic P–V relations (ESPVR and EDPVR) in control and diabetic mice treated with vehicle or cannabidiol (CBD). The shift of P-V loops right and changed slope of ESPVR and EDPVR in diabetic mice indicates decreased systolic and diastolic functions, which were less pronounced in diabetic mice treated with CBD (20 mg/kg/day) for 11 weeks. B) 12 weeks of diabetes was associated with decrease in left ventricular systolic pressure (LVSP), maximum first derivative of ventricular pressure with respect to time (+dP/dt), stroke work, ejection fraction, cardiac output, and load-independent indexes of contractility (preload-recruitable stroke work (PRSW), dP/dt–end-diastolic volume relation (dP/dt-EDV), and end-systolic pressure–volume relation (Emax), respectively), and an increase in left ventricular end-diastolic pressure (LVEDP) and prolongation of relaxation time constants (τ Weiss and Glantz), which were largely attenuated by CBD treatment (20 mg/kg/day I.P.) for 11 weeks. Results are mean±SEM of 8-11/group. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D].
CBD treatment attenuates diabetes-induced myocardial oxidative stress
There was increased accumulation of lipid peroxides (Fig.2A,B), protein carbonyls (Fig.2C), ROS generation (Fig.2D), expression of mRNA of various ROS generating NADPH oxidases (p22phox,p67phox, gp91phox; Fig.2E) with concordant decrease of GSH/GSSG ratio (Fig.2F) and attenuated activity of the superoxide eliminating enzyme, the superoxide dismutase (SOD) (Fig.2G) in hearts of diabetic mice. These changes were attenuated when mice were treated with CBD for 11 weeks during the course of the diabetes (Fig.2A-G).
Fig.2. CBD attenuates diabetes-induced myocardial oxidative stress.
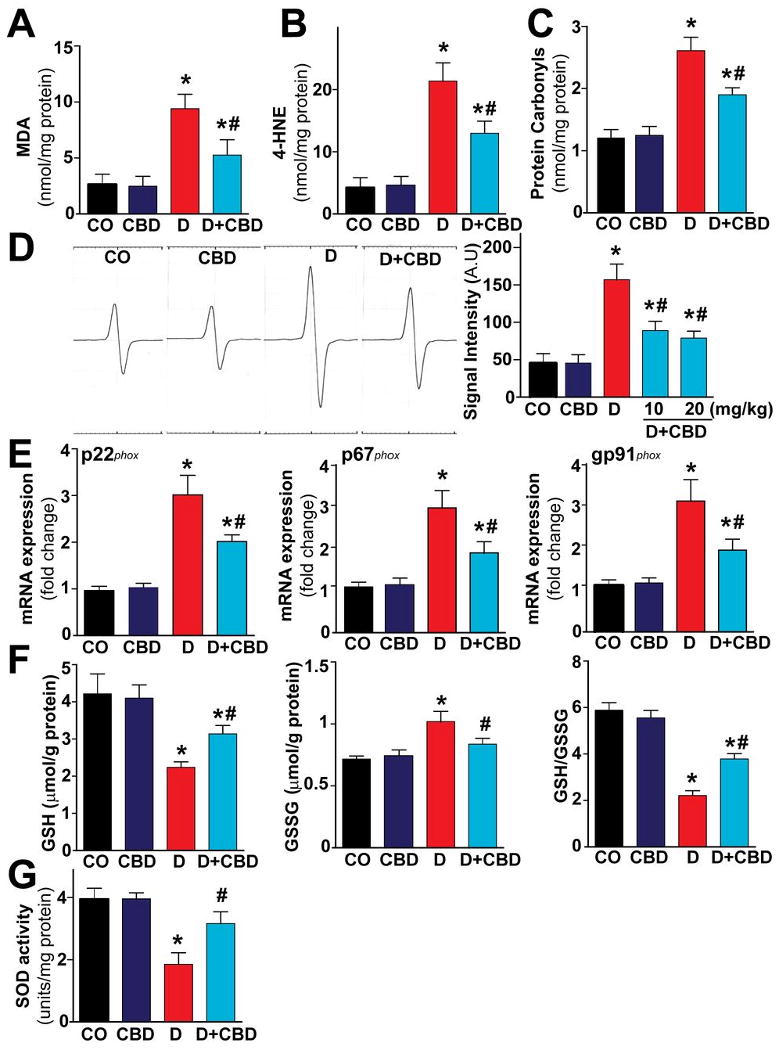
Oxidative stress in the myocardial tissues were determined by measuring (A) MDA, (B) 4-HNE, (C) protein carbonyls content and (D) ROS levels by EPR as described in the Methods section and the (E) NADPH oxidase subunits mRNA expression by real time RT-PCR (F) endogenous antioxidants (GSH) content and (G) SOD activity. *P<0.05 vs. vehicle control/CBD alone, #P<0.05 vs. diabetes [D], n=6-9/group.
CBD treatment attenuates diabetes-induced myocardial NF-κB activation and inflammation
As shown in (Fig.3A) there was a marked IκB-α degradation in the cytosol of diabetic hearts, with increased phosphorylation of IκB-α leading to release of active p65NF-κB, which subsequently translocates to the nucleus to induce the inflammatory and apoptotic gene expressions (Fig.3B). Gel shift assay also confirmed the NF-κB activation in diabetic hearts (Fig.3C). CBD treatment of diabetic mice inhibited the IκB-α and subsequent p65NF-κB nuclear translocation (Fig.3A-C). CBD treatment also inhibited the NF-κB-dependent mRNA and/or protein expression of adhesion molecules ICAM-1 and VCAM-1 (Fig.3D,F) and pro-inflammatory cytokine TNF-α (Fig.3E,G) respectively in the diabetic myocardial tissues.
Fig.3. CBD attenuates diabetes-induced myocardial NF-κB activation.
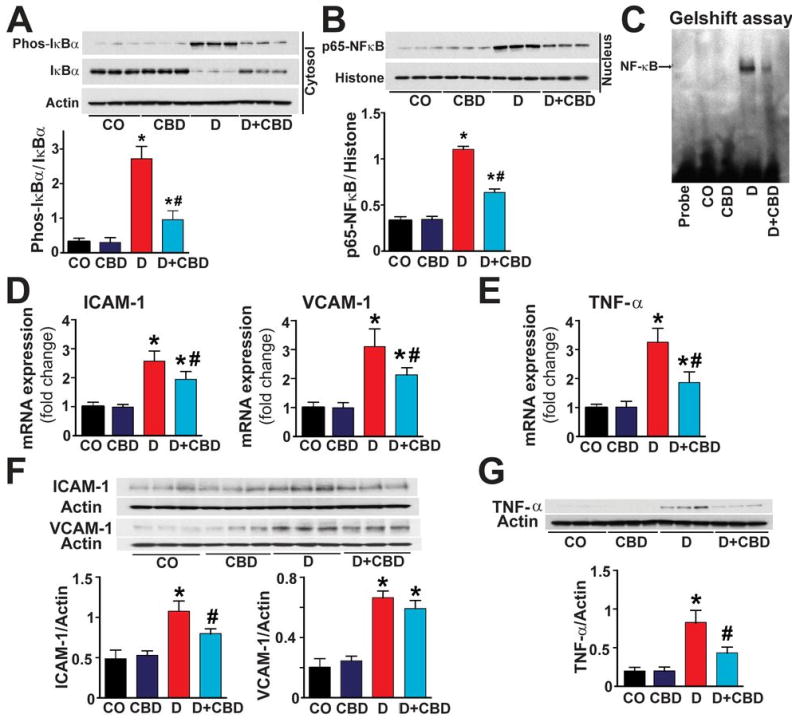
(A) Western blot analysis demonstrates IκB-α expression and its phosphorylation in the cytosolic fraction and (B) the nuclear translocation of p65NF-κB in the nuclear fraction of the heart tissue homogenates. (C) Shows the gel shift assay demonstrating NF-κB activation. (D) Shows the mRNA expression of ICAM-1/VCAM-1 (E) TNF-α in the respective groups as indicated (F) Shows the western blot analysis for the protein expression of ICAM-1/VCAM-1 and (G) TNF-α protein in the myocardial tissues. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n=6-9/group.
CBD treatments attenuates diabetes-induced nitrosative/nitrative stress
There was significant increase in inducible nitric oxide synthase (iNOS) expression (Fig.4A) and 3-nitrotyrosine (3-NT) accumulation (Fig.4B-E) in hearts of diabetic mice compared to vehicle or CBD alone treated mice. CBD treatment attenuated the diabetes-induced iNOS expression and 3-NT accumulation (marker of nitrosative/nitrative stress) (Fig.4B-E).
Fig.4. CBD inhibits diabetes-induced myocardial, iNOS expression and 3-NT accumulation.
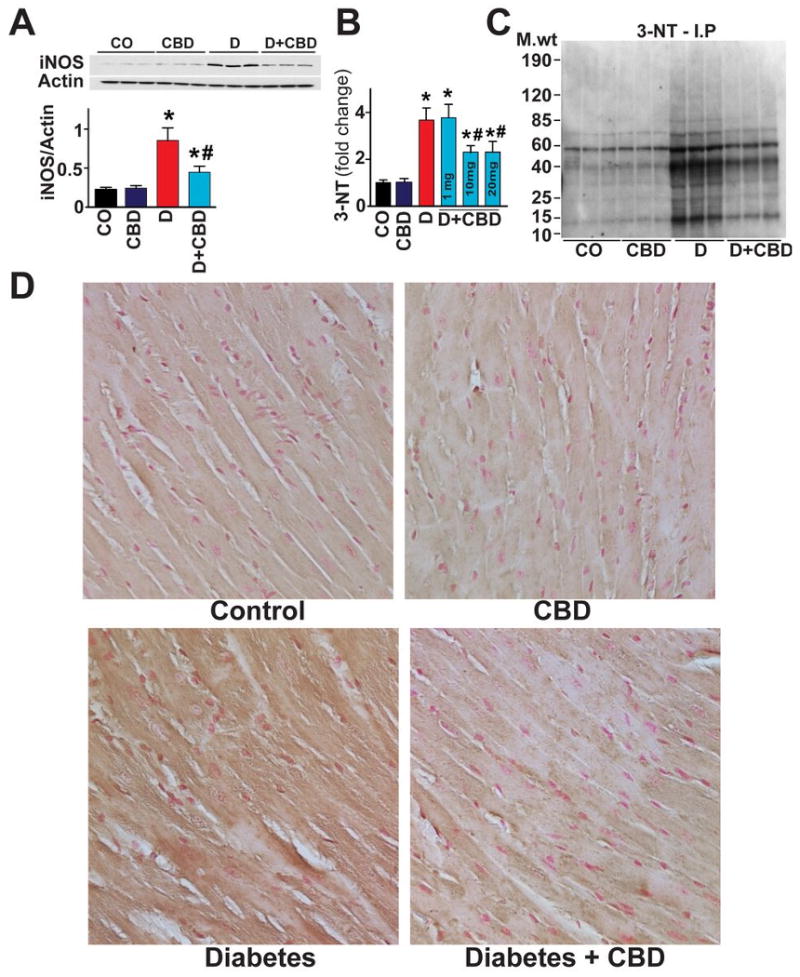
(A) iNOS expression was determined by Western immunoblot in the heart tissues (B) 3-NT levels in the heart samples were quantitatively determined by ELISA with indicated CBD concentration (mg/kg body weight) respectively. (C) Representative gel indicating the nitrated proteins analyzed by immunoprecipitation with 3-NT specific antibody (D) shows the representative images for the histochemical staining for 3-NT levels in the formalin-fixed myocardial tissues. (E) Depicts the immunofluorescence staining for 3-NT from frozen sections as described in methods. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n= 6-8/group.
CBD treatment attenuates diabetes-induced mitogen activated protein kinases (MAPKs) activation and apoptosis
There was marked increase in the p38MAPK (Fig.5A) and JNK (Fig.5B) activation in the myocardial tissues of diabetic mice. In addition, there was marked activation p38αMAPK (Fig.5C) and slightly diminished p38βMAPK (Fig.5C) in the diabetic myocardium. There was also activation of MAPKAPK-2 in the diabetic heart (Fig.5D). CBD treatment for 11 weeks significantly mitigated p38MAPK/JNK/p38αMAPK/MAPKAPK-2 activation, while it was not effective in restoring the p38βMAPK levels. In addition, Akt activation was also significantly hampered in the diabetic myocardium, which was attenuated with CBD treatment (Fig.5E). In diabetic myocardium there was marked increase in caspase 3 cleavage, caspase 3/7 activity (Fig.6A,B), chromatin fragmentation and PARP activity (Figs.6C,D), and enhanced apoptosis (Fig.6E and Fig.7); all these changes in diabetes were attenuated by CBD treatment.
Fig.5. CBD mitigates diabetes-induced myocardial activation of MAPKs and augments Akt activation.
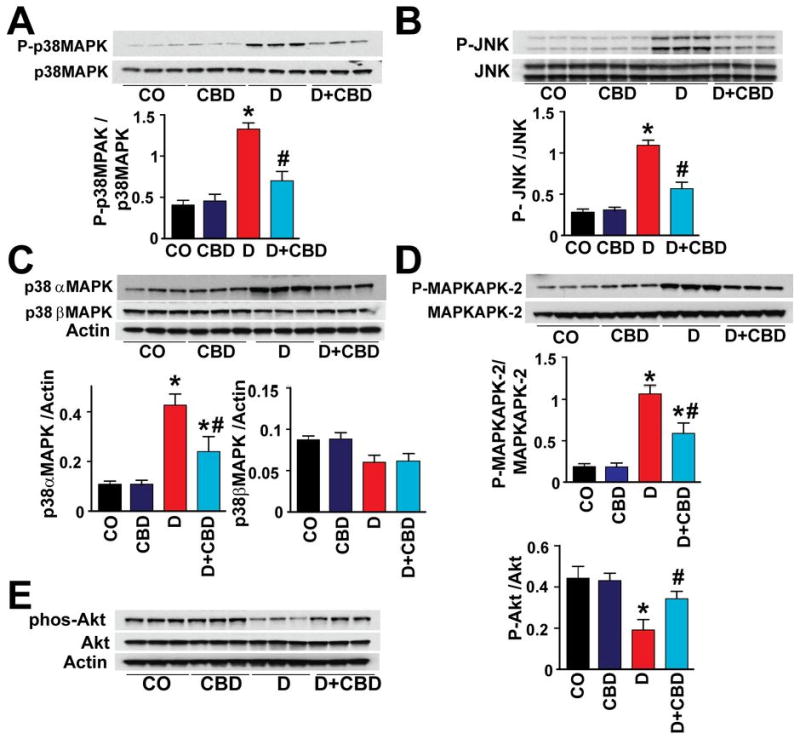
Western blot analysis shows the (A) p38MAPK (B) JNK (C) p38α/βMAPK (D) MAPKAPK-2 and (E) Akt activation in the myocardial tissues. P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n=6/group.
Fig.6. CBD mitigates diabetes-induced myocardial apoptosis and cell death.
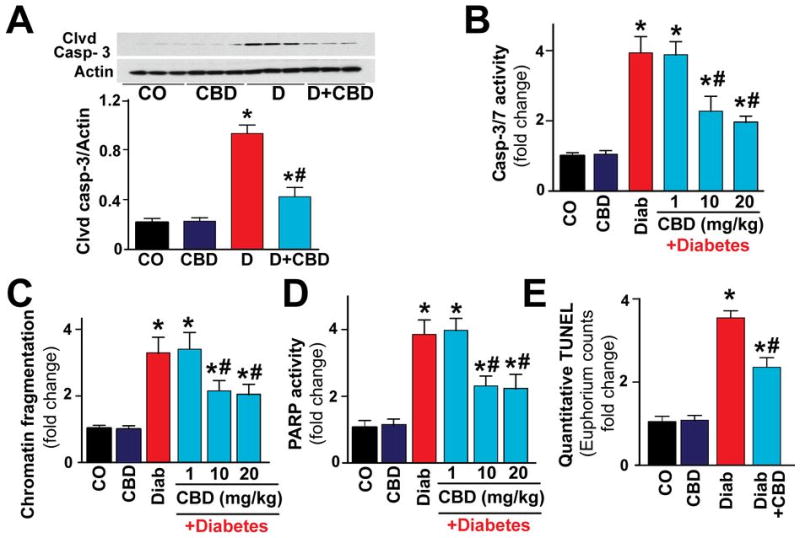
(A) Western blot analysis for the caspase 3 and (B) caspase 3/7 activity (C) chromatin fragmentation and (D) PARP activation and (E) quantitative TUNEL assay was performed as described in Methods. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n=6-9/group.
Fig.7. CBD mitigates apoptosis in the diabetic myocardium.
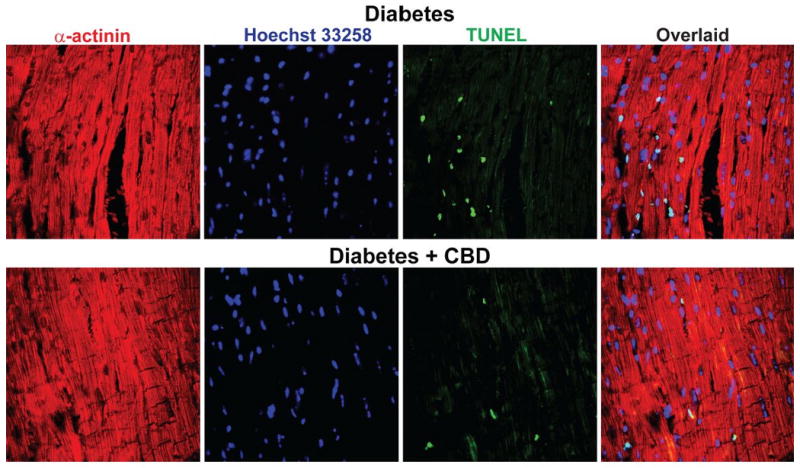
Shown are the representative TUNEL images in the diabetic myocardium and mice that were treated with CBD for 11 weeks. For details see the supplemental methods.
CBD treatment attenuates diabetes-associated myocardial fibrosis
Real time RT-PCR analysis revealed significant increases in the pro-fibrotic gene expressions (Fig.8A) and in collagen deposition (Fig.8B) in diabetic hearts, and these were attenuated by CBD (Fig.8).
Fig. 8. CBD attenuates diabetes-induced cardiac fibrosis.
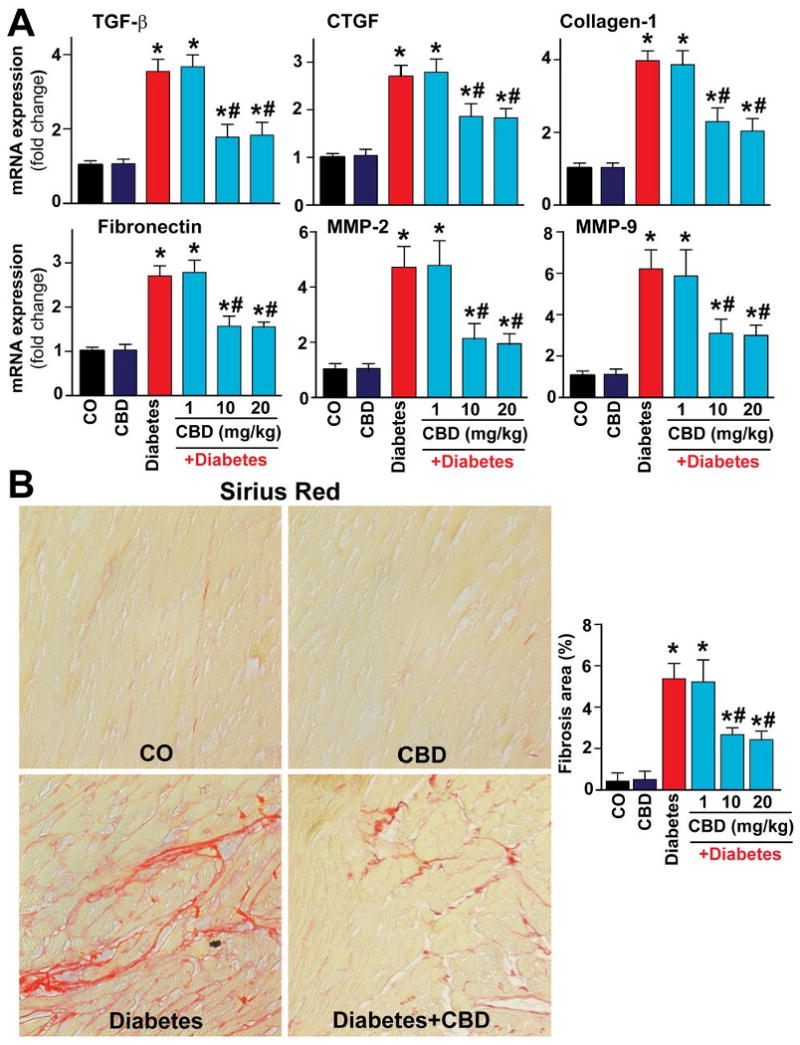
(A) Shows the mRNA expression of the pro-fibrotic genes in the myocardial tissues. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n=9/group. (B) Depicts the Sirius red staining indicating collagen deposition and implying and the extent of cardiac fibrosis. Images shown are representative from 4 independent experiments. *P<0.05 vs. vehicle control/CBD alone; #P<0.05 vs. diabetes [D], n= 4-6/group.
CBD post-treatment following the establishment of diabetic cardiomyopathy attenuates diabetes-induced myocardial oxidative/nitrosative stress, cell death and fibrosis
Remarkably, CBD 20 (mg/kg) treatment also attenuated the diabetes-induced increased myocardial nitrative stress, cell death (Suppl.Fig.4) and fibrosis (Suppl.Fig.5) when it was given for 4 weeks in 8 weeks diabetic mice.
CBD treatment attenuates high glucose (HG)-induced cytosolic and mitochondrial ROS generation and 3-NT formation in human cardiomyocytes (HCM)
HG treatment of HCM for 48 hrs markedly increased cytosolic (Suppl.Fig.6A) and mitochondrial (Suppl.Fig.6B) ROS/superoxide generation compared to cells treated with either D-glucose 5 mM, L-glucose 30 mM, or CBD (4μM) alone for the same duration. CBD markedly attenuated the HG-induced increased ROS generation (Suppl. Fig.6A,B) and 3-NT accumulation in HCM (Suppl. Fig.6C).
CBD mitigates HG-induced NF-κB activation and apoptosis in HCM
HG treatment induced NF-κB activation (Suppl.Fig.7A,B) and increased apoptosis and PARP-dependent cell death in cardiomyocytes (Suppl.Fig.8A,B); the anti-apoptotic activity of CBD was mediated, at least in part, via its ability to modulate Akt activity (Suppl. Fig.8A).
Discussion
Accumulating evidence suggests that increased oxidative/nitrosative stress coupled with activation of various downstream pro-inflammatory and cell death pathways play pivotal role in the development of complex biochemical, mechanical and structural alterations associated with diabetic cardiomyopathy (11,14,15) (3,4,6,12). However, in spite of the accumulating knowledge obtained during the past decades, the treatment of diabetic cardiomyopathy still remains poor and largely symptomatic (1,2).
Cannabidiol, a nonpsychoactive component of marijuana, has been shown to exert anti-inflammatory and antioxidant effects both in vitro and in various preclinical models of neurodegeneration and inflammatory disorders, independent from classical CB1 and CB2 receptors (20). Furthermore, CBD has recently been reported to lower the incidence of diabetes in non-obese diabetic mice (28) and to preserve the blood-retinal barrier in experimental diabetes (29).
In the present study, we have evaluated the effects of CBD treatment (for 11 weeks administered after the destruction of pancreatic beta cells and development of frank type 1 diabetes, as well as in 8 weeks diabetic animals for 4 weeks) on myocardial dysfunction, inflammation, oxidative/nitrosative stress, cell death and interrelated signaling pathways, using a mouse model of type I diabetic cardiomyopathy or primary human cardiomyocytes exposed to high glucose. Since significant cardiac dysfunction in this model starts to develop from 4 weeks of established diabetes (4,10) with gradually increasing fibrosis thereafter (12,15) (peaking around 8 weeks of established diabetes), in the first treatment protocol (Suppl.Fig.1A.) we aimed to study if CBD treatment can prevent the development of characteristic alterations of type I diabetic cardiomyopathy, in the second (Suppl.Fig.2A) if it is able to reverse these changes once they already developed.
Consistently with previous reports, diabetic cardiomyopathy was characterized by declined diastolic and systolic myocardial performance associated with enhanced myocardial expression of NADPH oxidase isoforms p22phox, p67phox, gp91phox, attenuated antioxidant defense (decreased glutathione content and SOD activity) coupled with increased myocardial reactive oxygen species generation and lipid peroxidation (4,6,10,12,15). The high glucose-induced ROS generation besides inducing lipid peroxidation may also initiate activation of various stress signaling pathways (e.g. jun Nterminal kinase and p38 MAPK). Our results are also in agreement with previous studies demonstrating enhanced activation of p38 MAPK and its downstream effector (p38 MAPKAPK-2) in diabetic cardiomyopathy models and demonstrating that pharmacological inhibition of p38 MAPK signaling attenuates the expression of cardiac inflammatory markers, such as TNF-α, IL-1β, and IL-6, and collagen content associated with diabetic cardiomyopathy (11,12). Likewise, with recent evidence supporting an emerging role of p38α activation in diabetic cardiomyopathy (12), in addition to its already established role in mediating cell death during myocardial ischemic-reperfusion injury. The high glucose induced-ROS generation also impairs important pro-survival signaling pathways such as Akt in diabetic hearts (13), activates pro-inflammatory and cell death pathways such as NF-κB (8,9) and nuclear enzyme poly(ADP)-ribose polymerase 1(10), which in turn regulate expression of important pro-inflammatory cytokines, cell adhesion molecules and iNOS. The latter results in increased nitrosative/nitrative stress, which is also implicated in cardiovascular complications of diabetes (5). A recent study has also suggested that the NF-κB activation may induce increased oxidative stress and contributes to mitochondrial and cardiac dysfunction in type II diabetes(9). Importantly, the oxidative-nitrosative stress, stress signaling and inflammatory pathways in diabetic cardiomyopathy are closely interrelated eventually promoting the development myocardial fibrosis (3,9,11,15,30).
CBD treatment (Suppl.Fig.1.) was able to attenuate the oxidative-nitrosative stress (decreased the myocardial ROS generation and expression of p22phox, p67phox, gp91phox, restored glutathione content and SOD activity, decreased 3-NT formation) and alterations of the above mentioned pro-survival (Akt) and stress signaling (p38, p38α, JNK) pathways in diabetic hearts. It also attenuated the NF-κB activation, expression of iNOS, TNFα, and ICAM-1, cell death and fibrosis in diabetic myocardium, and improved the associated characteristic functional alterations. Importantly, CBD treatment was able to attenuate/reverse (though to a lesser extent) some of the above mentioned diabetes-induced myocardial biochemical and functional changes following the establishment of diabetic cardiomyopathy with fibrosis (Suppl.Figs.2-5). CBD also attenuated the high glucose-induced increased reactive oxygen and nitrogen species generation, NF-κB activation and cell death in primary human cardiomyocytes (Suppl.Figs.6-8).
The above mentioned beneficial effects of CBD could be explain in part by its potent antioxidant properties, which was first suggested by the Nobel Prize winner Dr. Julius Axelrod (31). In Axelrod's study CBD was more protective against glutamateinduced neurotoxicity than any of the well-know antioxidants (e.g. ascorbate or α-tocopherol), indicating additional cytoprotective effects of CBD beyond its potent antioxidant properties (31). Indeed, our recent results suggest that CBD may exert potent effects on key pro-inflammatory pathways such as NF-κB and on pro-survival signaling such as Akt in vivo, which is most likely not related to its antioxidant effect. This is also supported by observations that CBD decreases inflammation in models in which conventional antioxidants are not very effective (e.g. in arthritis (20,32)), as well as by recent studies demonstrating that CBD is a potent inhibitor of bacterial lipopolysaccharide-activated NF-κB proinflammatory pathway in microglia cells(33). These results are also in support of the emerging role of the inflammation in the development and progression of diabetic cardiomyopathy (9,11,15,30).
Collectively, our results strongly suggest that CBD may have tremendous therapeutic potential in the treatment of diabetic cardiovascular and other complications by attenuating diabetes-induced oxidative/nitrosative stress, inflammation, cell death and fibrotic pathways.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of NIH/NIAAA (to P.P.) and by NIDA grant #9789 (to R.M.). Dr. Béla Horváth is a recipient of a Hungarian Research Council Scientific Research Fund Fellowship. Authors are indebted to Dr. Murali C. Krishna for generously providing his resources and expertise with EPR measurements, Drs. Sergey Dikalov and Kathy K. Griendling for sending the EPR probe during the time when it was not commercially available, and to Dr. George Kunos for providing key resources and support. Dr. Pacher dedicates this study for the 80th birthday of Professor Raphael Mechoulam and to Dr. Julius Axelrod.
This study was supported by the Intramural Research Program of the NIH/NIAAA (to P.P.) and NIDA DA9789 (to R.M.)
Dr. Veves receives funding from Novartis for investigator-initiated research grant—unrelated to this study
List of abbreviations
- CBD
cannabidiol
- ICAM-1
Inter-Cellular Adhesion Molecule 1 or CD54
- iNOS
inducible nitric oxide synthase
- 3-NT
3-nitrotyrosine
- MAPKs
mitogen-activated protein kinases (e.g. p38 and JNK)
- MMP 2 and 9
matrix metalloproteinases
- NF-κB
nuclear factor kappa B
- NADPH oxidases
nicotinamide adenine dinucleotide phosphate-oxidases
- PARP
poly(ADP-ribose) polymerase
- TNF-α
tumor necrosis factor alpha
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- VCAM-1
vascular cell adhesion molecule-1 or CD106
Footnotes
No conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fein FS. Diabetic cardiomyopathy. Diabetes Care. 1990;13:1169–79. doi: 10.2337/diacare.13.11.1169. [DOI] [PubMed] [Google Scholar]
- 2.Regan TJ, Ahmed S, Haider B, Moschos C, Weisse A. Diabetic cardiomyopathy: experimental and clinical observations. N J Med. 1994;91:776–8. [PubMed] [Google Scholar]
- 3.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 4.Kajstura J, Fiordaliso F, Andreoli AM, et al. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–24. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 5.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L, Wang Y, Zhou G, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Feng W, Xue W, et al. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009;58:1391–402. doi: 10.2337/db08-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragno M, Mastrocola R, Medana C, et al. Oxidative Stress-Dependent Impairment of Cardiac-Specific Transcription Factors in Experimental Diabetes. Endocrinology. 2006;147:5967–5974. doi: 10.1210/en.2006-0728. [DOI] [PubMed] [Google Scholar]
- 9.Mariappan N, Elks CM, Sriramula S, et al. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–83. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–21. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 11.Westermann D, Rutschow S, Van Linthout S, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 12.Thandavarayan RA, Watanabe K, Ma M, et al. Dominant-negative p38{alpha} mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H911–919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 13.Van Linthout S, Spillmann F, Riad A, et al. Human Apolipoprotein A-I Gene Transfer Reduces the Development of Experimental Diabetic Cardiomyopathy. Circulation. 2008;117:1563–1573. doi: 10.1161/CIRCULATIONAHA.107.710830. [DOI] [PubMed] [Google Scholar]
- 14.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 15.Westermann D, Rutschow S, Jager S, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56:641–6. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009;31:63–77. doi: 10.1007/s00281-009-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;7:607–15. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 19.Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–92. [PubMed] [Google Scholar]
- 20.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–85. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 22.Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40:701–8. doi: 10.1016/0091-3057(91)90386-g. [DOI] [PubMed] [Google Scholar]
- 23.Durst R, Danenberg H, Gallily R, et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H3602–7. doi: 10.1152/ajpheart.00098.2007. [DOI] [PubMed] [Google Scholar]
- 24.Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–24. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay P, Bátkai S, Rajesh M, et al. Pharmacological Inhibition of CB1 Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protocols. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss L, Zeira M, Reich S, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–9. doi: 10.1016/j.neuropharm.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–44. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–7. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 31.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collageninduced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–6. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616–26. doi: 10.1074/jbc.M109.069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


