Abstract
Certain forms of inflammation of an allograft are highly detrimental to the induction and maintenance of transplant tolerance as they foster stable commitment to graft-destructive, not graft-protective, forms of T-cell immunity. Hence, a reduction in adverse tissue inflammation may prove crucial in facilitating the induction and maintenance of a long-lasting state of transplant tolerance.
Introduction and context
Upon activation by donor alloantigens, recipient naive T cells can differentiate into a variety of graft-destructive, effector T cell or graft-protective, regulatory T cell (Treg) phenotypes. These T-cell commitments are determined largely by the texture of the innate immune milieu in which T-cell activation occurs. Both cytokines and Toll-like receptor agonists are of great importance. A milieu in which transforming growth factor β1 (TGFβ1) is expressed in the absence of proinflammatory cytokines promotes the commitment of alloactivated T cells into a tissue-protective, forkhead box P3 (Foxp3)+ Treg phenotype. In contrast, a milieu in which proinflammatory cytokines are abundant prevents the generation of Foxp3+ Tregs and instead directs T-cell commitment into the tissue-destructive T helper 1 (Th1), Th2, or Th17 phenotypes [1-4]. Unfortunately, a robust expression of proinflammatory cytokines is typical for recently engrafted organ transplants. The inflamed state, highly detrimental to the Treg induction and immunoregulatory function [1,3,5], is a consequence of innate immune activation in response to the ischemia and reperfusion injury [5,6]. Although conventionally used agents such as corticosteroids are perhaps useful in restricting the early graft inflammation [7-9], their broad immunosuppressive action, including blocking the expression of TGFβ1, does not facilitate tolerance [10,11]. Hence, we believe that reducing the adverse forms of inflammation but allowing activity of TGFβ1 and other inhibitory cytokines within a graft may prove to be a key approach for the induction and maintenance of allograft tolerance. We suspect that the creation of an intra- and peri-graft milieu devoid of proinflammatory cytokines will serve to guide the majority of donor-activated T cells into a TGFβ1-incited, Treg tissue-protective phenotype. Indeed, our own results and the results of others support this concept [12-15]. Therapies that primarily block adverse inflammation, such as alpha1-antitrypsin, anti-interleukin (IL)-6, or anti-tumor necrosis factor-alpha, have been successfully utilized by our group in tilting the balance of the allograft response toward tolerance, or in restoring tolerance in the non-obese diabetic (NOD) model of type 1 diabetes, even in NOD mice with frank diabetes [14,15]. Given the availability of therapies (many of them approved by the US Food and Drug Administration) that are suitable for blocking the adverse forms of inflammation, rapid translation into the clinic seems possible for the treatment of certain immune-inflammatory disorders. We are aware that a pure prevention or a blockade of inflammation may not be sufficient to achieve lasting transplant tolerance in humans. Additional lymphocyte-depleting measures are required first of all in order to prevent rejection in pre-sensitized recipients [16]. Nonetheless, we believe that favorably tipping the balance of pro- and anti-inflammatory cytokines in the milieu in which non-depleted T cells re-populate will foster T-cell commitment into a tissue-protective mode and promote tolerance. Our current efforts at blocking TIM4 (T-cell immunoglobulin and mucin domain-containing protein 4), a molecule upregulated by antigen-presenting cells exposed to proinflammatory cytokines and Toll-like receptor agonists [17,18], have proven equally successful in promoting long-term engraftment in preclinical models of transplantation and autoimmunity, although this method is not yet ready for testing in humans. If confirmed that TIM4 blockade leaves T-cell anti-viral responses largely intact, it may be an especially attractive strategy to promote tolerance in patients with chronic viral infections. Collectively, our own studies and the studies of others establish ‘adverse’ inflammation as a key therapeutic target in the quest for transplant tolerance.
Major recent advances
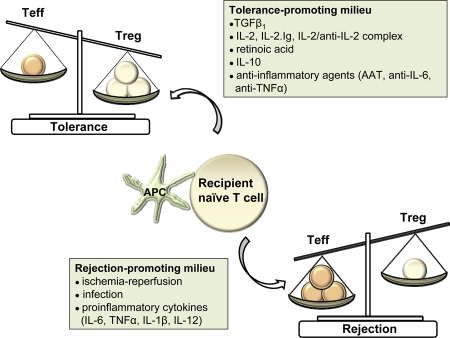
Induction and maintenance of transplant tolerance require that graft-protective Foxp3+ Tregs efficiently and durably restrain the pool of graft-destructive effector T cells after anti-rejection therapy is withdrawn [19] (Figure 1). For tolerance to be permanent, the Foxp3-dependent Treg immunoregulatory phenotype must be stabilized. Why? A stable expression of Foxp3, the Treg lineage specification transcription factor, is required to maintain Treg function and thereby maintain transplant tolerance [20,21]. Loss of Foxp3 gene expression, such as that occurring in the inflamed, IL-6-rich environments, can destabilize Treg molecular phenotype and immunoregulatory function, thereby undermining the maintenance of transplant tolerance [22,23]. With the loss of immunoregulatory function among these destabilized Tregs, rejection occurs as the immunoregulatory restraints upon donor-reactive effector T cells are released.
Figure 1. Impact of local cytokine milieu on the balance between effector and regulatory T cells.
A milieu dominated by anti-inflammatory cytokines or inflammation-dampening agents promotes the commitment of donor-activated T cells into a regulatory T-cell phenotype and thereby fosters transplant tolerance. A milieu dominated by proinflammatory cytokines promotes effector T cell generation and rejection of the allograft. AAT, alpha-1-antitrypsin; APC, antigen-presenting cell; IL, interleukin; Teff, effector T cell (T helper 1 [Th1], Th2, and Th17); TGFβ1, transforming growth factor β1; TNFα, tumor necrosis factor-alpha; Treg, regulatory T cell.
How is Foxp3 expression stabilized? Foxp3 expression in Tregs is regulated, in part, by epigenetic modifications of the Foxp3 chromosomal locus [24]. The methylation status of CpG-sensitive residues upstream of the transcriptional start site (Exon1) is an important regulator of Foxp3 expression. Methylation of these residues represses Foxp3 gene expression while complete demethylation is required for optimal Foxp3 gene expression [25,26]. Other epigenetic mechanisms, such as histone methylation and acetylation, also modulate Foxp3 stability [26,27]. Of note, therapeutic agents that directly foster maintenance of demethylated Foxp3 promote robust expression of Foxp3-sensitive genes, immunoregulatory T-cell function, and tolerance [26,28]. For this purpose, some inhibitors of DNA methyltransferases, such as nucleoside analog 5-azacytidine, have been successfully used in experimental studies [28]. Perhaps owing to the known toxicity of demethylating agents, formal clinical trials in transplantation have not yet been undertaken. Why are these observations potentially important for clinical application? That Treg infusions have the potential to prolong allograft survival and induce transplant tolerance is widely acknowledged [29]. However, the clinical use of Tregs for the adoptive transfer into transplant recipients is hindered by the inherent instability of the Foxp3-dependent Treg phenotype upon exposure of Tregs to inflamed environments. As noted, in the proinflammatory milieu, Foxp3 expression is diminished, inhibitory function is compromised, and some Tregs even convert to T effector-like phenotypes [22,30-32]. Such Treg instability has been associated with the activation of DNA methyltransferases and the consequent remethylation of CpG residues [26]. It therefore seems intuitive that therapeutic strategies able to maintain Foxp3 in a demethylated state may be essential for the effective application of Treg therapy in the clinic. While inhibitors of DNA methyltransferases and of histone deacetylase are not without toxicity, the addition of safe agents that synergize or supplant these drugs as a means to regulate epigenetic expression of Foxp3 should prove extremely valuable in the long-standing quest to induce tolerance.
Tregs and conventional effector T cells take important cues from their microenvironment, which influences their commitment into regulatory or effector phenotypes. Among extracellular stimuli, IL-2-triggered expression of STAT5 (signal transducer and activator of transcription 5) likely plays a major role in stabilizing Tregs [33]. STAT5, activated downstream of IL-2 receptor complex signaling, binds to the promoter region of the Foxp3 gene and thereby activates its transcription [34]. By enhancing transcription of Foxp3, the STAT5 pathway serves to maintain a stable Treg phenotype. We and others have utilized IL-2, IL-2 anti-IL-2 complexes, or IL-2.Ig either alone or in conjunction with rapamycin and other agents to promote tolerance induction [14,35,36]. Such therapies have successfully induced transplant tolerance or restored self-tolerance in the NOD model of type 1 diabetes. In addition to activating the STAT5 pathway and expression of Foxp3, IL-2 signal, if delivered by a long-lived IL-2 anti-IL-2 complex or IL-2.Ig, also causes apoptosis of repeatedly activated effector (but not regulatory) T cells [37]. The fact that calcineurin inhibitors such as cyclosporin negatively impact Treg function and activation-related effector T-cell apoptosis [19] may indeed be a result of suppressed IL-2 signals. We have previously shown that cyclosporin does prevent tolerance induced by co-stimulation blockade in a cardiac transplant model. Rapamycin, on the other hand, did not impede the induction of such achieved tolerance and, as we showed later, does promote the generation of induced Tregs [38,39]. The imbalance of tissue-protective Tregs and tissue-destructive Th17 cells was recently implicated in the pathogenesis of autoimmunity and certain types of rejection [1]. Of note, the commitment of naive T cells to the Th17 phenotype (a subset with aggressive cytodestructive properties) is inhibited by IL-2, and the expression of STAT5 negatively regulates Th17 cells [40]. It is therefore reasonable to posit that the activation of the IL-2/STAT5 pathway promotes tolerance by favorably tilting the balance between Th17 cells and Tregs toward Treg dominance.
Future directions
Strategies used to obtain transplant tolerance have primarily targeted T cells. These strategies have centered upon attempts to delete or at least deplete donor-reactive T cells or alter the early events of T-cell activation such as through co-stimulation blockade. The notion that graft inflammation is the major impediment to transplant tolerance prompts us to further investigate the means of its most efficient therapeutic targeting. It may not be possible to create transplant tolerance unless the inflammatory milieu in which donor-reactive T cells perceive donor antigen is modified. We believe that blocking ‘adverse’ inflammation in the peri-transplant period can guide the majority of donor-activated naive T cells into the graft-protective Treg mode. In addition, strategies that foster the expression of Treg Foxp3 by epigenetic modification may be utilized in conjunction with anti-inflammatory agents to further stabilize Tregs and tolerance.
Acknowledgments
TBS has received grant support from National Institutes of Health (NIH PO1 AI041521, NIH PO1 AI073748 and NIH U01 AI066705) and Juvenile Diabetes Research Foundation (JDRF 4-2004-368). MK has received grant support from NIH (PPG U19 DK080652, PO1 AI073748 and PO1 AI041521) and JDRF (4-2004-368 and 1-2007-524). DH has received grant support from NIH (F32 AI084373-01).
Abbreviations
- Foxp3
forkhead box P3
- IL
interleukin
- NOD
non-obese diabetic
- STAT5
signal transducer and activator of transcription 5
- TGFβ1
transforming growth factor β1
- Th
T helper
- TIM4
T-cell immunoglobulin and mucin domain-containing protein 4
- Treg
regulatory T cell
Competing Interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/83
References
- 1.Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15:411–5. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- 2.Strom TB, Koulmanda M. Recently discovered T cell subsets cannot keep their commitments. J Am Soc Nephrol. 2009;20:1677–80. doi: 10.1681/ASN.2008101027. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Ahmed E, Wang T. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–25. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Mohamed Sayegh 08 Jun 2009
- 4.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]; F1000 Factor 19Evaluated by Marsha Wills-Karp 18 May 2006, Christian Engwerda 24 May 2006, Torben Lund 26 May 2006, Rachel Caspi 05 Jun 2006, Stefan Kaufmann 06 Jun 2006, Paul Gleeson 21 Jun 2006, Andrew Weinberg 29 Jun 2006
- 5.Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, Brayman KL. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29:603–30. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Krämer BK, Colvin RB, Heeger PS, Murphy BT, Schröppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund T, Fosby B, Korsgren O, Scholz H, Foss A. Glucocorticoids reduce pro-inflammatory cytokines and tissue factor in vitro and improve function of transplanted human islets in vivo. Transpl Int. 2008;21:669–78. doi: 10.1111/j.1432-2277.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 8.Kainz A, Wilflingseder J, Mitterbauer C, Haller M, Burghuber C, Perco P, Langer RM, Heinze G, Oberbauer R. Steroid pretreatment of organ donors to prevent postischemic renal allograft failure: a randomized, controlled trial. Ann Intern Med. 2010;153:222–30. doi: 10.7326/0003-4819-153-4-201008170-00003. [DOI] [PubMed] [Google Scholar]
- 9.Pulitanò C, Aldrighetti L. The protective role of steroids in ischemia-reperfusion injury of the liver. Curr Pharm Des. 2008;14:496–503. doi: 10.2174/138161208783597353. [DOI] [PubMed] [Google Scholar]
- 10.Bolkenius U, Hahn D, Gressner AM, Breitkopf K, Dooley S, Wickert L. Glucocorticoids decrease the bioavailability of TGF-beta which leads to a reduced TGF-beta signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2004;325:1264–70. doi: 10.1016/j.bbrc.2004.10.164. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Sun J, Sheil AG, McCaughan GW, Bishop GA. A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplantation. 2001;72:44–51. doi: 10.1097/00007890-200107150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007;84:1375. doi: 10.1097/01.tp.0000281384.24333.0b. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EC, Mizrahi M, Toledano M, Defelice N, Wright JL, Churg A, Shapiro L, Dinarello CA. Alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A. 2008;105:16236–41. doi: 10.1073/pnas.0807627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koulmanda M, Budo E, Bonner-Weir S, Qipo A, Putheti P, Degauque N, Shi H, Fan Z, Flier JS, Auchincloss H, Jr, Zheng XX, Strom TB. Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proc Natl Acad Sci U S A. 2007;104:13074–9. doi: 10.1073/pnas.0705863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koulmanda M, Bhasin M, Hoffman L, Fan Z, Qipo A, Shi H, Bonner-Weir S, Putheti P, Degauque N, Libermann TA, Auchincloss H Jr, Flier JS, Strom TB. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105:16242–7. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Mark Atkinson 04 Nov 2008
- 16.Le Moine A, Vokaer B, Charbonnier LM. A brief focus on memory cells in transplantation. Transplant Proc. 2009;41:3361–2. doi: 10.1016/j.transproceed.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, Anderson AC, Sobel RA, Hafler DA, Strom TB, Kuchroo VK. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–13. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–64. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Richard L Stevens 03 Jun 2005
- 19.Strom TB. 2006 Homer W. Smith Lecture: Taming T Cells. J Am Soc Nephrol. 2007;18:2824–32. doi: 10.1681/ASN.2007070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Thomas Huenig 01 Mar 2007
- 21.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–213. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JI, Lee MK, Moore DJ, Sonawane SB, Duff PE, O’Connor MR, Yeh H, Lian MM, Deng S, Caton AJ, Markmann JF. Regulatory T-cell counter-regulation by innate immunity is a barrier to transplantation tolerance. Am J Transplant. 2009;9:2736–44. doi: 10.1111/j.1600-6143.2009.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Stephen Cobbold 15 Jul 2008
- 24.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–9. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 25.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 26.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–35. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Bruce Blazar 27 Nov 2007
- 28.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C, García JL, Carrancio S, Hernández-Campo P, González FJ, Flores T, Ciudad L, Ballestar E, Del Cañizo C, San Miguel JF, Pérez-Simon JA. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–21. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 29.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JPM. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Mohamed Sayegh 12 May 2008
- 30.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–8. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Bailey-Bucktrout SL, Jeker LT Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 10Evaluated by Kenneth Tung 13 Aug 2009
- 32.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]; F1000 Factor 17Evaluated by Fiona Powrie 24 Feb 2003, Thomas Huenig 25 Feb 2003, Peter Jensen 06 Mar 2003, Matthias von Herrath 21 Mar 2003, Paul Lyons 24 Mar 2003
- 33.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Kendall Smith 30 Jan 2007
- 34.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Kendall Smith 28 Mar 2007, Richard Williams 30 Mar 2007, Mercedes Rincon 10 Apr 2007
- 35.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 2002;51:638–45. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 37.Strom TB, Koulmanda M. Cytokine related therapies for autoimmune disease. Curr Opin Immunol. 2008;20:676–81. doi: 10.1016/j.coi.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 39.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by Michel Goldman 30 May 2007