Abstract
High Fat Diet (HFD)-induced obesity is a major contributor to diabetes and cardiovascular disease, but the underlying genetic mechanisms are poorly understood. Here, we use Drosophila to test the hypothesis that HFD-induced obesity and associated cardiac complications have early evolutionary origins involving nutrient-sensing signal transduction pathways. We find that HFD-fed flies exhibit increased triglyceride (TG) fat and alterations in insulin/glucose homeostasis, similar to mammalian responses. A HFD also causes cardiac lipid accumulation, reduced cardiac contractility, conduction blocks and severe structural pathologies, reminiscent of diabetic cardiomyopathies. Remarkably, these metabolic and cardiotoxic phenotypes elicited by HFD are blocked by inhibiting insulin-TOR signaling. Remarkably, reducing insulin-TOR activity by TSC1-2, 4EBP, FOXO) or increasing lipase expression in the myocardium suffices to efficiently alleviate cardiac fat accumulation and dysfunction induced by HFD. We conclude that deregulation of insulin-TOR signaling due to a HFD is responsible for mediating the detrimental effects on metabolism and heart function.
Introduction
Obesity has grown to epidemic proportions globally, with more than 1.5 billion adults overweight and 400 million of them considered obese. Increasing evidence indicates that excessive dietary accumulation of lipids (ei. ‘obesity’) is a high risk factor in causing deleterious effects on the metabolism and heart function and has been strongly linked to the progression of heart disease and type 2 diabetes (Szendroedi and Roden 2009; van Herpen and Schrauwen-Hinderling 2008). Investigating the origin and deleterious effects of high fat diet (HFD)-induced obesity and its genetic mediators is an important step in understanding the mechanisms that contribute to obesity-associated secondary diseases, led by heart disease.
However, the mechanisms that underlie HFD pathophysiology have yet to be elucidated and include theories that the cause of obesity is a recent evolutionary genetic adaptation to alternating periods of famine and excess (Neel et al. 1998). Alternatively, the potential for HFD-induced obesity may have arisen early in evolution via nutrient sensing pathways that coordinate metabolism. Here, we used the Drosophila model to investigate the genetic mechanisms that may underlie HFD-induced obesity and cardiac dysfunctions. Drosophila is well suited to study this hypothesis. For example, the way the human heart is specified during embryogenesis relies on mechanisms and gene networks that were first elucidated in Drosophila (Bodmer 1995; Harvey 1996), which has subsequently been validated in many ways in mammals (Cripps and Olson 2002; Zaffran and Frasch 2002; Ocorr et al. 2007a; Neely et al. 2010). Not only is the embryology of cardiac specification equivalent between vertebrates and invertebrates, but nearly the entire complement of control genes and interactions is conserved and even partially interchangeable between vertebrates and flies (Bodmer and Venkatesh 1998; Cripps and Olson 2002). Since there is such a deep evolutionary conservation in the formation of the heart, it is likely that many aspects of heart function are also conserved, including the mechanisms that control or influence how the heart responds to HFD-induced obesity. In addition, Drosophila has already been established as an excellent model for studying the genetic control of metabolism and nutrient sensing pathways (Baker and Thummel 2007; Leopold and Perrimon 2007; Kim and Rulifson 2004; Oldham and Hafen 2003). The insulin and TOR pathways are highly conserved regulators in the control of metabolism. Although the molecular basis of regulating and coordinating metabolic homeostasis is far from being understood, manipulating insulin or TOR signaling in species ranging from yeast to humans dramatically influences metabolic responses, such as lipid and glucose homeostasis (Arking et al. 2005; Saltiel and Kahn 2001; Tatar et al. 2003; Vellai et al. 2003; Wang et al. 2005).
The genetic simplicity of the Drosophila model combined with recently established heart function assays (Ocorr et al. 2007b,c; Taghli-Lamallem et al. 2008; Wessells et al. 2004; Fink et al. 2009) make it possible to probe the genetic mechanisms of how a HFD affects heart function. Our findings indicate that flies on a HFD become obese which is dependent on insulin-TOR signaling as well as regulators of lipid metabolisms (triacylglycerol lipase and fatty acid synthase). We show that both systemic and (adipose) tissue-specific manipulations can protect the fly from HFD-induced systemic lipid accumulation and other deleterious effects of high dietary fat, such as heart dysfunction. Moreover, cardiac-specific inhibition of TOR downstream of TSC1-2, overexpression of FOXO or lipase protects the heart dysfunction in otherwise obese flies due to exposure to HFD conditions. Importantly, our evidence suggests that the metabolic and cardiac dysfunction caused by a HFD is evolutionarily and functionally conserved and mediated by a nutrient-sensitive circuit, which includes insulin-TOR signaling and the control of lipid metabolism. Thus, targeted inhibition of the TOR pathway or lipid biogenesis may provide new therapeutic interventions in obesity and its adverse effects on heart function.
Results
Flies exhibit similar phenotypes as Mammalian Metabolic Syndrome
To investigate the potentially conserved genetic mechanisms involved in lipid accumulation affecting heart function in the Drosophila model, we first determined the effects of a HFD on the fly’s metabolism. We found a dose-dependent increase in whole body TG content per body mass when flies were fed diets containing 3–30% saturated fats from coconut oil for 5 days (Figure 1A, S1A). This indicates that the fly accumulates lipids in a dose dependent fashion. Next, we tested if the major individual fatty acids (FAs) in the coconut oil could also cause an increase in TGs. We found that either lauric or myristic acid (the main components of coconut oil) caused a significant increase in whole body TGs (Figure 1B, S1B).
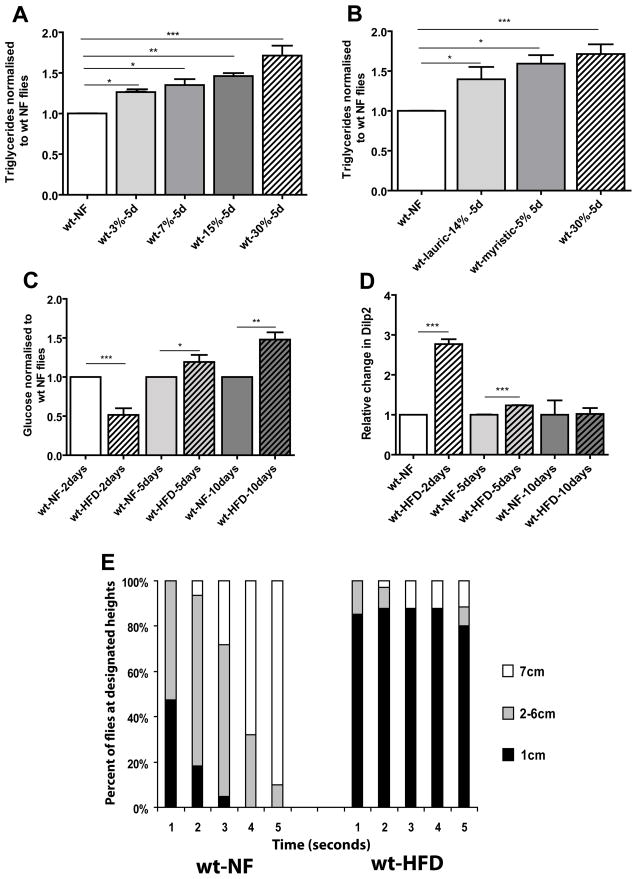
Figure 1. HFD-induced obesity leads to metabolic syndrome in flies.
A) Triglyceride content (expressed as relative change from NF flies) of 10–15 day old females on HFD for 5 days. The w1118 fly strain was used as wildtype (wt) in all experiments unless indicated otherwise. wt flies were used under 4 different concentrations of saturated fats. At least 3 independent experiments were done for each time point for all TG experiments. The concentrations used were 3 (n=46), 7 (n=49), 15 (n=31), 30% (n=81) for 5 days. wt type flies (n=107) showed a dose dependent increase in TGs (* = p<0.05, ** = p<0.01, ***= p<0.0001). B) Triglyceride content (expressed as relative change from NF flies) of 10–15 day old females on HFD for 5 days. The w1118 fly strain was used for the wildtype (wt) flies. wt flies were used two different types of saturated fats. Myristic and Lauric acid are both the major components of coconut oil. The amount of each fatty acid corresponds to its respective amount found in the 30% HFD. At least 3 independent experiments were done for each time point for all TG experiments. The concentrations used were lauric acid 14% (n=36), while myristic acid was 5% (n=35) and finally 30% of the original coconut oil mixture for 5 days. wt type flies showed a dose dependent increase in TGs (* = p<0.05, ** = p<0.01, ***= p<0.0001). C) Glucose content of female wt flies fed a 30% HFD (normalized to wt NF-fed flies of each appropriate age). Trehalose present in the hemolymph was converted to glucose (see M&M). Since 30% HFD gave us the strongest most consistent results we used 30% for all of the remaining experiments in this study. At least 3 independent experiments were done for each time point. After 2 days glucose decreases then rapidly increases after 5 and 10 days (* = p<0.05, ** = p<0.01, *** = p<0.0001). A minimum of 35 flies were used for each variable at each time point. D) Relative Dilp2 transcript levels in wt flies fed a 30% HFD for 2, 5 and 10 days. Dilp2 levels rapidly increase after 2 days then begin to decrease the longer the fly remains on a HFD (*** = p<0.0001). All qPCR were done in triplicate. E) Graphical representation of the effects of time and Geotaxic activity. Flies were filmed for 5 seconds then the movie was analyzed and individual flies were counted at each height 1cm being the lowest portion of the vial with 7 cm being the highest part of the vial. A minimum of 150 flies were used for each variable. All flies eventually moved to the top of the vial but we only counted the position of the flies to the allotted 5 second time span. Flies on a 30% HFD showed a significant decrease in geotaxic activity with approximately 80% remaining in the bottom of the vial for the allotted time span.
In mammals increased levels of TGs have been found to be a major risk factor for metabolic syndrome, insulin resistance and the onset of type 2 diabetes (Ouwens et al., 2005; Van Gaal et al., 2006). In mammals after initial exposure to HFD, insulin release is increased, which corresponds to a decrease in glucose levels. However upon chronic exposure to HFD, glucose levels begin to increase as the periphery becomes more insulin resistant. To determine if changes in insulin and glucose homeostasis by HFD-induced obesity are functionally conserved, we tested changes in glucose and insulin homeostasis in flies fed a HFD. Indeed, after two days on a 30% HFD, flies showed a decrease in total glucose accompanied by an increase in Drosophila insulin-like peptide 2 (Dilp2) transcript levels (Figure 1C,D). After 5 and 10 days on HFD, glucose levels rose while Dilp2 levels continued to drop (Figure 1C,D). Another marker for monitoring insulin-glucose homeostasis is phosphorylated Akt (pAkt), which decreases under HFD conditions thereby lowering glucose uptake in mammals (Manning, 2004). In flies on a HFD pAkt is progressively reduced (Figure S2A), compared to flies on a normal food (NF) diet. We also find that under a HFD the TOR pathway is activated, as measured by increased 4EBP and S6K phosphorylation (Figure S2B). Together, these data suggest that the Drosophila model exhibits central aspects of metabolic syndrome and obesity upon HFD.
Excess dietary fat consumption has been linked with increased non-adipose fat deposition which is thought to be a contributing factor in instigating several secondary diseases such as type II diabetes, non-alcoholic fatty liver disease, colon cancer and cardiovascular disease (Schaffer 2003, Unger 2003, van Herpen and Schrauwen-Hinderling 2008). Therefore we investigated the accumulation of lipids in both adult adipose tissue (fatbody, FB) and the midgut, which are the fly’s major organs for lipid storage and utilization. Using Oil red O to measure the changes in fat accumulation, we find an increase in lipid droplet staining in HFD treated flies compared to NF fed controls both in FB and gut (Figure S3). These findings indicate that when fed a HFD flies accumulate excess fat in both adipose and non-adipose tissue, as seen in mammals.
A recent study found that upon fat accumulation activity levels tend to decrease (Wareham, 2007). Therefore, we determined the activity levels of flies under HFD conditions. Normally flies prefer to move against gravity (negatively geotaxic) (Gargano et al., 2005). When testing for changes in geotaxic activity we found that HFD-fed flies exhibit a substantial reduction in geotaxic behaviour (Movies S1,2), with most flies remaining at the bottom of the vial during the experimental time (Figure 1E). They will eventually climb to the top, albeit more slowly and less vigorously. These data suggest that consumption of a HFD has adverse, lethargic-like effects on the flies’ activity levels.
Increased dietary fats causes severe functional and structural changes in the fly heart
When rodents are fed a HFD they show signs of increased accumulation of cardiac TGs, accompanied by hypertrophy and decreases in fractional shortening, a relative measure of cardiac contractility (Fang et al., 2008; Sowers, 2003). It may be the increased adipose and circulating lipid levels that cause a disturbance of cardiac performance. For example, increased expression of lipid transporters in the heart leads to elevated fat in the myocardial cells accompanied by cardiac dysfunction (Chiu et al., 2005), although the underlying mechanisms have yet to be elucidated. To determine if ‘fat’ flies also show increased cardiac TG levels and exhibit deteriorating heart function, we examined them under HFD conditions. After 5 days on a HFD we observed elevated cardiac TG levels (Figure 2A) and progressively altered contraction patterns (Figure 2B–G, Movies S3–5). We then investigated other cardiac dysfunctions and observed a reduced fractional shortening (FS) due to a smaller diastolic diameter (Figure 4C,D), reminiscent of a restrictive cardiac phenotype (see Cammarato et al., 2008). In addition, we found non-contractile portions of the heart, dysfunctional inflow valves (ostia) and increased incidences of partial conduction blocks (anterior and posterior heart beats at a different rate) (Figure 2D–G, Movies S6–8). We also found severe defects and disorganization in the myofibrillar structure of the heart of flies fed a HFD (Figure 2H1–2″). Taken together these results dramatically illustrate that a HFD in Drosophila adversely affects heart function, and that in both mammals and flies a HFD compromises cardiac activity and structural integrity.
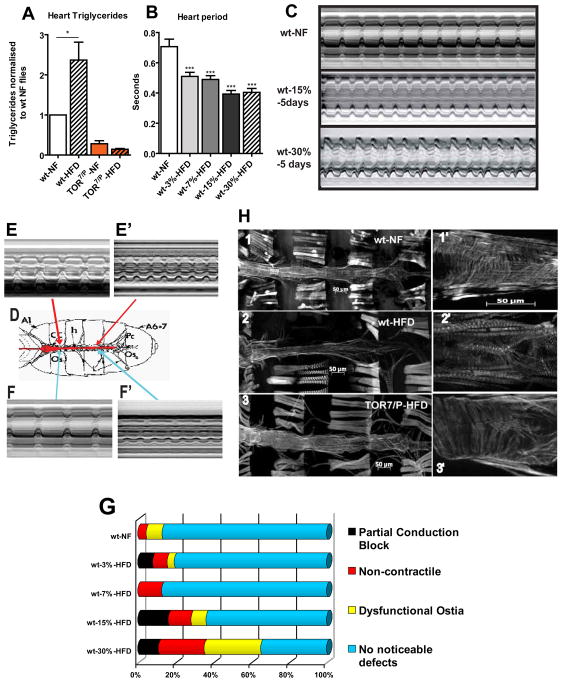
Figure 2. HFD treatment causes cardiac dysfunction.
A) Triglyceride content from female hearts on NF and HFD for 5 days (normalized to wt NF hearts). TOR7/P mutant flies were examined under the same food conditions. At least 3 independent experiments were done for each time point for all TG experiments (with approximately 90 hearts were used for each condition and genotype). wt type flies showed an increase in TGs (* = p<0.05) while the TOR mutants had significantly lower levels of TGs that did not increase on a HFD. B) Bar graph representation of changes in heart period for a population of approximately 24 flies hearts from each food type. A dose dependent effect is shown for the increase in dietary fat content (***p<0.0001). A minimum of 22 flies were used for each dietary condition. C) M-mode traces prepared from high speed movies of semi-intact preparations on NF, 15%, and 30% HFDs for 5 days. Note the changes in the M-mode traces as the concentration of fat increases. Red bars indicate systolic and diastolic diameters. D) A sketch of a dissected semi-intact heart preparation. Arrows indicate to the area the corresponding M-modes shown in E–F′. E–E′) Representation of partial conduction blocks of hearts using M-modes from different portions of the same heart. E) M-mode from the anterior portion of the heart displaying a regular heart beat. E′) M-mode from the posterior portion of the same heart displaying a faster and erratic heart beating pattern, compared to I. F–F′) Representation of a portion of the heart that is non-contractile hearts using M-modes of different heart regions. F) M-mode taken from the anterior portion of the heart displays regular beating pattern. F′) M-mode taken from the posterior portion of the same heart showing poor or no contractions. G) Side bar graph of heart phenotypes seen under HFD conditions with increasing amount of fat. The heart phenotypes used in this graph are partial conduction blocks, non-contractile myocardial cells, dysfunctional ostia and no noticeable defects. The instances of all 3 phenotypes increase in a fat dose-dependent fashion. A minimum of 26 heart movies were analysed for each dietary condition. H) Fluorescent micrographs of hearts on NF and HFD for 5 days at 10x and 25x optical magnification. Adult hearts are stained with AlexaFlourR 594 phalloidin. 1) Adult heart of a wt flies on NF. 1′) Magnified anterior NF-fed wt heart region. Note the regular arrangements of myofibrillar organization. 2) Adult heart of a wt flies on HFD for 5 days. Note the degeneration of the regular myofibrillar structure and the decrease in heart tube diameter. 2′) Magnified anterior wt heart region on HFD. Note the disorganization of the circular myocardial myofibrils. 3) Adult heart of a TOR7/P mutant on a HFD for 5 days. Note that there is little to no degradation of heart structure and the diameter is not constricted. 3′) Magnified anterior heart region of a TOR7/P mutant. There is little change in myocardial structure under HFD conditions compared to NF-fed wt (1′).
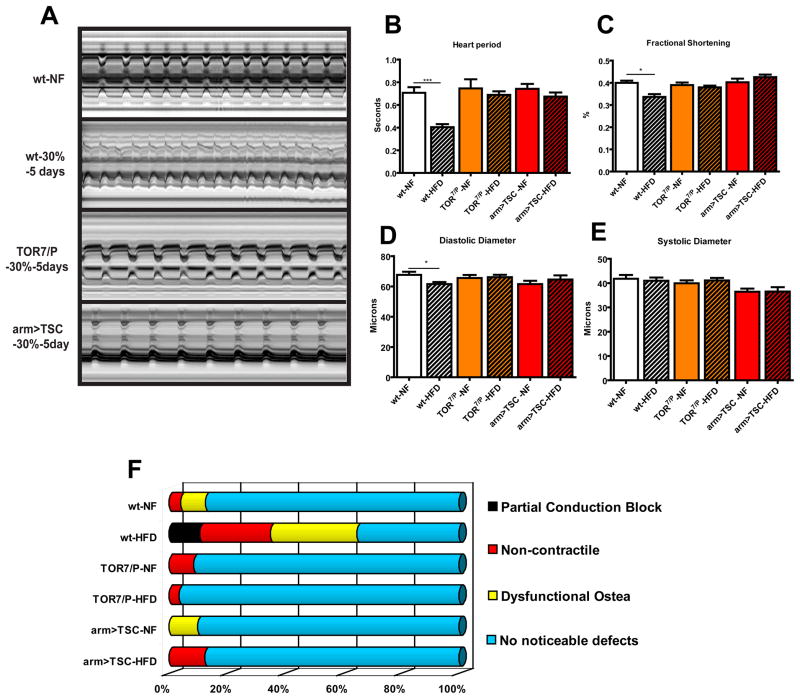
Figure 4. Reducing TOR function prevents HFD-induced obesity cardiac dysfunction.
A) M-mode traces of dissected wt flies on NF and 30% HFD, TOR7/P mutants and arm>TSC1-2 on NF and 30% HFD. No significant change was seen in TOR mutant nor arm<TSC heart M-modes on HFD when compared to wt on NF. B) Bar graph of cumulative heart periods for wt, TOR mutants (n=25, 36 respectively) and arm>TSC1-2 flies (n= 20 (NF), 21 (HFD)) under NF and HFD conditions. No change in heart period from wt under NF was seen in TOR mutants and arm>TSC1-2 flies under HFD conditions. C) Bar graph of changes in Fractional Shortening. A decrease in Fractional Shortening in wt flies was seen after 5 days on a 30% HFD (p<0.001). While no change was seen in fractional shortening in neither the TOR mutants nor the arm>TSC1-2 flies under a HFD. D) Bar graph of combined diastolic diameter data of wt and TOR mutant and arm>TSC1-2 hearts under NF and 30% HFD conditions. No change was seen in Diastolic Diameter in TOR mutants and arm>TSC1-2 flies under a HFD. E) Bar graph of systolic diameter of fly heart as in (F). No decrease in Systolic Diameter was seen after 5 days on a 30% HFD for any of the strains tested. F) Graphical representation of heart phenotypes as in Fig. 2G. No significant change the heart phenotype of TOR7/P mutants or with systemic TSC1-2 overexpression under HFD could be detected, when compared to wt flies. A minimum of 20 individual fly heart movies were analyses.
Systemic inhibition of the TOR pathway prevents excess fat accumulation
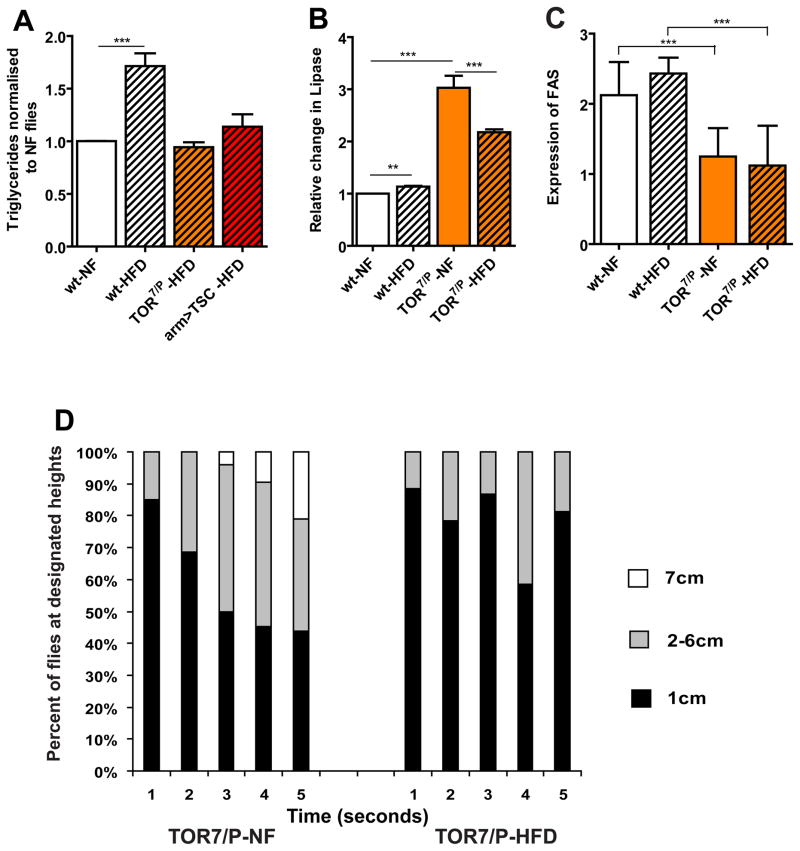
It is unclear how obesity increases the risk of heart disease. Since the TOR pathway has been linked to nutrient sensing and the modulation of aging heart function in Drosophila (Luong et al., 2006; Wessells et al., 2009), we tested whether TOR signalling is involved in the regulation of lipid levels related to cardiac dysfunction. To determine if reducing TOR function can alter the effects of HFD-induced obesity, we fed hypomorphic TOR7/P mutant flies (Luong et al., 2006) a HFD and observed no increase in TG levels, compared to flies on NF (Figure 3A). The TOR mutants show lower TG levels on both NF and a HFD, compared to wildtype on NF (Figure S1C). These results suggest that reducing TOR function is either accelerating fat catabolism, or decreasing lipid anabolism or storage. To test possible downstream mechanisms that may contribute to this phenotype, we tested the change in transcript levels of genes involved in lipid metabolism: Brummer (ATGL) lipase (Bmm) (Gronke et al., 2005) and Fatty Acid Synthase (FAS) (Valet et al., 2002). We found that Bmm transcript levels are significantly increased, whereas FAS levels are decreased, in TOR7/Pmutants under both NF and HFD conditions (Figure 3B,C), suggesting that TOR activity regulates the balance between lipid anabolism and catabolism, possibly due to increased lipase and decreased FAS activity. We tested this idea further by using Oil Red O, a lipid droplet marker, to investigate the changes in fat accumulation in both the FB and gut. Indeed, HFD-fed TOR mutants showed no increase in the quantity of lipid droplets in both the FB and gut, and fat levels were generally lower (Figure S3).
Figure 3. Reducing TOR function prevents HFD obesity.
A) Comparison of changes in TG levels (expressed as relative change from NF flies) between wt, TOR7/P and systemic TSC1-2 overexpression (arm>TSC1-2 flies). At least 3 independent experiments were performed for each time point for all TG experiments. A significant increase in TGs were seen in wt flies (*** p<0.0001) fed a 30% HFD for 5 days, while TOR mutants (n=46) started with lower levels of TG under NF and these levels did not increase even on 30% HFD (n=48). arm>TSC flies (n=30) had similar levels as the wt control flies but these levels did not increase when placed on a HFD. B) Relative Bmm (AGTL Lipase) mRNA transcript levels. There is a slight increase in Lipase levels in wt under 30% HFD conditions (p<0.05). In contrast, TOR7/P mutants have a 3-fold increase in Bmm levels under NF conditions and remain high under HFD conditions. All qPCR were done in triplicate. C) Expression of FAS transcript levels in wt and TOR7/P mutants under both NF and 30% HFD conditions for 5 days. TOR7/P flies show significantly lower FAS mRNA levels than the corresponding wt flies on the same diets (** p<0.01). All qPCR were done in triplicate. D) Graphical representation of the effects of time and Geotaxic activity. Flies were filmed for 5 seconds then the movie was analyzed and individual flies were counted at each height 1cm being the lowest portion of the vial with 7 cm being the highest part of the vial. A minimum of 150 flies were used for each variable. All flies eventually moved to the top of the vial but we only counted the position of the flies to the allotted 5 second time span. Unlike wt, TOR7/P seem to have an inherent defect in their geotaxic yet this phenotype was exacerbated when fed a HFD.
To determine if TOR-dependent prevention of HFD induced obesity extends to other HFD mediated phenotypes, we tested the geotactic activity of the TOR mutants under NF and HFD conditions. We find that in TOR mutants these activity levels are already impaired under NF, and this phenotype is exacerbated when flies are placed on a HFD (Figure 3D), which suggests that the protection by the ubiquitous inhibition of TOR activity is not a global response but may be selective to tissue type and lipid metabolism. These data are consistent with mice that are deficient in TOR complex 1 (TORC1) in skeletal muscles, which exhibit a 40% decrease in voluntary wheel activity (Bentzinger et al., 2008).
Systemic inhibition of the TOR pathway protects the heart against HFD-induced cardiac dysfunction
Next, we tested whether global reduction of TOR function protects the heart’s performance under HFD conditions. First, we tested whether the elevated TGs due to a HFD are reduced in the heart itself in TOR7/P mutants. Indeed, reduced systemic TOR function causes a significant decrease in cardiac TGs, which remains low even under HFD conditions similar to the results from whole body TGs (Figure 2A). We then show that the TOR mutants exhibit considerable protection against deterioration of heart function when placed on a HFD. Remarkably, flies with reduced TOR function exhibit unchanged heartbeat and contractility parameters under NF or HFD, comparable to wildtype under NF conditions (Figure 4A–E, Movie S9). Similarly, the incidences of conduction blocks, dysfunctional ostia and non-contractile myocardial regions were as low as in the TOR mutants under NF or HFD conditions compared to wildtype flies on NF (Figure 4F). We also find that the myocardial structure remains intact and similar to wt on NF (Figure 2H3-3′). These findings suggest that a decrease in TOR activity initiates lipid breakdown and decreased lipid synthesis, which may help prevent the adverse effects of a HFD on the heart.
Metabolic homeostasis is a balance between storage and expenditure that is regulated by genetic and environmental interactions, both polygenic and multi-organ in nature. To elucidate how reduced TOR signalling alters the HFD response at the tissue level, we used the UAS/Gal4 system (Brand and Perrimon, 1993) to inhibit TOR pathway components both in the whole organism and in selective tissues. First, we overexpressed the TOR inhibitor TSC1-2 ubiquitously using the arm-Gal4 driver, which resulted in a similar rescue of the HFD-induced increase in TGs and aberrant heart phenotypes (Figure 4), as with TOR loss-of-function mutants. Moreover, global inhibition of TOR translational effectors (eIf4E and S6K) by 4EBP (Miron et al., 2001) or dominant-negative S6K overexpression (S6KDN) (Hennig and Neufeld, 2002), respectively, also elicits a protective cardiac phenotype against HFD-induced abnormalities (not shown), similar to TSC1-2 overexpression (Figure 4). These results indicate that systemic inhibition of TOR signalling protects the animal against the adverse cardiac effects of a HFD and that multiple TOR-dependent effectors may contribute to TG accumulation and cardiac dysfunction.
Adipose-specific manipulation of insulin-TOR signalling affects obesity phenotypes
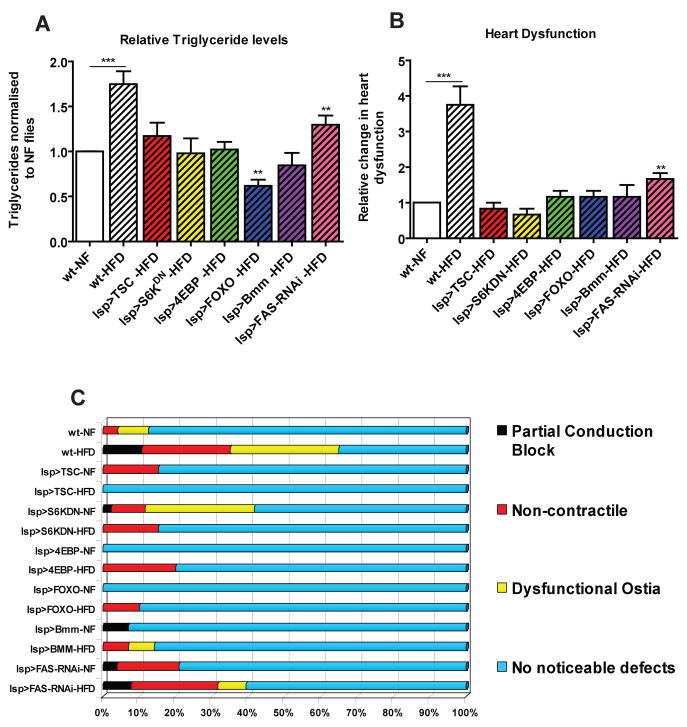
In insects the FB is an important organ for lipid storage and hormonal regulation and is functionally comparable to the vertebrate liver and adipose tissue. Therefore, we tested whether inhibition of TOR signalling in the fly’s FB can prevent the HFD-induced obesity and heart phenotypes. Expression of TSC1-2, S6KDN, and 4EBP specifically in the FB showed a phenotype similar to the ubiquitous inhibition of TOR exposed to a HFD. Specifically, FB-restricted expression abolished the normally observed increase in TGs, and there are no significant changes in cardiac function of all these genotypes when subjected to HFD conditions (Figure 5, S4). Thus, genetic manipulations inhibiting TOR pathway activation specifically in the FB resulted in protection from HFD-induced fat accumulation and cardiac dysfunction. This result also suggests that manipulation of TOR in the FB regulates the organism’s systemic lipid metabolism, which in turn influences proper heart function.
Figure 5. Fatbody-specific inhibition of TOR prevents obesity and heart phenotypes.
A) Changes in TG content of wt and fatbody-specific (lsp-Gal4) expression of TSC1-2 (n=36), S6KDN (n=36), 4EBP (n=34), FOXO (n=48), Bmm (n=36) and FAS-RNAi (n=34). wt flies show a significant increase in TGs while all of the other flies show no significant increase in TG levels after 5 days on 30% HFD. B) Bar graph of relative change in heart dysfunction for fatbody-specific (lsp-Gal4) expression of TSC1-2 (n= 24), S6KDN (n=36), 4EBP (n=24), UAS-FOXO (n=36), UAS-Bmm (n=36) and UAS-FAS-RNAi (n=24). Only FAS-RNAi had a moderate increase in the severity of all 3 cardiac dysfunction phenotypes (see C) when compared to the same genotype under HFD. C) Side Bar graph of individual aberrant heart phenotypes for wt and fatbody-specific expression of TSC1-2, S6KDN, 4EBP, FOXO; FAS-RNAi and Bmm. Only FAS-RNAi had a moderate increase in heart abnormalities under a HFDA minimum of 24 individual fly heart movies were analysed for each genotype and diet variation.
Since we find that TOR mutants exhibit an increase in Bmm and a decrease in FAS transcript levels (Figure 3B,C), we tested if expression of Bmm or FAS-RNAi in the FB protects against HFD-induced increases in body fat and heart dysfunction. Indeed, we find that expression of Bmm in the FB prevents systemic TG accumulation and protects against abnormalities in heart function under HFD, similar to FB expression of TSC1-2 (Figure 5, S4). When we tested the effects of FAS knockdown in the FB, we found a partial decrease in TG levels and a moderate attenuation of HFD-associated heart defects (Figure 5, S4). These findings suggest that prevention of fat accumulation by increasing lipolysis or reducing lipogenesis can mimic the metabolic and cardiac protection from HFD observed by reduced TOR signaling.
It has been shown that loss of insulin signaling in the murine adipose leads to lower circulating insulin levels and prevents obesity (FIRKO mouse; Bluher et al., 2002). Therefore we tested if altering insulin signaling in the FB had physiological consequences similar to TOR inhibition. To achieve this, we expressed the negative effector of insulin signaling, FOXO, in the FB. This also limited the accumulation of lipids, as we observed for TOR inhibition and Bmm lipase expression, and protected the heart from the adverse effects of excess lipid accumulation (Figure 5, S4). Thus, reduced insulin-TOR signaling in the FB prevents systemic dyslipidemia and protects the heart (and presumably) other organs from lipid overload.
Autonomous protection of the heart from HFD by cardiac inhibition of insulin-TOR signaling
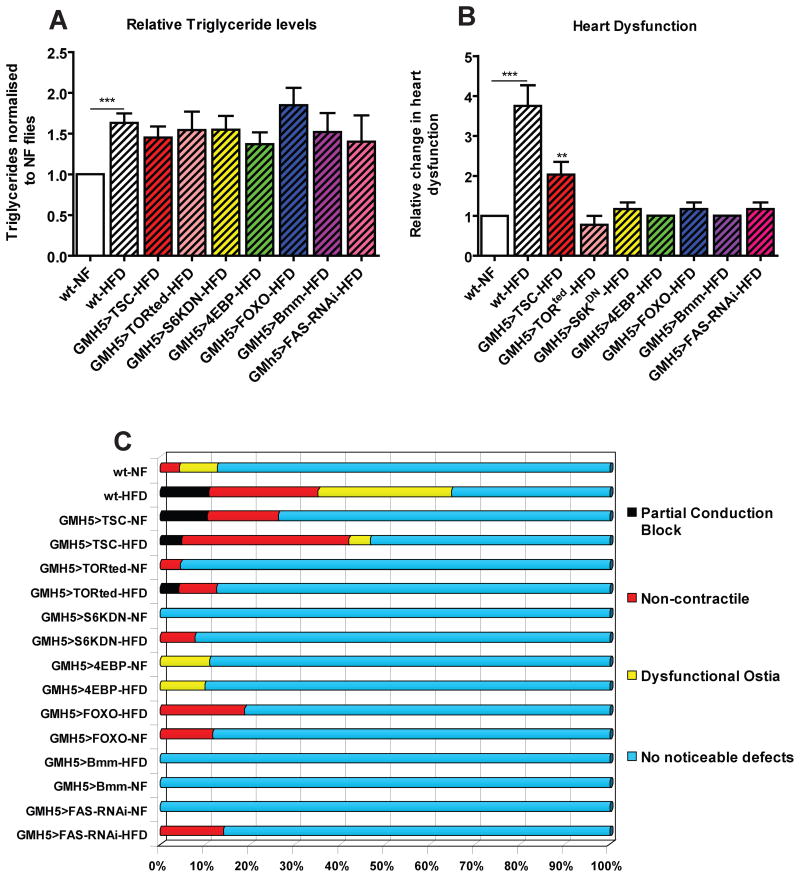
The above findings raise the question whether manipulating insulin-TOR signaling in the heart itself is sufficient to protect the heart against the effects of a HFD. To test this, we expressed TSC1-2, 4EBP and S6KDN specifically in the myocardial cells of the heart, and found that the systemic TG levels show the same increase when fed a HFD, as did wildtype flies (Figure 6A, S5A). Remarkably, we also observed that myocardial expression of both 4EBP and S6KDN were sufficient to protect against HFD-induced cardiac abnormalities, despite elevated systemic lipid levels (Figure 6, S5B). However, unlike all other TOR inhibitory manipulations, overexpression of TSC1-2 in the heart did not confer a robust protection from a HFD-inflicted insult to the heart (Figure 6, S5B). To further confirm that inhibition of TOR itself was sufficient to prevent HFD-induced cardiac dysfunction, we expressed a dominant-negative form of TOR, TORTED (Hennig and Neufeld, 2002), in the heart, and found similar cardiac protection, as with 4EBP and S6KDN, against the adverse effects of a HFD (Figure 6), comparable to the systemic or FB-specific manipulations (Figure 5).
Figure 6. Myocardial-specific inhibition of TOR autonomously blocks HFD heart effects despite obesity.
A) Relative changes in TG content of wt and Myocardial-specific expression (GMH5-Gal4) of TSC1-2 (n=36), TORTed (n=36), S6KDN (n=48), 4EBP (n=36), FOXO (n=35), Bmm (n=48) and FAS-RNAi (n=36). All flies tested show a significant increase in TGs levels after 5 days on 30% HFD. B) Bar graph of relative change in heart dysfunction for myocardial-specific (GMH5) expression of TSC1-2 (n=23), TORted (n=24), S6KDN (n=35), 4EBP (n=42), FOXO (n=22), Bmm (n=33) and FAS-RNAi (n=25). Only TSC1-2 had an increase in the incidences of all 3 phenotypes when compared to the same genotype under HFD. C) Side Bar graph of individual aberrant heart phenotypes for myocardial-specific (GMH5) expression of TSC1-2, TORted, S6KDN, 4EBP, FOXO and Bmm. Only TSC1-2 had an increase in the incidences of all 3 phenotypes when compared to the same genotype under HFD. A minimum of 22 individual fly heart movies were analysed for each genotype and diet variation.
To determine if elevated lipase activity is a possible mechanism downstream of reduced TOR activity in the protection of the heart, we directly expressed the Bmm lipase in the myocardial cells of flies exposed to a HFD. We found that increased cardiac lipase expression protected the hearts against HFD-associated dysfunction, yet the systemic TG levels were increased under a HFD, as in wildtype (Figure 6A, S5B). We also tested heart-specific FAS knockdown and observed similar protective phenotypes to those observed with Bmm overexpression or inhibition of TOR signaling downstream of TSC1-2 (Figure 6, S5B). These findings strongly imply that reduced cardiac TOR signaling autonomously protects the heart’s susceptibility to a HFD by altering lipid metabolism.
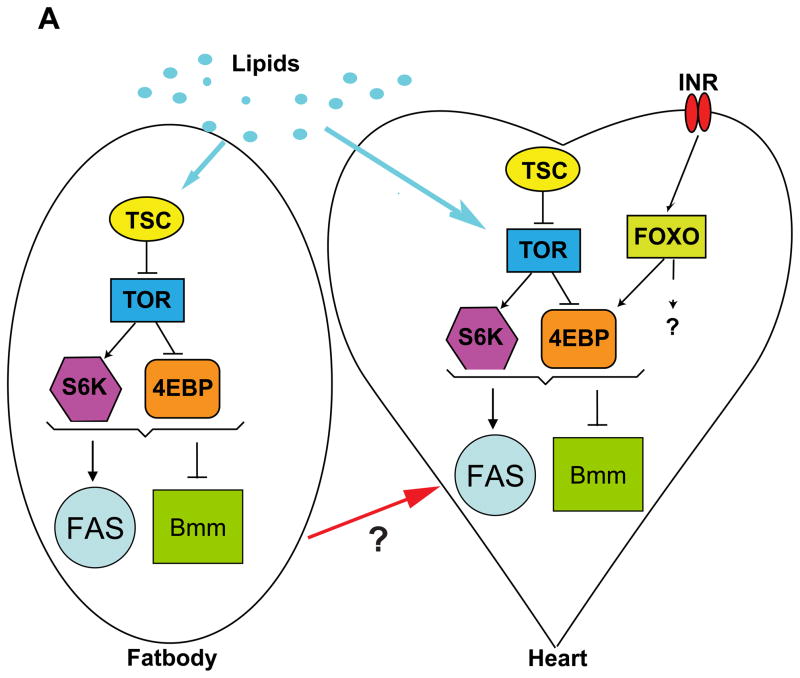
Since adipose FOXO expression protects against HFD-induced cardiac malfunction (Figure 5B,C) and cardiac FOXO expression protects against cardiac aging (Wessells et al., 2004), we also tested whether FOXO activity in the heart under HFD conditions is also protective. Indeed, we find that the expression of FOXO in myocardial cells autonomously protects the heart from the adverse effects of a HFD, but as expected not against overall body fat accumulation (Figure 6A). We then tested if altering downstream insulin-TOR signaling in the heart tissue would affect lipid accumulation with the heart. We found no significant increase in heart-specific TG accumulation in HFD hearts when overexpressing either FOXO or Bmm (Figure S5C). We also found that under NF conditions Bmm overexpression had significantly lower levels of heart TGs under NF conditions then wt controls (Figure S5C). These findings correlate well with our previous data showing a decrease in TG accumulation when inhibiting insulin-TOR or increasing Lipase activity. Taken together, these results suggest that moderate reduction in insulin-TOR signaling prevents HFD-induced obesity and cardiac dysfunction (Figure 7). It will be interesting to see whether FOXO acts upstream of TOR effectors (ei. 4EBP) in protecting the heart from lipotoxicity, as it does in reducing the decline of cardiac performance with age (Wessells et al., 2009).
Figure 7. Model of insulin-TOR pathway funxction in HFD-induced obesity.
A) Model for the effects of increased lipids on the insulin-TOR pathway in the fatbody and the heart.
Discussion
HFD-induced obesity is associated with an increased risk for diseases, including cancer, diabetes, and heart disease. The polygenic and/or multi-organ nature of HFD-induced obesity makes it difficult to determine the relative contribution to each of these diseases. In order to examine these complicated interactions in a simple system, we established a HFD-induced obesity model in Drosophila to elucidate the underlying mechanisms. We used the genetic versatility of the Drosophila model along with sophisticated cardiac function assays to investigate the effects of insulin-TOR mediated metabolic regulation and the crosstalk between organs exposed to excess dietary fat. We find that HFD-fed flies become obese, develop metabolic syndrome and exhibit severe symptoms of cardiac lipotoxicity. The deleterious HFD-induced effects are alleviated by genetic manipulations of metabolic regulators or by directly altering lipid metabolism either systemically, in adipose tissue, or specifically (autonomously) in the heart.
We provide evidence that Drosophila fed a HFD exhibit central features of mammalian metabolic syndrome, including elevated lipid levels and changes in insulin and glucose homeostasis. Furthermore, the effects of the HFD-induced obesity on the heart are profound, including diminished contractility, conduction blocks and structural defects. The HFD-induced elevation in TG levels is an important marker for this collection of phenotypes as high TG levels are associated with disruptions of lipid and glucose homeostasis, mitochondrial function and other processes (Schaffer 2003, Unger 2003, Ouwens et al., 2005; Van Gaal et al., 2006, van Herpen and Schrauwen-Hinderling 2008), all of which may contribute to high lipid accumulation and heart phenotypes.
Because the insulin-TOR pathway is a key integrator of metabolism, we initiated a comprehensive investigation of both the systemic and tissue-specific effects of altering insulin-TOR signaling under HFD conditions. We found that reduction of pathway activity blocks HFD-induced increased lipid levels in Drosophila. Recent studies have begun to connect insulin-TOR signaling to the regulation of lipid metabolism in flies and mammals (Luong et al., 2006; Li et al. 2010; Lee et al. 2010; this study). For example, the increased lipid synthesis caused by insulin treatment is blocked by reduction of TOR function, and the activation of the TOR pathway leads to fat accumulation (Luong et al., 2006; Porstmann et al., 2008; Li et al., 2010). In addition, TOR function in lipogenesis (and heart function) may be mediated by activation of Sterol Regulatory Element Binding Protein (SREBP) and its lipogenic target genes, including FAS (HY Lim, R.T.B., S.O. and R.B., unpubl.). Therefore, modulation of lipid metabolism is a likely mechanism that mediates the effect of TOR signaling on the HFD phenotype.
To determine the contributions of lipid metabolism to the HFD-induced obesity phenotypes, we examined a key regulator of lipid utilization: the Bmm (ATGL) gene is required for the breakdown of lipid droplets, and encodes a triacylglycerol lipase that is conserved from nematodes to mammals (Gronke et al., 2005; 2007; this study). Drosophila Bmm mutants show significant increases in TGs, while ectopic expression results in the reverse effect of lowering TG levels. Previous studies in mice have shown that systemic mutants of ATGL caused increases in lipid accumulation in non-adipose such as the heart (Haemmerle et al., 2006; Hirano et al., 2008). Here, we find that reduction of TOR function leads to increased Bmm RNA levels and Bmm overexpression prevents the HFD-induced elevation of TG levels. In the heart, Bmm overexpression protects against cardiac dysfunction and fat accumulation inflicted by a HFD. These protective effects by Bmm may stem from modified lipid utilization by the mitochondria. Additionally, lipid droplet lipolysis may also liberate fatty acid ligands for nuclear receptors, which may contribute to changes in mitochondrial biogenesis (Palanker et al., 2009; Finck et al., 2003). Altered insulin-TOR signaling is likely to also likely to increase activity of translation factors involved in mitochondrial function (Zid et al. 2009). Lastly, increases in autophagy have been implicated in the regulation of lipid metabolism via the breakdown of lipid droplets (Kovsan et al., 2009). Thus, reduction of insulin-TOR signaling can coordinately lead to changes in lipid metabolism, which in turn affects organismal physiology under excess dietary fat conditions.
A critical step in the regulation of lipid synthesis involves SREBP, which in flies responds to decreased levels of the major membrane lipid phosphatidylethanolamine (PE) by increasing FAS expression (Rawson, 2003). In examining the role of lipid synthesis in relation to the HFD-induced obesity phenotypes, we observed that the transcript levels of FAS decrease in TOR mutants, and that FAS knockdown reduces the deleterious effects of HFD, possibly because of reduced lipid synthesis. Consistent with this idea, loss of SREBP and FAS function in mammalian models leads to low TG levels (Valet et al., 2002; Bentzinger et al., 2008), and loss of Drosophila easily shocked (eas), which encodes an ethanolamine kinase critical for PE synthesis, leads to increased levels of the active form of SREBP, increased FAS expression and thus elevated TG levels (HY Lim and R. B., unpubl.). In addition, this effect is also observed in mammalian hepatocytes where TOR function is required for SREBP activation (Li et al., 2010), and it has been proposed that TOR serves as an important fork in the road of diabetic insulin resistance separating gluconeogenesis from lipogenesis (Li et al. 2010; Laplante and Sabatini 2009; 2010). Collectively, these data support the idea that TOR is required to mediate HFD-induced obesity and ensuing (cardiac) organ defects via multiple mechanisms. Interestingly, reducing or blocking fat accumulation mimics the protective effects of lowered insulin-TOR signaling under a HFD. However, it remains to be determined what it is about the accumulating fat that is detrimental to an organism: Is the fat itself or a side effect of its accumulation that is causing metabolic and physiological dysregulation?
Our data indicate that heart dysfunction due to the HFD treatment are the result of autonomous changes within the heart, as evidenced by the increased cardiac TG levels; and cardiac-only reduction of insulin-TOR signaling protects the heart from dysfunction and fat accumulation. Importantly, our findings show that inhibition of insulin-TOR signaling or ensuing fat accumulation in the heart itself can significantly prevent cardiac dysfunction despite the presence of elevated systemic TG levels. In addition, HFD-induced obesity also influences heart function via the adipose tissue since blocking insulin-TOR function in the adipose can also prevent the HFD obesity heart phenotypes. Thus, non-autonomous cross-talk factors, which may include hormones or metabolites, also contribute to HFD-induced heart dysfunction. The Drosophila model will help to understand the basis of such cross-talk.
The fact that flies become obese on a HFD has important implications on its own. Theories for the increased frequency and appearance of obesity include the thrifty gene hypothesis, which tries to understand why the incidence of obesity may be increasing despite its deleterious effects. These ideas state that recent evolutionary selection pressure on people experiencing frequent famines concentrated genetic variants that increased the ability to provide sufficient nutrients to an organism in times of lean, but also predisposed to obesity in times of plenty. Alternatively, the potential for HFD-induced obesity may have arisen early in evolution, perhaps independently of external selection pressure, via deregulation of metabolic responses in a multi-cellular organism. In this report, we provide evidence to suggest that the capacity for HFD-induced obesity and its associated complications are evolutionary ancient and is latent in core nutrient sensing pathways that becomes manifest upon exposure to dietary extremes that bypass the homeostatic threshold and dynamic range.
The discovery of these HFD-induced obesity phenotypes in the Drosophila genetic model will permit a detailed dissection of obesity phenotypes, especially with regard to the cardiac lipotoxicity effects (and possibly mimicking aspects of diabetic cardiomyopathy). In particular, we can now attempt to understand the various contributions of insulin resistance, fat accumulation, and fatty acid oxidation to the HFD-induced obesity phenotypes, including timing requirements. In summary, the advent of the Drosophila HFD-induced obesity model opens up many new horizons to study deregulated processes and diseases of chronic lipid excess.
Materials & Methods
Fly Stocks
We obtained w1118 flies from Bloomington’s stock center and used these as wildtype controls. We used multiple drivers for each tissue type. For driving expression ubiquitously, we used arm-Gal4 (Bloomington); in the fatbody we used the lsp-Gal4 (Cherbas et al. 2003) and the DCG-Gal4 (provided by Jon Graff and J. Suh); for the heart we used GMH5 (Wessells et al. 2004) and Hand-Gal4 (Han et al. 2006). We found similar trends for corresponding drivers for each tissue. S6KDN (Mary Stewart), and UAS-d4EBP (Miron et al. 2001), UAS-FAS-RNAi (Vienna RNAi stock center), UAS-Bmm (Gronke et al. 2005) were used. UAS-TORTED and UAS-TSC1-2 were a donation by (Tom Neufeld). The TOR mutants are from Luong et al. (2006). To test a negative effector of the insulin signaling pathway we used UAS-FOXO (Wessells et al. 2004). All flies were maintained on normal food (NF) source made from a combination of yeast, corn starch and molasses. High fat diet (HFD) was made by mixing either 3,7,15 or 30% coconut oil was added to the food in a weight to volume ratio with the NF.
Heart Assays
For a detailed analysis of the cardiac contractions it is necessary to surgically expose the fly’s heart in order to make it accessible for high-resolution video microscopy (Vogler and Ocorr 2009). In this analysis we used the previously publish heart assay and analysis (Ocorr et al., 2007a,b,c: Fink et al., 2009). In brief, dissections to expose the fly’s heart within the abdomen were kept in oxygenated saline and high-speed digital movies were taken, and then analyzed for heart rate, arrhythmia, contractility, fractional shortening, etc. (Ocorr et al., 2007a,b,c: Fink et al., 2009).\
HFD feeding regime
Bottles of flies were emptied and dated then 5 days after emptying the vial all flies were taken and placed and a new vial of NF and aged 5 more days. This population was then spilt into two populations one on NF and one on the designated concentration of HFD for either 2, 5 or 10 days. We used 30% HFD for 5 days for the majority of the experiments since it gave strong and reproducible phenotypes.
Statistical Analysis
All statistical analysis was done using student t-tests and the analysis was performed using GraphPad Prism version 5.00 for Windows, (GraphPad Software, San Diego California USA, www.graphpad.com).
Supplementary Material
Acknowledgments
We would like to thank J Graff, J Suh, Laurent Perrin, M Stewart, Tom Neufeld, N Sonenberg, for reagents and fly stocks. We would also like to thank Daniel Kelly, Timothy Osborne and Michael Karin for critical reading of the manuscript. We thank Lisa Elmén for technical assistance and fly stock curating. R.T.B. was supported by fellowships from CIRM and from the Sanford Child Health Center at the Sanford-Burnham Institute. K.O. was supported by a Scientist Development Grant of the American Heart Association (AHA). S.O. was supported by AHA and NHLBI of NIH. R.B. was supported by NHLBI of NIH, MDA and the Ellison Foundation.
Footnotes
Authors Contributions
R.T.B designed and performed experiments, analyzed data and wrote the paper; J.C., K.R., J.R., S.G., S.D., performed experiments; K.O. developed analytical tools and helped analyze data; and R.B and S.O supervised the project, analyzed data and wrote the paper.
Additional methods are in Supplementary Information available online
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Bodmer R. Heart development in Drosophila and its relationship to vertebrate systems. Trends Cardiovasc Med. 1995;5:21–28. doi: 10.1016/1050-1738(94)00032-Q. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev Genet. 1998;22:181–186. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cammarato A, Dambacher CM, Knowles AF, Kronert WA, Bodmer R, Ocorr K, Bernstein SI. Myosin transducer mutations differentially affect motor function, myofibril structure, and the performance of skeletal and cardiac muscles. Mol Biol Cell. 2008;19:553–562. doi: 10.1091/mbc.E07-09-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008;295:H1206–H1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gronke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, Jackle H, Kuhnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Han Z, Yi P, Li X, Olson EN. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development. 2006;133:1175–82. doi: 10.1242/dev.02285. [DOI] [PubMed] [Google Scholar]
- Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- Hennig KM, Neufeld TP. Inhibition of cellular growth and proliferation by dTOR overexpression in Drosophila. Genesis. 2002;34:107–110. doi: 10.1002/gene.10139. [DOI] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kovsan J, Bashan N, Greenberg A, Rudich A. Potential role of autophagy in modulation of lipid metabolism. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00562.2009. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3281–3282. doi: 10.1073/pnas.1000323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron M, Verdu J, Lachance PE, Birnbaum MJ, Lasko PF, Sonenberg N. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat Cell Biol. 2001;3:596–601. doi: 10.1038/35078571. [DOI] [PubMed] [Google Scholar]
- Neel JV, Weder AB, Julius S. Type II diabetes, essential hypertension, and obesity as “:syndromes of impaired genetic homeostasis”: the “thrifty genotype” hypothesis enters the 21st century. Perspect Biol Med. 1998;42:44–74. doi: 10.1353/pbm.1998.0060. [DOI] [PubMed] [Google Scholar]
- Neely GG, Kuba K, Cammarato, et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Akasaka T, Bodmer R. Age-related cardiac disease model of Drosophila. Mech Ageing Dev. 2007a;128:112–116. doi: 10.1016/j.mad.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, Bodmer R. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci U S A. 2007b;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr KA, Crawley T, Gibson G, Bodmer R. Genetic variation for cardiac dysfunction in Drosophila. PLoS ONE. 2007c;2:e601. doi: 10.1371/journal.pone.0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RB. The SREBP pathway--insights from Insigs and insects. Nat Rev Mol Cell Biol. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Therapeutic lifestyle changes in the management of obesity. Endocr Pract. 2003;9(Suppl 2):97–98. doi: 10.4158/EP.9.S2.97. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]
- Taghli-Lamallem O, Akasaka T, Hogg G, Nudel U, Yaffe D, Chamberlain JS, Ocorr K, Bodmer R. Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell. 2008;7:237–249. doi: 10.1111/j.1474-9726.2008.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- Valet P, Tavernier G, Castan-Laurell I, Saulnier-Blache JS, Langin D. Understanding adipose tissue development from transgenic animal models. J Lipid Res. 2002;43:835–860. [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Vogler G, Ocorr K. Visualizing the beating heart in Drosophila. J Vis Exp. 2009 doi: 10.3791/1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang Y, Li H, Chieu HK, Munn AL, Yang H. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. EMBO J. 2005;24:2989–2999. doi: 10.1038/sj.emboj.7600764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham NJ. Epidemiological studies of physical activity and diabetes risk, and implications for diabetes prevention. Appl Physiol Nutr Metab. 2007;32:778–782. doi: 10.1139/H07-032. [DOI] [PubMed] [Google Scholar]
- Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Xu X, Lo PC, Lee HH, Frasch M. Cardiogenesis in the Drosophila model: control mechanisms during early induction and diversification of cardiac progenitors. Cold Spring Harb Symp Quant Biol. 2002;67:1–12. doi: 10.1101/sqb.2002.67.1. [DOI] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.