Abstract
Glycogen synthase kinase-3 (GSK-3) is a well-established downstream component of the phosphatidylinositol 3-kinase (PI3K) signalling pathway but is also a key enzyme in negatively regulating the canonical Wnt/β-catenin signalling pathway. Several recent studies argue that PKB (protein kinase B)-mediated inhibition of GSK-3 leads to β-catenin accumulation, but whether cross-talk actually exists between these two pathways is controversial. To elucidate the mechanisms of shared signalling components, further studies taking into account different components of the PI3K signalling pathway and different pools of GSK-3 or β-catenin are required.
Introduction and context
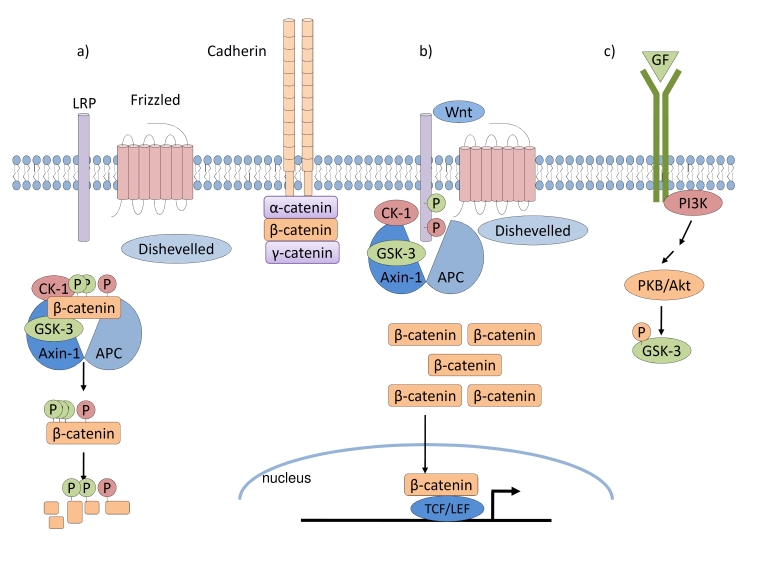
Glycogen synthase kinase-3 (GSK-3) is a constitutively active and ubiquitously expressed serine/threonine kinase [1]. In addition to playing a well-defined role in suppressing the canonical Wnt/β-catenin signalling pathway [2,3], it functions in Hedgehog, Notch, and several growth factor signalling pathways [4]. Large amounts of β-catenin typically are associated with cadherin complexes at the plasma membrane of adherent cells. However, in the absence of Wnt signalling, GSK-3 phosphorylates cytosolic β-catenin within a complex that includes adenomatous polyposis coli, Axin-1, casein kinase-1 (CK-1), and other proteins and targets it for ubiquitin-mediated degradation (Figure 1a). In this manner, very low levels of non-cadherin-associated β-catenin are maintained in the cell. Upon Wnt binding to Frizzled receptors and low-density lipoprotein receptor-related protein (LRP) co-receptors and subsequent engagement of the intracellular protein Dishevelled, the destruction complex is disrupted, GSK-3 and CK-1 activities are diverted to LRP co-receptors at the membrane, and cytoplasmic β-catenin avoids phosphorylation. As a result, β-catenin accumulates and enters the nucleus to regulate gene expression via binding to the TCF/LEF (T-cell factor/lymphoid enhancer-binding factor) DNA-binding proteins (Figure 1b).
Figure 1. GSK-3 in Wnt and growth factor signalling pathways.
(a) In the absence of Wnt signalling, the destruction complex comprised of APC, Axin-1, CK-1, and GSK-3 promotes the phosphorylation and subsequent ubiquitin-mediated degradation of β-catenin. (b) Upon Wnt stimulation, Dishevelled is engaged, the destruction complex is disrupted, and CK-1 and GSK-3 activities are diverted to LRP at the cell membrane. Unphosphorylated β-catenin may accumulate and enter the nucleus to regulate gene expression upon binding to TCF/LEF DNA-binding proteins. (c) Upon growth factor stimulation, activation of the PI3K signalling cascade leads to PKB-mediated phosphorylation of GSK-3 and inhibition of its kinase activity. APC, adenomatous polyposis coli; CK-1, casein kinase-1; GF, growth factor; GSK-3, glycogen synthase kinase-3; TCF/LEF, T-cell factor/lymphoid enhancer-binding factor; LRP, low-density lipoprotein receptor-related protein; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B.
Physiological levels of GSK-3 do not limit the capacity of the destruction complex to mediate β-catenin degradation; only a small percentage (<10%) of the total GSK-3 in the cell is associated with Axin and engaged in canonical Wnt signalling [5,6]. Other pools of GSK-3 are additionally used in several other pathways. Upon insulin stimulation and consequent phosphatidylinositol 3-kinase (PI3K) activation, for example, the serine/threonine kinase protein kinase B (PKB)/Akt phosphorylates GSK-3 and negatively regulates its kinase activity (Figure 1c) [7]. A similar mechanism of negative regulation of GSK-3 has been demonstrated in other growth factor pathways [8-11]. Regulation of β-catenin has also been demonstrated in many of these pathways [8-12]. However, the direct convergence of Wnt and growth factor pathways on β-catenin regulation remains a controversial topic of discussion.
Several human cancers tend to involve separate mutations of both pathways [13]. If PI3K activation were sufficient to activate Wnt signalling, why would additional mutations that lead to β-catenin stabilization be needed? Several studies have also shown that growth factor stimulation that leads to GSK-3 inhibition through PI3K signalling does not result in stabilization of β-catenin in the cell [14-16]. Furthermore, GSK-3 molecules that have mutations in the PKB phosphorylation sites and thus that are insensitive to inhibition by PI3K signalling are still inhibited by Wnt signalling [15-17]. It would appear that, although PI3K and Wnt signalling pathways share a core regulatory protein, the insulation of these pathways is sufficient to prevent cross-talk [18]. As demonstrated by Ng and colleagues [16], Axin may shield the associated GSK-3 within the destruction complex from protein kinases (such as PKB) that otherwise would phosphorylate and inactivate GSK-3. Although GSK-3 that is not directly associated with the complex may still be phosphorylated and inhibited, sufficient levels remain bound to the Axin complex to suppress the accumulation of β-catenin. It should be noted that chemical inhibitors of GSK-3 affect all GSK-3 molecules within a cell. Hence, use of such reagents eliminates any selectivity gained by compartmentalization of the kinase. This is also true for genetic knockout or RNAi (RNA interference) suppression of each of the two isoforms (GSK-3α and -β), although suppression must exceed 75-80% to have an impact on β-catenin [17].
Major recent advances
A recent report by Maes and colleagues [11] describes the effects of induction of vascular endothelial growth factor (VEGF) overexpression in the osteo-chondroprogenitor cells of adult mice. VEGF overexpression caused vascular defects, bone marrow fibrosis, hematological abnormalities, and increased bone mass, similar to the bone phenotype observed upon β-catenin overexpression in the osteo-progenitor cells of mice [19,20]. VEGF overexpression correlated with increased PI3K signalling via VEGFR-2 (VEGF receptor-2), leading to enhanced GSK-3 phosphorylation, and the authors also observed β-catenin stabilization. These results support the idea that receptor tyrosine kinase-mediated growth factor signals act through PI3K to induce β-catenin. However, there are other explanations. Accumulation of β-catenin in the mouse tissues could be due to indirect activation of conventional Wnt signalling through induction of Wnt ligands or modulators. Although the authors show that β-catenin induction is sensitive to infusion of the PI3K antagonist wortmannin in the VEGF-expressing mice, this does not exclude an indirect pathway. Another possible mechanism is suggested by the known effect of VEGF promotion of vascular permeability, a process that is preceded by VEGF-dependent vascular endothelial-cadherin (VE-cadherin) phosphorylation and dissolution of the VE-cadherin complex comprised of adherens junction proteins p120, β-catenin, plakoglobin, and α-catenin [21]. Thus, excess levels of cytosolic β-catenin released from the membrane compartment, together with increased stress on the destruction complex, may contribute to VEGF-mediated effects on β-catenin stabilization. Furthermore, nuclear localization of β-catenin has been reported to be promoted by PKB phosphorylation of Ser552 [22]. Clearly, it is not trivial to exclude alternative or indirect effects but it is somewhat easier to assume that the correlation between PI3K-induced inhibition of GSK-3 and accumulation of β-catenin is actually a direct linkage.
Future directions
Insulation mechanisms play critical roles in permitting shared signalling components between pathways yet maintain the integrity of these pathways and resultant biological processes. Under normal circumstances, signalling specificity appears well insulated and can withstand precise experimental pressure. However, these mechanisms may be overwhelmed by unnatural genetic and chemical perturbation or by disease mutations. Furthermore, the insulating mechanisms may not be relevant in all tissues or cell types. For example, both PKB and GSK-3 interact with the scaffold protein DISC-1 (disrupted-in-schizophrenia-1) in neurons and this protein, when overexpressed, has been shown to lead to accumulation of β-catenin and subsequent signalling [23-25]. It remains to be seen whether DISC-1 might mediate cross-talk between the PI3K and Wnt pathways in a more biologically relevant context since DISC-1 protein levels do not change significantly and the amount of GSK-3 bound by DISC-1 is only a small fraction of the cellular total – similar to the amount associated with Axin – and therefore is unlikely to be competitive. The small GTPase Cdc42 (cell division control protein-42 homolog), through its regulation of the PAR/aPKC (partitioning defective/atypical protein kinase C) complex, has been proposed to inhibit GSK-3 and stabilize β-catenin in the skin [26]. Activation of Wnt signalling by aPKC raises questions not unlike those associated with the effect of PKB on GSK-3 and β-catenin but also raises the possibility that PI3K may activate Cdc42 [27,28] and impinge on Wnt signalling in a PKB-independent manner. To definitively address the mechanisms and circumstances by which PI3K or other components of this pathway might impact β-catenin regulation, future experiments will need to be conducted with cells or tissues that possess depleted levels of GSK-3 or β-catenin and thus exhibit increased sensitivity to upstream manipulation of growth factor or Wnt signalling. Under these sensitized conditions, cross-talk may be more biologically relevant and detectable. Likewise, if such experiments fail to demonstrate interplay, there may be greater appreciation of the compartmentalization of signalling events under physiological conditions. Generation of cell lines in which elements of the PI3K pathway can be rapidly activated (e.g., PKB-estrogen receptor fusions) should allow investigators to evaluate whether β-catenin levels are induced within the expected time frame (β-catenin accumulation requires >30 minutes). The potential for different pools of GSK-3 or β-catenin to supplement each other in the cell must also be carefully evaluated in such experiments. Importantly, the dynamics of any observed changes must be taken into consideration to exclude one pathway from leading to changes in the levels of agonists and antagonists of another pathway such as through transcriptional regulation or micro-RNA expression. There are many examples of shared components in signalling pathways with little consideration of distinct pools or mechanisms to chaperone and isolate fractions of these molecules. Often, the simplest and most direct path is not taken in biology, leading to erroneous assumptions and conclusions. Indeed, understanding why a more convoluted route has been chosen can reveal new discoveries and previously overlooked components.
Acknowledgments
The authors acknowledge funding by the Canadian Institutes of Health Research (CIHR) Operating Grant FRN 74711 (to JRW) and a Heart and Stroke Foundation (Ontario, Canada) postdoctoral fellowship (to DV).
Abbreviations
- aPKC
atypical protein kinase C
- Cdc42
cell division control protein-42 homolog
- CK-1
casein kinase-1
- DISC-1
disrupted-in-schizophrenia-1
- GSK-3
glycogen synthase kinase-3
- LRP
low-density lipoprotein receptor-related protein
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- VE-cadherin
vascular endothelial-cadherin
- VEGF
vascular endothelial growth factor
Competing Interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/82
References
- 1.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–8. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–14. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nature Rev Neuro. 2010;11:539–51. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 9Evaluated by Randall Moon 24 Oct 2003, Jeffrey Axelrod 30 Oct 2003
- 6.Benchabane H, Hughes EG, Takacs CM, Baird JR, Ahmed Y. Adenomatous polyposis coli is present near the minimal level required for accurate graded responses to the Wingless morphogen. Development. 2008;135:963–71. doi: 10.1242/dev.013805. [DOI] [PubMed] [Google Scholar]
- 7.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 8.Ishibe S, Haydu JE, Togawa A, Marlier A, Cantley LG. Cell Confluence Regulates Hepatocyte Growth Factor-Stimulated Cell Morphogenesis in a beta-Catenin-Dependent Manner. Mol Cell Biol. 2006;26:9232–43. doi: 10.1128/MCB.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu D, Yu B, Zhao C, Ye W, Lv Q, Hua Z, Ma J, Zhang Y. The effect of pleiotrophin signaling on adipogenesis. FEBS Letters. 2007;581:382–8. doi: 10.1016/j.febslet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. PNAS. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I, Moermans K, Nyabi O, Haigh K, Naessens M, Haenebalcke L, Tuckermann JP, Tjwa M, Carmeliet P, Mandic V, David JP, Behrens A, Nagy A, Carmeliet G, Haigh JJ. Increased skeletal VEGF enhances β-catenin activity and results in excessively ossified bones. EMBO J. 2010;29:424–41. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–beta-catenin signalling. Nat Gen. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–33. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8Evaluated by Patrice Morin 26 Jun 2007
- 14.Ding VW, C RH, McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–81. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 15.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–83. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, Schutte M, Clevers H. Phosphatidylinositol 3-kinase signaling does not activate the Wnt cascade. J Biol Chem. 2009;284:35308–13. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–71. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeill H, Woodgett JR. When pathways collide: collaboration and connivance among signalling proteins in development. Nature Rev Mol Cell Biol. 2010;11:404–13. doi: 10.1038/nrm2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 21.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–22. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 22.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–73. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto A, Asai N, Namba T, Wang Y, Kato T, Tanaka M, Tatsumi H, Taya S, Tsuboi D, Kuroda K, Kaneko N, Sawamoto K, Miyamoto R, Jijiwa M, Murakumo Y, Sokabe M, Seki T, Kaibuchi K, Takahashi M. Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron. 2009;63:774–87. doi: 10.1016/j.neuron.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 13Evaluated by Valera Vasioukhin 23 Mar 2009, Orly Reiner 06 Apr 2009
- 26.Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 2006;20:571–85. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez C, Portela RA, Mellado M, Rodríguez-Frade JM, Collard J, Serrano A, Martínez-A C, Avila J, Carrera AC. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol. 2000;151:249–62. doi: 10.1083/jcb.151.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–45. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]