Abstract
Clearing agents are often used in pretargeting despite the potential for decreased tumor accumulation of the effector. However, according to the authors' semiempirical model, a clearing agent should not necessarily decrease tumor accumulation. In this study, the authors have added a clearing step to their model—morpholino phosphorodiamidate oligomer (MORF)/complement MORF (cMORF) pretargeting system—to confirm this prediction. The CC49 antibody was conjugated with both biotin and an 18 mer MORF. The influence of avidin on antibody clearance was first evaluated in normal mice in which each animal received 30 μg of MORF-CC49-biotin, 0–70 μg of avidin 1 day later, and 1.2 μg of 99mTc-cMORF 3 hours later, with sacrifice at 3 hours. Thereafter, a pretargeting study in mice bearing an LS174T tumor was performed at a 34 μg avidin dosage. In normal mice, the blood level of 99mTc-cMORF fell by 60% at an avidin dosage of 10 μg or higher. In tumored mice, avidin produced a similar reduction in blood but had no influence on tumor level, which remained at 6.30% ID/g as predicted. In conclusion, in addition to the expected reduced effector levels in blood and normal tissues, a reduction in tumor accumulation was avoided when adding a clearing agent as predicted.
Key words: clearing agent, prediction model, pretargeting, tumor accumulation
Introduction
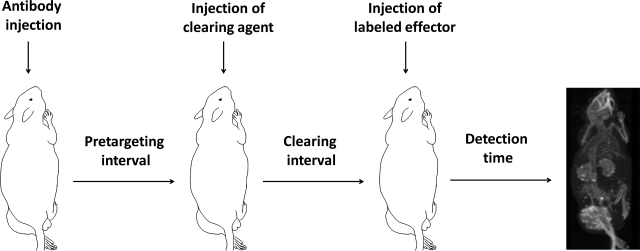
The use of a clearing agent in pretargeting, illustrated in Figure 1, has been extensively considered.1–8 Examples of clearing agents include avidin,9 antibodies against the pretargeting antibody,10,11 and galactose.12 Adding a clearing agent increases the complexity of an already complex protocol and makes the already difficult dosage and timing optimization more challenging. These difficulties coupled with the complexities may account for the discrepancies between different laboratories employing similar clearing agents. For example, a biotin–galactose–HSA clearing agent was reported to be ineffective,6 whereas the same agent was reported in another study to reduce the antibody levels in circulation by more than 90%.7 However, perhaps the most serious concern is the possibility that a clearing agent may reduce the tumor accumulation of an effector in a pretargeted tumor.
FIG. 1.
The schematic pretargeting process with a clearing (or chase) step.
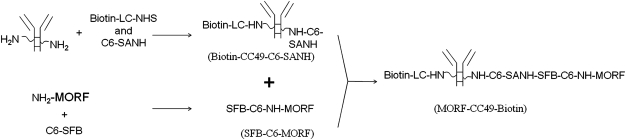
In this investigation, avidin was selected as a clearing agent for the biotinylated antibody because of its high binding affinity for biotin and its heavy glycosylation. Avidin is efficiently deposited in liver and may be expected to clear any biotinylated antibody bound to it as well. The biotinylated antibody CC49 used in this study is an antiTAG-72 antibody conjugated with both biotin and morpholino phosphorodiamidate oligomer (MORF) as shown in Figure 2.
FIG. 2.
Schematics showing the conjugation of the CC49 antibody with both biotin and morpholino phosphorodiamidate oligomer (MORF).
A semiempirical model based on the two-step conventional pretargeting procedure has been established earlier.13–16 In the present study, the authors intended to confirm whether this model can correctly predict the influence of a clearing agent on the tumor accumulation of the effector. Predictions on the effector levels in normal tissues would have required accurate measurements of the pharmacokinetics of the pretargeting antibody following the addition of the clearing agent, and were beyond the scope of this limited study. As such, predictions on the influence of a clearing agent were confined to tumor accumulation.
Materials and Methods
The base sequences of MORF and its complement (cMORF) were, respectively, 5′-TCTTCTACTTCACAACTA-linker-amine and 5′-TAGTTGTGAAGTAGAAGA-linker-amine (GeneTools). The murine anti-TAG-72 antibody CC49 was produced by Strategic Biosolutions from the CC49 murine hybridoma cell line, a gift from Dr. Schlom (Laboratory of Tumor Immunology and Biology, NCI, NIH). The Hydralink kit consisting of C6-SANH and C6-SFB was from Solulink Biosciences. The NHS-LC-biotin and the EZTM Biotin Quantitation Kit were both from Pierce. The Sephadex G-100 resin was from Pharmacia Biotech, and the PD-10 columns were from NeoRx Corp. The 130 mL Bellco tissue sieve kit was from Bellco Glass. The 99Mo-99mTc generator was from Perkin Elmer Life Science. All other chemicals were of reagent grade and used without purification. 99mTc-cMORF was prepared as previously described.17
Antibody conjugation and testing
As illustrated in Figure 2, the NHS-biotin was added to the antibody solution together with an active NHS carboxylate ester linked with an acetone-protected pyridinyl hydrazine (C6-SANH). Briefly, to 1.5 mL of a CC49 antibody solution (1.7 mg/mL) in 20 mM PBS (pH 7.2), 100 μg C6-SANH and 75 μg NHS-LC-biotin in 3 μL DMF/H2O (v/v = 17/13) were added. The sample was brought to room temperature for half an hour after overnight incubation at 4°C and loaded onto a 9 mL commercial PD-10 column. At the same time, the C6-SFB was conjugated to MORF as previously described.18 The subsequent combination of the pooled peak fractions of the SANH-CC49-biotin with the solution of the SFB-modified MORF and the purification of the reaction mixture were performed as earlier.19 The average number of MORF groups per antibody (gpm of MORF) was determined as described,20 whereas the average gpm of biotin was determined following a slightly modified protocol from the manufacturer of EZTM Biotin Quantitation Kit.
Both the LS174T cells and tumor fragments were used to evaluate the TAG-72 binding of the modified CC49. Three 12-well plates were seeded with LS174T cells and were used after the cells reached 70% confluence. Added to each well in triplicate was 150 μL of culture medium (MEM Earle's No. 11095 PB buffer with 1% fetal bovine serum, 1% penicillin–streptomycin mixture, and 10 mM nonessential amino acids formulation) containing 0–170 μg of native CC49 antibody, 0.4 μg MORF-CC49-biotin (0.67 gpm of MORF, 3.41 gpm of biotin), and 3.5 ng (5.5 μCi) 99mTc-cMORF. After incubation on ice for 1 hour, the medium was removed, the wells were washed twice with culture medium, and the cells were lysed with 0.2 M NaOH/1% SDS. The radioactivity in the cells and medium was counted in an NaI(Tl) well counter (Cobra II automatic gamma counter; Packard Instrument Company). A control group of three wells were treated in an identical fashion but without addition of antibody.
Tumor fragments were recovered from a mouse with an LS174T tumor of 1–2 g after euthanization under anesthesia. Working throughout at 4°C, the tumor was cut in a Petri dish into pieces of 2–3 mm in 15 mL working medium (RPMI-1640 with 1% FBS). The pieces were rinsed three times with the working media and were then ground into fragments. After passing through a 80 mesh sieve, the slurry was washed with the working media until all red blood cells were removed. The tumor fragment concentration was quantitated by weighing the pellet from 1 mL of suspension after centrifugation.
For the binding study, 27 microcentrifuge tubes coated with BSA were divided into nine groups (n = 3). An aliquot of 500 μL medium (1% BSA in Dulbecco's PBS) containing 20 mg of LS174T fragments was added to each tube, followed by 250 μL medium containing 0–85 μg of native CC49, 5.8 ng of the MORF-CC49-biotin, and 0.23 ng (0.4 μCi) of 99mTc-cMORF. These conditions differ slightly from those used in the above cell study because of the adjustment based on the results from that cell study. After incubation on ice for 1 hour, the tubes were centrifuged at 900 g and the pellets were washed twice with 1 mL medium. The radioactivity in the medium and pellets was counted. A control group of three tubes were treated in an identical fashion but without addition of the antibody.
Clearance study in normal mice
The dosages and timing were selected as described previously.21 As always, any convenient dosage of the pretargeting antibody may be selected, as long as it does not saturate the tumor antigen. It has been shown for the LS174T tumor model that a 30 μg dosage of MORF-CC49-biotin satisfies this condition. Therefore, this dosage was selected for the normal animal study to determine the influence of avidin. The clearing agent should be added when the tumor accumulation of the antibody has reached its maximum level. Previous experiences indicated that the accumulation of the CC49 antibody in LS174T tumors peaks at 1 day22 when blood levels are still high. As the pharmacokinetics of the MORF-CC49-biotin antibody would not differ greatly from that of the MORF-CC49 antibody in this measure, 1-day pretargeting interval was used. The clearance efficacy of avidin was evaluated after 3 hours by measuring the antibody blood level because it has been reported that there are no further reductions in antibody concentration in blood beyond 3 hours.8 In this research, the measurement of the blood antibody level at 3 hours was accomplished by in vivo labeling in which a dosage of 99mTc-cMORF in excess with respect to circulating antibody was first injected. As the unbound excess 99mTc-cMORF cleared rapidly, only the 99mTc-cMORF bound to MORF-CC49 remained in circulation to be counted.
Thus, 20 CD1 mice, in groups of 4, each received intravenously 30 μg MORF-CC49-biotin. At 21 hours, group 1 received nothing, whereas groups 2–5 received 3.35, 6.70, 33.5, and 67.0 μg of avidin, respectively. Three (3) hours later, each mouse received 1.2 μg of 99mTc-cMORF. The mice were euthanized at 3 hours after the 99mTc-cMORF injection by heart puncture and exsanguination under anesthesia. Samples of blood and other organs were removed, weighed, and counted in a well gamma counter with a standard of the injectate. Blood and muscle were assumed to constitute 7% and 40% of body weight, respectively.
Clearance studies in tumored mice
The tumor accumulation of the effector will be at its maximum (MPTA) as long as its dosage is below the tumor-saturating dosage. It has been previously shown that the MPTA of any effector is equal to the product of the fraction (f) of cardiac output (F) to tumor, the reciprocal of the tumor weight (W−1), the tumor trapping fraction of the effector (E), and the area under the blood curve of the effector21:
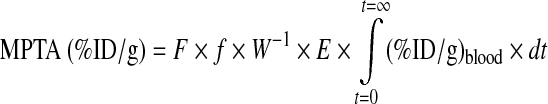 |
If a clearing step is added when the antibody concentration in blood is minimal, no parameter in this equation will change (based on the authors' experience, the standard deviation in the accumulation of the effector in tumor is 17%; therefore, the antibody blood concentration is considered to be minimal when less than 10% of the injected effector is bound to circulating antibody).
For a convenient dosage of 30 μg of MORF-CC49-biotin, the tumor saturating (and therefore optimum) dosage of the cMORF effector is 2.2 μg, based on the authors' semiempirical model, if the tumor accumulation of MORF-CC49-biotin is identical to that of MORF-CC49 measured previously.21 However, as the tumor accumulation of MORF-CC49-biotin may be slightly altered by the biotin modification, to guarantee that the effector accumulation in tumor does not exceed saturation, about half of the calculated saturating dosage (1.2 μg) was used.
Eight NIH Swiss nude mice bearing LS174T tumors each received intravenously 30 μg of MORF-CC49-biotin. At 21 hours thereafter, half the animals received 33.5 μg of avidin, a dosage determined earlier in normal mice (see below). The other half did not receive avidin. Subsequent steps were identical to those used in the normal mice study. Tumor excision was performed as described earlier.20 It has been previously measured the effector MPTA as a function of tumor weight in this model system is 4.51 × (tumor weight in grams)−0.66, with an SD of about 17% of the measured MPTA.14,22 The predicted accumulation of the effector in tumor for any tumor size can be calculated from this equation. After sacrifice, the predicted tumor accumulations from this MPTA–tumor weight relationship were compared with the measured tumor accumulations.
Results
Antibody conjugation and testing
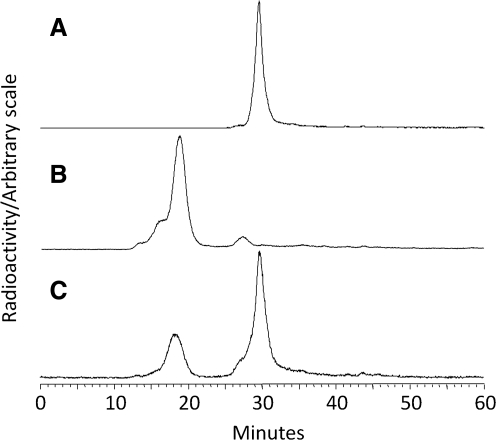
Figure 3 presents size-exclusion HPLC radiochromatograms of labeled cMORF (panel A) obtained on a Superose-12 column with a 0.05 M phosphate buffer eluant (pH 7.2) at a flow rate of 0.60 mL/min. The complete shift to higher molecular weight (panel B) following the addition of excess MORF-CC49-biotin demonstrates high radiochemical purity. The small peak at 28 minutes is due to labeled cMORF binding to the free MORF remaining after antibody purification. By adding a known amount of labeled cMORF in excess of that required to saturate the MORF-CC49-biotin, an average value of 0.65 ± 0.03 MORFs per CC49 was calculated from the partial shift (panel C). Following instructions from the manufacturer of EZTM Biotin Quantitation Kit, an average value of 3.41 biotins per CC49 was obtained.
FIG. 3.
Size-exclusion HPLC radiochromatograms of labeled cMORF before (A) and after (B) adding excess MORF-CC49-biotin and after adding limited MORF-CC49-biotin (C). The cMORF/MORF molar ratios in B and C are 0.04 and 2.19, respectively.
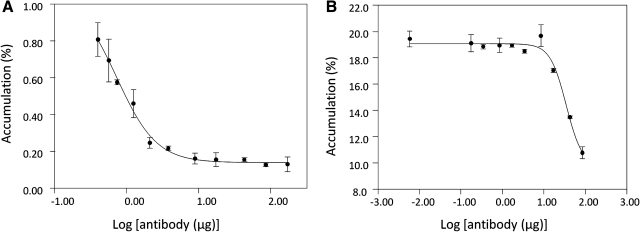
Figure 4 shows that after conjugating with both MORF and biotin, the specific binding of the CC49 antibody to TAG-72 is preserved. In both cases, the rapid drop in cell-associated radioactivity with increasing concentration of native antibody confirms specific binding.
FIG. 4.
Accumulation of labeled MORF-CC49-biotin with increasing native antibody concentration in cells (A) and tumor fragments (B). The error bars represent one standard deviation (n = 3).
Clearance study in normal mice
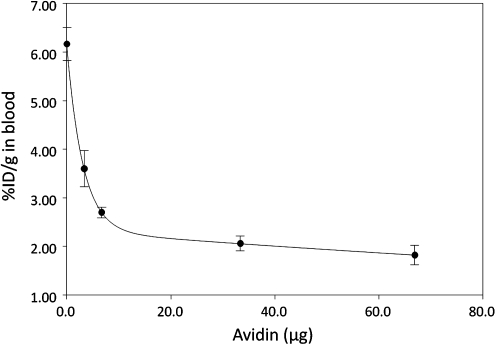
Figure 5 shows the influence of the avidin dosage on the blood level in normal mice of labeled cMORF (by inference, the blood level of the pretargeting antibody). As determination was conducted at 3 hours after the 99mTc-cMORF injection and all free 99mTc-cMORF should have cleared (<0.04% ID/g),20 the blood level of 99mTc-cMORF measures the antibody level in circulation. As shown, increasing the avidin dosage is highly effective in reducing the blood level at low dosages, but the effect diminishes rapidly after about 10 μg. Thus, at 21 hours postantibody administration, a clearance efficiency of over 60% is achievable for an avidin dosage of 10 μg or above. This observation may suggest that not all of the antibody molecules in circulation were accessible.
FIG. 5.
The blood level of effector at 3 hours postradioactivity injection (by inference, that of the pretargeting antibody) in normal mice receiving in sequence 30 μg MORF-CC49-biotin, increasing dosages of avidin administered 21 hours later, and 1.2 μg 99mTc-cMORF 3 hours thereafter. The error bars represent one standard deviation (n = 4).
Clearance study in tumored mice
Table 1 presents the biodistributions at 3 hours of 99mTc-cMORF in nude mice with LS174T tumors and in normal CD1 mice for comparison. Each mouse received 30 μg of MORF-CC49-biotin antibody, followed at 3 hours later by 33.5 μg of avidin. Also included are the results in both normal and tumored animals conducted identically except for the omission of the avidin. The biodistributions in normal organs and blood are reasonably similar in normal and tumored mice. The clearance efficiency at 62% in normal mice and 69% in nude mice are also similar (calculated from the blood values in Table 1). As the avidin-clearing agent is clearing the unlabeled MORF-CC49-biotin but not the radiolabeled cMORF effector into the liver, the liver radioactivity levels do not show any increase with the use of avidin. In previous studies of MORF/cMORF pretargeting, it has been observed that MORF-conjugated antibodies, when deposited into normal tissues such as liver, become “invisible” to the effector. Presumably, the same phenomenon is occurring here.
Table 1.
Biodistribution of 99mTc-cMORF in Normal CD1 Mice and in LS174T Tumor–Bearing Nude Mice, Receiving MORF-CC49-Biotin with and Without the Avidin as Clearing Agent
| |
Tumored mice with avidin |
Tumored mice without avidin |
Normal mice with avidin |
Normal mice without avidin |
||||
|---|---|---|---|---|---|---|---|---|
| Organ | Average | SD | Average | SD | Average | SD | Average | SD |
| Liver | 1.19 | 0.14 | 1.63 | 0.38 | 1.14 | 0.25 | 1.74 | 0.16 |
| Heart | 0.47 | 0.04 | 0.95 | 0.10 | 0.56 | 0.05 | 1.40 | 0.27 |
| Kidney | 3.19 | 0.32 | 3.75 | 0.65 | 4.00 | 0.28 | 5.41 | 2.82 |
| Lung | 0.63 | 0.06 | 1.80 | 0.96 | 0.74 | 0.07 | 2.02 | 0.28 |
| Spleen | 1.09 | 0.13 | 1.12 | 0.08 | 1.65 | 0.49 | 1.19 | 0.12 |
| Muscle | 0.25 | 0.02 | 0.33 | 0.02 | 0.40 | 0.09 | 0.63 | 0.12 |
| Salivary | 0.50 | 0.08 | 0.88 | 0.08 | 0.77 | 0.08 | 1.33 | 0.12 |
| Blood | 1.56 | 0.20 | 4.11 | 0.54 | 2.06 | 0.15 | 6.17 | 0.34 |
| Tumor | 6.30 | 0.92 | 6.30 | 1.79 | N/A | N/A | N/A | N/A |
| Tumor (g) | 0.68 | 0.18 | 0.75 | 0.21 | N/A | N/A | N/A | N/A |
The values are presented as an average of the percentage of injected dose per g (n = 4).
SD, standard deviation; N/A, not applicable.
Tumor accumulations are predicted to be independent of the clearance step, provided that the dosage of the effector is below the saturating dosage (i.e., at its MPTA). As such, the tumor accumulations of the effector in groups with and without avidin are predicted to be identical for the same tumor size. The average tumor weight for the two groups was similar at 0.68 versus 0.75 g, and therefore, the tumor accumulations are predicted to be similar. As shown in Table 1, the tumor accumulations are in gratifying agreement at 6.30% ± 0.92% versus 6.30% ± 1.79% ID/g (p = 0.50).
In addition to the agreement between the tumor accumulations with and without a clearing agent, these values are in agreement with the calculated values from the MPTA–tumor weight relationship: MPTA = 4.51 × (tumor size/g)−0.66, with an SD of 17%.14,22 For tumors of 0.68 and 0.75 g, the calculated tumor accumulations are 5.82% ± 0.99% and 5.45% ± 0.92% ID/g and therefore in satisfying agreement with the experimental values of 6.30% ± 0.92% and 6.30% ± 1.79% ID/g.
Discussion
Adding a clearing agent in a pretargeting study can improve the tumor/nontumor ratios by decreasing blood levels.1–12 However, depending upon the method of clearance and the dosage of the clearing agent, there remains a possibility that by adding the clearing agent, along with the desired reduction of effector accumulation in normal tissues, the tumor accumulation will also be reduced. This will certainly be the case if the clearing agent binds to the same target as the effector. Thus, when a peptide nucleic acid (PNA) polymer was cleared with avidin–cPNA, tumor accumulation of the cPNA effector decreased because of the occupation of the effector-binding sites.4 This competition can be avoided by the use of a pretargeting MORF-antibody conjugated with a second, independent group such as biotin to permit clearance of the antibody by avidin without any influence on the radiolabeled cMORF effector. Avidin has been previously considered to clear biotinylated antibodies23 in connection with pretargeting. Because of its four binding sites, avidin can also be used as a bridge between the antibody and effector.24,25 In this case, the avidin has two functions, to bind the biotin–antibody in circulation (to encourage its clearance into the liver) and to bind the biotin–antibody in tumor (to provide binding sites for a labeled biotin effector). In the present study, the authors have selected to pursue an alternative approach in which biotin is conjugated to the antibody and avidin serves only as a clearing agent.8
The results of the present investigation confirm the prediction that tumor accumulation of the effector will be unaffected as long as its dosage is below that required to saturate the accessible antibody in tumor.21 Mirallie et al. used avidin as a clearing agent in a pretargeting strategy using bispecific antibodies and also did not observe a decrease in tumor accumulation of the effector.8 Although there are other literature reports on changes in percentage of tumor accumulations when clearing agents are used,3,4,6 these tumor accumulations may not have been at their MPTAs.
Conclusions
A MORF–antibody–biotin construct has been synthesized and used in a pretargeting strategy along with avidin as a clearing agent. Under the conditions of this study, tumor accumulation of the effector was unaffected by addition of a clearing step as predicted. This observation may be applicable to other pretargeting systems, although this must be experimentally confirmed.
Acknowledgments
The authors are grateful to Dr. Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) for providing the CC49 hybridoma. Financial support was in part from the National Institutes of Health (CA94994, CA107360, and DK082894).
Disclosure Statement
No institutional or commercial affiliations would pose a conflict of interest regarding the publication of this article.
References
- 1.Goodwin DA. Meares CF. McCall MJ, et al. Pre-targeted immunoscintigraphy of murine tumors with indium-111-labeled bifunctional haptens. J Nucl Med. 1988;29:226. [PubMed] [Google Scholar]
- 2.Goodwin DA. Meares CF. Watanabe N, et al. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994;54:5937. [PubMed] [Google Scholar]
- 3.Yao Z. Zhang M. Kobayashi H, et al. Improved targeting of radiolabeled streptavidin in tumors pretargeted with biotinylated monoclonal antibodies through an avidin chase. J Nucl Med. 1995;36:837. [PubMed] [Google Scholar]
- 4.Wang Y. Chang F. Zhang Y, et al. Pretargeting with amplification using polymeric peptide nucleic acid. Bioconjug Chem. 2001;12:807. doi: 10.1021/bc0100307. [DOI] [PubMed] [Google Scholar]
- 5.Karacay H. Sharkey RM. Govindan SV, et al. Development of a streptavidin-anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer. Reagent development. Bioconjug Chem. 1997;8:585. doi: 10.1021/bc970102n. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey RM. Karacay H. Griffiths GL, et al. Development of a streptavidin-anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer. Studies in a human colon cancer xenograft model. Bioconjug Chem. 1997;8:595. doi: 10.1021/bc970101v. [DOI] [PubMed] [Google Scholar]
- 7.Axworthy DB. Reno JM. Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci USA. 2000;97:1802. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirallie E. Sai-Maurel C. Faivre-Chauvet A, et al. Improved pretargeted delivery of radiolabelled hapten to human tumour xenograft in mice by avidin chase of circulating bispecific antibody. Eur J Nucl Med Mol Imaging. 2005;32:901. doi: 10.1007/s00259-005-1811-2. [DOI] [PubMed] [Google Scholar]
- 9.Sinitsyn VV. Mamontova AG. Checkneva YY, et al. Rapid blood clearance of biotinylated IgG after infusion of avidin. J Nucl Med. 1989;30:66. [PubMed] [Google Scholar]
- 10.Sharkey RM. Primus FJ. Goldenberg DM. Second antibody clearance of radiolabeled antibody in cancer radioimmunodetection. Proc Natl Acad Sci USA. 1984;81:2843. doi: 10.1073/pnas.81.9.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin DA. Meares CF. Diamanti C, et al. Use of specific antibody for rapid clearance of circulating blood background from radiolabeled tumor imaging proteins. Eur J Nucl Med. 1984;29:226. doi: 10.1007/BF00448541. [DOI] [PubMed] [Google Scholar]
- 12.Ong GL. Ettenson D. Sharkey RM, et al. Galactose-conjugated antibodies in cancer therapy: properties and principles of action. Cancer Res. 1991;51:1619. [PubMed] [Google Scholar]
- 13.Liu G. He J. Dou S, et al. Further investigations of morpholino pretargeting in mice-establishing quantitative relations in tumor. Eur J Nucl Med Mol Imaging. 2005;32:1115. doi: 10.1007/s00259-005-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G. Dou S. He J, et al. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging. 2007;34:237. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G. Dou S. Pretorius PH, et al. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2008;35:272. doi: 10.1007/s00259-007-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G. Dou S. Rusckowski M, et al. An experimental and theoretical evaluation of the influence of pretargeting antibody on the tumor accumulation of effector. Mol Cancer Ther. 2008;7:1025. doi: 10.1158/1535-7163.MCT-07-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G. Dou S. He J, et al. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity. Appl Radiat Isot. 2006;64:971. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J. Liu G. Dou S, et al. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G. Dou S. Pretorius PH, et al. Tumor pretargeting in mice using MORF conjugated CC49 antibody and radiolabeled complementary cMORF effector. Q J Nucl Med Mol Imaging. 2009 [Epub ahead of print]. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G. He J. Dou S, et al. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur J Nucl Med Mol Imaging. 2004;31:417. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu G. Hnatowich DJ. A semiempirical model of tumor pretargeting. Bioconjug Chem. 2008;19:2095. doi: 10.1021/bc8002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G. Dou S. Liang M, et al. The ratio of maximum percent tumour accumulations of the pretargeting agent and the radiolabelled effector is independent of tumour size. Eur J Cancer. 2009;45:3098. doi: 10.1016/j.ejca.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H. Sakahara H. Endo K. Yao Z-S. Toyama S. Konishi J. Repeating the avidin “chase” markedly improved the biodistribution of radiolabelled biotinylated antibodies and promoted the excretion of additional background radioactivity. Eur J Cancer. 1995;31:1689. doi: 10.1016/0959-8049(95)00244-d. [DOI] [PubMed] [Google Scholar]
- 24.Hnatowich DJ. Virzi F. Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294. [PubMed] [Google Scholar]
- 25.Paganelli G. Pervez S. Siccardi AG, et al. Intraperitoneal radio-localization of tumors pre-targeted by biotinylated monoclonal antibodies. Int J Cancer. 1990;45:1184. doi: 10.1002/ijc.2910450632. [DOI] [PubMed] [Google Scholar]