Abstract
Asymmetric cell division – where two dissimilar daughter cells are produced – relies on asymmetric positioning of the telophase spindle midzone, which specifies the cleavage furrow. Ou et al. (2010) now report in Science a mechanism of asymmetric midzone positioning driven by a polarized cortical distribution of the contractile motor myosin-II.
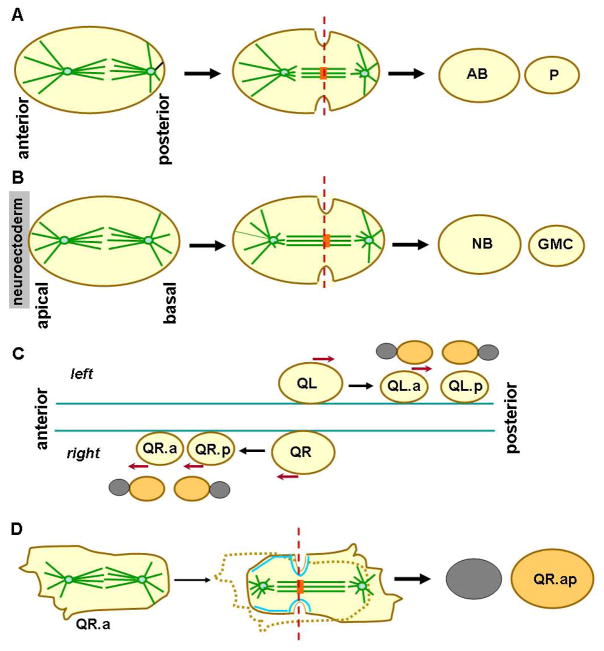
A widely observed mechanism leading to simultaneous increase in both cell number and cell fate complexity during development is asymmetric cell division, the process where a single cell division generates two daughter cells differing in their molecular contents and often also in their size at birth. The location of the plane of cytokinesis in a dividing animal cell, positioned by different microtubule arrays associated with the spindle (Werner and Glotzer, 2008), determines the sizes of the products of division. In particular, the midzone region of the telophase spindle, composed of a dense packing of microtubules, plays a key role in localizes many molecules controlling the formation and final closure of the cleavage furrow. Two general schemes have been observed that can lead to asymmetric positioning of the midzone. The first scheme, as best studied in the first division of the C. elegans zygote, the anaphase spindle, oriented along the anterior-posterior (A-P) and elongates symmetrically, but the entire spindle is moved closer to the posterior cortex as a result of dynein-mediated microtubule pulling forces to generate a larger anterior blastomere and a smaller posterior blastomere (Grill and Hyman, 2005) (Fig.1A). A distinct second scheme of asymmetric midzone positioning was observed in Drosophila embryonic neuronal progenitor cells (neuroblasts). The metaphase spindle, orientated along the apical-basal axis, is positioned symmetrically at first, but during anaphase, the apical side of the spindle half elongates considerable more than the basal side, leading to the formation of a midzone skewed toward the basal cortex and generating a small ganglion mother cell (GMC) and a larger neuroblast (Kaltschmidt et al., 2000) (Fig.1B).
Figure 1. Comparison of spindle asymmetry establishment mechanisms for unequal cell division.
(A) Asymmetric midzone positioning in the C. elegans zygote. The entire spindle is shifted toward the posterior cortex during anaphase by a biased pulling force exerted on astral microtubules (green lines). The resulting asymmetric position of the midzone (orange block) leads to cell division (red line) that generates a large AB blastomere and small P blastomere. (B) Asymmetric cell division in Drosophila embryonic neuroblasts. The metaphase spindle is positioned symmetrically along the apical-basal axis, but during anaphase, more extensive elongation of the apical side of the spindle skews the midzone toward the basal cortex. This results in cell division that generates a large neuroblast (NB) and a small GMC cell. (C) Cell migration and asymmetric divisions of Q neuroblast in C. elegans L1 larva. QL and QR cells migrate (red arrows) and generate QL.a and QL.p, QR.a and QR.p, respectively. After further migration (except for QL.p), each of the four neuroblasts divides asymmetrically (as shown above each) to generate a large cell (orange) and a small cell (grey). (D) Detailed observation of QR.a cell division revealed that the spindle starts out symmetrically positioned, as in Drosophila embryonic neuroblasts, but asymmetric spindle elongation coupled with anterior cortical contraction, driven by a polarized distribution of myosin-II (blue) and posterior expansion, lead to a biased position of the spindle midzone toward the anterior cortex.
The new work by Ou et al (2010), recently published in Science, presents mechanistic insight into another example of asymmetric cell division resembling the second mode described above in the less well studied system of Q neuroblasts in the C. elegans L1 larva stage. Unlike Drosophila neuroblasts, which delaminate from but remain in contact with the epithelium of the neuroectoderm, the Q neuroblasts are migratory cells that undergo several rounds of asymmetric cell divisions intermittent with movement along the A-P axis (Ou and Vale, 2009; Sulston and Horvitz, 1977). In the first round of these divisions, a pair of Q neuroblasts divides to generate four neuroblasts: the more anteriorly located QR.a and QL.a, and the more posteriorly located QR.p and QL.p (Fig.1C). Each of the four neuroblasts then undergoes an asymmetric division to generate a large cell, which gives rise to neuron(s), and a small cell, which soon undergoing apoptosis. Ou and colleagues used fluorescence video microscopy to follow the asymmetric division of Q neuroblasts in live animals and saw two distinct behaviors for the neuroblasts. QR.p and QL.p cells appear to generate daughter cells of unequal sizes following a route similar to that in the zygote: the spindle elongates symmetrically but the entire spindle is positioned asymmetrically along the A-P axis, biasing cell division toward the posterior pole (generating a small, apoptotic posterior daughter). In contrast, QR.a (and most likely also QL.a) divides in a way similar to that of Drosophila embryonic neuroblasts: the metaphase spindle is placed centrally, but during anaphase, the posterior half of the spindle elongates while the length of the anterior spindle half remains constant (Fig.1D). As the spindle asymmetry develops, the anterior cell cortex moves toward the anterior spindle pole while the posterior cortex expands. These coordinated movements lead to the formation of a midzone skewed toward the anterior cell pole and produce a small anterior and a large posterior cell after cytokinesis.
Prior to division, the QR.a cell moves anteriorly, and its anterior cortex maintains protrusive activity, like the leading edge of a migrating cell. However, after anaphase onset, the anterior cortex gradually ceases protrusion and starts contracting, resembling the retracting tail of a crawling cell. Meanwhile, the posterior cortex actively expands, forming a new leading edge. Supporting a critical role for the observed cortical dynamics of the dividing cell in the asymmetric division, the authors observed that myosin-II, the actin-based motor protein underlying cortical tension and contractile forces, is enriched in the anterior cortex, the side that gives rise to the smaller cell (Fig.1D). Moreover, local inactivation of this population of myosin-II by chromophore assisted laser inactivation (CALI) disrupted the asymmetric cell division, resulting in daughters of roughly equal sizes, while CALI of a control fluorescence protein associated with the anterior cortex produced no such effect. These observations led the authors to propose that a polarized distribution of myosin-II results in a contracting anterior cortex, which “squeezes” the cytoplasm toward the posterior pole and thus promotes its expansion while the spindle midzone stays put.
Although myosin II in a variety of cell types has previously been implicated in the establishment of cell polarity, this finding by Ou et al (2010) represents the first example of myosin involvement in setting up spindle asymmetry during anaphase. An obvious question is whether this involvement also occurs during Drosophila neuroblast asymmetric division, where spindle asymmetry is also established during anaphase. However, unlike in QR.a cells, myosin-II is found to concentrate in Drosophila embryonic neuroblasts at the side of the cortex corresponding to the large cell before anaphase onset. During cytokinesis, myosin-II concentrates mostly to the cleavage furrow with no apparent basal enrichment (Barros et al., 2003), suggesting that that myosin-II does not have the same role. An important difference between QR.a and Drosophila neuroblast may lie in the origin of these cells: the former are migratory cells, whereas the latter originate from an epithelium and exhibit polarity along the apical-basal axis. In fact, the coordinated contraction of the anterior cortex and expansion of the posterior cortex reflects a posterior-directed cell movement (Fig.4D).
Finally, it remains an intriguing question why these cell divisions must generate cells of unequal sizes, if the purpose of the asymmetry is simply to differentially segregate cell fate determinants between the daughters. In the case of Q neuroblasts, the smaller progenies of the asymmetric divisions normally undergo apoptosis and are quickly cleared away by neighboring phagocytic cells. When the size asymmetry in QR.a cell division was disrupted by myosin-II CALI, the now larger anterior cell (the one normally being cleared away) sometimes escaped apoptosis and differentiated into neuron-like cells. This suggests that cell size may play a role in cell fate determination. However, since polarity in migratory cells, as shown in neutrophils, is promoted by mutually inhibitory interactions between the pathways that establish the myosin-II-rich contractile back and that generate the protrusive actin leading edge (Xu et al., 2003), myosin-II CALI may disrupt cell polarity in general, which may prevent the proper segregation of cell fate determinants. This question of whether and why size matters has yet to be resolved even in the well studied Drosophila neuroblast system, as no mutation is known to only disrupt size asymmetry without affecting cell polarity or spindle orientation. However, the mere fact that such a separation of function mutation has yet to be found does point to an intimate mechanistic link between the physical and molecular asymmetries in these cell divisions, much of which remains to be unraveled.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barros CS, Phelps CB, Brand AH. Dev Cell. 2003;5:829–840. doi: 10.1016/s1534-5807(03)00359-9. [DOI] [PubMed] [Google Scholar]
- Grill SW, Hyman AA. Dev Cell. 2005;8:461–465. doi: 10.1016/j.devcel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt JA, Davidson CM, Brown NH, Brand AH. Nature cell biology. 2000;2:7–12. doi: 10.1038/71323. [DOI] [PubMed] [Google Scholar]
- Ou G, Stuurman N, D’Ambrosio M, Vale RD. Science. doi: 10.1126/science.1196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Vale RD. J Cell Biol. 2009;185:77–85. doi: 10.1083/jcb.200812077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Werner M, Glotzer M. Biochemical Society transactions. 2008;36:371–377. doi: 10.1042/BST0360371. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]