Abstract
Objective
To analyze insulin resistance (IR) and determine the need for a 2-hour oral glucose tolerance test (OGTT) for the identification of IR and impaired glucose tolerance (IGT) in lean nondiabetic women with polycystic ovary syndrome (PCOS).
Methods
This was a cross-sectional analysis of treatment-naive women with PCOS who enrolled in a university-based clinical trial. Nondiabetic women with PCOS based on the Eunice Kennedy Shriven National Institute of Child Health and Human Development (NICHD) definition, aged 18–43 years and weighing ≤113 kg, were evaluated. Glucose and insulin levels were assessed at times 0, 30, 60, 90, and 120 minutes after a 75-g glucose load. Lean was defined as body mass index (BMI) <25 kg/m2. Multiple linear regression was performed.
Results
A cohort of 78 women was studied. The prevalence of IR was 0% among lean women vs. 21% among nonlean subjects based on fasting insulin I0 and 40%–68% based on two different homeostatic model assessment (HOMA) cutoff points (p < 0.005). All women with IR had a BMI ≥ 28. Controlling for age and race, BMI explained over 57% of the variation in insulin fasting (Io), glucose fasting/Io (Go/Io), the qualitative insulin sensitivity check index (QUICKI), and HOMA and was a highly significant predictor of these outcomes (p < 0.0001). Only 1 of 31 (3%) of the lean PCOS women had IGT based on a 2-hour OGTT, and no lean subjects had IGT based on their fasting blood glucose.
Conclusions
Diabetes mellitus, IGT, and IR are far less common in young lean women with PCOS compared with obese women with PCOS. These data imply that it is unnecessary to routinely perform either IR testing or 2-hour OGTT in lean women with PCOS; however, greater subject accumulation is needed to determine if OGTT is necessary in lean women with PCOS. BMI is highly predictive of both insulin and glucose levels in women with PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of reproductive-aged women and is the most common endocrine-associated cause of infertility.1 Approximately 6.5% of women of reproductive age have PCOS,2,3 which is characterized clinically by irregular or absent menstrual periods and hyperandrogenic manifestations, such as acne and hirsutism.4 Although many women with PCOS are also obese, obesity per se is not a part of the diagnostic criteria for PCOS. In fact, it is well known that women who are lean can also have PCOS.
Women with PCOS are known to be at increased risk for insulin resistance (IR). In lean women with PCOS, this IR is thought to be similar in severity to that in women who are obese and do not have PCOS.5 Because of their IR, women with PCOS are at increased risk for type 2 diabetes mellitus.6 Based on this information, a committee opinion from the Androgen Excess Society recommended that all obese women with PCOS be screened for impaired glucose tolerance (IGT) and diabetes mellitus using a 2-hour oral glucose tolerance test (OGTT).7 Their recommendation for screening only obese women with PCOS and not lean women with PCOS was based on the increased risk of type 2 diabetes in obese women with PCOS compared with their lean counterparts. This concept is based on the idea that obese women with PCOS have an additive IR effect from their obesity and from having PCOS. Because of insufficient data, however, it is much less clear if women with PCOS who are lean should be routinely screened for glucose intolerance. There are also limited data in regard to the prevalence of IR in lean women with PCOS, the various methods of detecting IR in this population, and which, if any, demographic factors affect insulin sensitivity in this population of women.
When a clinician makes a diagnosis of PCOS in a lean individual, that clinician must then decide what further metabolic evaluation, if any, to obtain. A fasting blood glucose (FBG) level is a simple test to assess glucose intolerance and to rule out diabetes mellitus. However, if this test is normal, do lean women with PCOS require routine screening with a 2-hour OGTT? If a test that is specific for IR is to be done, which test should be chosen? Clearly, a diagnosis of diabetes warrants directed therapy. Furthermore, the presence of IGT helps to identify women at increased risk for developing diabetes and allows clinicians to initiate treatment regimens, including lifestyle modifications, that can reduce that risk. The clinical utility of a diagnosis of IR is not as well established, and differentiating subjects who are IR from those who are not is problematic because insulin sensitivity is difficult to define and quantify. However, IR is associated with an increased risk of type 2 diabetes, hypertension, dyslipidemia, obesity, hypercoagulability, endothelial dysfunction, and cardiovascular disease (CVD).8 Therefore, one could use this rationale to advocate IR screening in a high risk population.
The objectives of this study were multiple. First, we sought to determine the prevalence of IR and IGT in a cohort of lean women with PCOS who were not diabetic based on a fasting serum glucose level. Second, we aimed to evaluate a variety of homeostatic and minimal model tests to determine their use in assessing IR in lean women with PCOS. Third, we wanted to compare our findings in lean women with PCOS to a group of nonlean women with PCOS. Finally, we wanted to develop a model using body mass index (BMI) to predict absolute changes in insulin and glucose levels.
Materials and Methods
The population studied comprised treatment-naïve women diagnosed with PCOS who had agreed to participate in a complementary and alternative therapy (CAM) randomized clinical trial. This article is a cross-sectional analysis of baseline data collected before any intervention, restricted to the participants who qualified for the study between January 2006 and June 2009 and who had complete data points. The University of Virginia Internal Review Board approved this protocol (UVA IRB 12045), and all study participants signed an informed consent.
Enrollment criteria
Inclusion criteria for the CAM study were (1) a diagnosis of PCOS, confirmed through the study using the Eunice Kennedy Shriven National Institute of Child Health and Human Development (NICHD) criteria of oligomenorrhea, nondiabetic with self-reported hirsutism or acne, and elevated free testosterone,9 (2) aged 18–43 years, (3) weight of ≤250 pounds (113 kg), and (4) at least one menses in the past 6 months but no more than eight periods in the most recent 12 months without hormonal intervention. Free testosterone was calculated from testosterone and sex hormone-blinding globulin (SHBG) levels,10–12 using >6.8 pg/mL as the definition of an elevated level among females.13 Exclusion criteria other than contraindications for the CAM intervention were (1) the use of metformin, other oral hypoglycemic agents, insulin, or any hormonal contraceptives in the 60 days before enrollment, (2) currently pregnant or breastfeeding during the prior 30 days, (3) any hormonal intervention in the prior 60 days, (4) immune deficiency, (5) FBG level >125 mg/dL, and (6) hemoglobin A1C (HgbA1C) level >6.0%.
Data sources
Demographic, lifestyle, and gravidity data were self-reported. HgbA1C levels were measured along with FBG levels up to 2 weeks before the OGTT. A standard 2-hour OGTT was administered using a 75-g glucose load (either glucola drink or jellybeans14) in a fasting state. Blood draws by trained nursing staff were taken at times 0, 30 (allowing 10 minutes for glucose consumption), 60, 90, and 120 minutes.
Venous blood glucose values were assayed by the glucose-oxidase method (Yellow Spring Instruments) and subsequently converted to plasma glucose. Serum insulin samples were stored at −80°C and subsequently batch assayed with a chemiluminescent immunoassay (Immulite 2000 Automated Immunoassay Analyzer). The minimum detection for insulin was 2.0 mIU/mL. Internal laboratory experience has demonstrated that hemolyzed insulin samples can run low. One of the insulin samples (at time 120) was run on hemolyzed serum and appeared low relative to the woman's other longitudinal samples after review by a single investigator (L.M.P.). That insulin result was excluded from the corresponding measures in this analysis.
All assays (except HgbA1C) were run by the University of Virginia General Clinical Research Center Core Lab. Height and weight were measured by a research nurse for all study participants. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Insulin and glucose calculations
This analysis used a wide variety of glucose and insulin measures, both with fasting and 2-hour insulin (microIU/mL) and glucose (mg/dL) values, follows:
Glucose: fasting (G0), time 120 (G120), area under the curve (AUCG)
Insulin: fasting (I0), time 120 (I120), AUCI
Glucose/insulin ratio: fasting (G0/I0), time 120 (G120/I120), G0/I0 (AUCG/I)
Homeostatic model assessment (HOMA-IR) = (I0* G0)/405, from Matthews et al.15
Insulin sensitivity index (ISI0,120), by Gutt et al.16
Quantitative insulin sensitivity check index (QUICK) I = 1/(log(I0) + log(G0)), from Katz et al.17
IR was defined as an elevated fasting insulin (I0 > 20)18 or an elevated HOMA-IR value. Two cutoff points were used for HOMA-IR: > 2.5 based on the original HOMA research15 and > 3.8 based on a cohort of white women with PCOS.18 An abnormal ratio of glucose insulin was defined as (1) G0/I0 < 7.219 and (2) G120/I120 ≤ 1.0.20 IGT was defined as an elevated fasting glucose (110 mg/dL ≤ G0 ≤ 125 mg/dL) or an elevated 2-hour glucose (140 mg/dL ≤ G120 ≤ 199 mg/dL).21 Area under the curve (AUC) for insulin and glucose were calculated by the trapezoid method.
Statistical analyses
This analysis stratified lean, defined as BMI < 25 kg/m2 and nonlean (BMI ≥ 25 kg/m2) women with PCOS. Participant demographics, lifestyle, and gravidity variables were compared between the two BMI groups with Wilcoxon, chi-squared, or Fisher's exact tests. Means and standard deviations (SD) were calculated for each continuous measure of insulin, glucose, IR, and IGT. The various glucose and insulin levels between the two BMI groups were compared with Wilcoxon nonparametric (continuous measures) or Fisher's exact (dichotomous measures) tests. An alpha level of 0.05 was used to evaluate statistical significance.
With respect to the multivariate analyses, the primary goal was to examine how well various glucose and insulin measures could be predicted based on BMI (continuous measure). To this end, the insulin and glucose measurements were transformed to the natural log scale in order to model an end point with a normal distribution. The initial linear regression model of log-transformed fasting insulin adjusted for BMI, age, race (white/nonwhite), smoking, gravidity (nulligravid/multigravid), single marital status, and regular exercise (yes/no). Only two potential confounders were included in the final models: race because it was marginally significant (p = 0.085) and age because of the known confounding between age and BMI in our cohort (Table 1). For consistency, all insulin and glucose final models included the same covariates. The models were evaluated with R2 coefficients and the Wald p value for the BMI coefficient. All modeling was performed with SAS (version 9.1.3, Cary, NC).
Table 1.
Demographics and Reproductive History of Study Population of Women with Polycystic Ovary Syndrome Stratified by Body Mass Index
| Total (n = 78) | BMI < 25(n = 31) | BMI ≥ 25(n = 47) | p value, test | |
|---|---|---|---|---|
| Age, years, mean (SD) Range | 26.6 (6.4) 18–43 | 23.9 (5.3) 18–38 | 28.3 (6.4) 18–43 | 0.002, Wilcoxon |
| Race | ||||
| White African | 59 (76%) | 21 (68%) | 38 (81%) | 0.19, chi-square |
| American | 10 (13%) | 4 (13%) | 6 (13%) | White vs. nonwhite |
| Asian | 5 (6%) | 5 (16%) | 0 (0%) | |
| Other | 4 (5%) | 1 (3%) | 3 (6%) | |
| Hispanic ethnicity | 5 (6%) | |||
| Education | ||||
| High school or less | 11 (14%) | 2 (6%) | 8 (17%) | 0.26, Fisher |
| Some college | 34 (44%) | 13 (42%) | 21 (46%) | |
| At least college degree | 33 (42%) | 16 (52%) | 17 (37%) | |
| Single | 46 (59%) | 25 (80%) | 21 (45%) | 0.015, chi-square |
| Live alone | 11 (14%) | 6 (19%) | 5 (11%) | 0.33, Fisher |
| Student | ||||
| Full-time | 29 (37%) | 21 (68%) | 8 (17%) | < 0.0001, Fisher |
| Part-time | 7 (9%) | 0 (0%) | 7 (15%) | |
| Not a student | 42 (54%) | 10 (32%) | 32 (68%) | |
| Employed for pay | 49 (63%) | 15 (48%) | 34 (72%) | 0.032, chi-square |
| Smoke currently | 16 (21%) | 3 (10%) | 13 (28%) | 0.08, Fisher |
| Nulligravid | 55 (71%) | 27 (87%) | 28 (60%) | 0.011, Fisher |
| Exercise in past month | 63 (81%) | 28 (90%) | 35 (74%) | 0.14, Fisher |
| BMI, mean (SD) | 29.2 (6.5) | 22.6 (1.7) | 33.5 (4.6) | |
| Range | 18.6–43.1 | 18.6–24.9 | 25.3–43.1 | N/A |
| HgbA1C at enrollment, mean (SD) | 5.3 (0.3) | 5.3 (0.3) | 5.3 (0.4) | 0.24, Wilcoxon |
| Range | 4.5–6.2 | 4.5–5.9 | 4.5–6.2 | |
BMI, body mass index; HgbA1C, hemoglobin A1C; N/A, not available; SD, standard deviation.
Results
Between January 2006 and June 2009, 83 women were screened and had complete data points for the CAM study. During screening, 5 nonlean women were found to be ineligible for the protocol and this analysis because of elevated HgbA1C levels and were subsequently found to be diabetic by their healthcare provider. The study population's BMI ranged from 27 to 41 kg/m2. Included in our study population was 1 woman with a HgbA1C level of 6.2%. This patient was allowed to remain in the analysis because her two preintervention FBG results were normal (94 and 104 mg/dL). Thus, the final analytic cohort consisted of 78 women with PCOS, of whom 31 were lean and 47 were not.
The study recruited both university students and women from the community. Several demographic factors strongly differed between the two BMI groups (<25 kg/m2 and ≥ 25 kg/m2). The lean BMI group was younger, more likely to be students, less likely to be working for pay, and less likely to have ever been pregnant than the nonlean group (p < 0.04) (Table 1).
As evidence of eligibility for the entire study population, the mean serum dehydroepi and roster one sulfate (DHEAS) level was 188 μg/dL (range 27–479), mean serum prolactin level was 9.9 ng/mL (range 3–25), mean thyroid-stimulating (TSH) was 1.50 μIU/mL (range 0.44–3.6), mean serum total testosterone level was 58 ng/dL (range 18–161), and mean serum 17-hydroxyprogesterone level was 119 ng/dL (range 40–250).
All insulin and glucose measures were significantly different in the lean women with PCOS compared to with the nonlean women with PCOS (p ≤ 0.01) (Table 2), with all measures reflecting more favorable metabolism in the lean subjects. None of the lean women with PCOS (0%) were IR based on I0 or HOMA measures (Table 3). In comparison, the prevalence of IR among the nonlean women ranged from 21% to 68% depending on the method used to assess IR, with HOMA15 yielding the highest rate. All women with IR had a BMI ≥ 28. If BMI ≥ 28 was the clinical criterion used to assess IR with the HOMA, in a population similar to our study cohort, the specificity would be 100% (95% confidence interval [CI] 92%–100%) and the sensitivity would be 26% (95% CI 15%-40%) using the Matthews et al.15 cutoff point and 56% (95% CI, 41%–70%) using the Kauffman et al. cutoff point.19 Only 1 of the lean women with PCOS had IGT based on a 2-hour serum glucose level. None of the lean women with PCOS met the criteria for type 2 diabetes (2-hour blood glucose > 199 mg/dL).
Table 2.
Various Glucose and Insulin Measures in Women with Polycystic Ovary Syndrome Stratified by Body Mass Index (n = 78)
| BMI < 25 Median (25% and 75% interquartile range) | BMI ≥ 25 Median (25% and 75% interquartile range) | p values (Wilcoxon) | |
|---|---|---|---|
| Fasting glucose (mg/dL) | 88 (83–93) | 95 (92–100) | 0.0002 |
| AUC glucose | 13,170 (11,460–14,565) | 15,645 (13,275–17,130) | 0.0075 |
| G120 (mg/dL) | 91 (79–107) | 109 (93–136) | 0.0055 |
| Fasting insulin (μIU/mL) | 4.44 (2.6–6.5) | 14.1 (9.15–18.74) | <0.0001 |
| I120 (μIU/mL) | 34.2 (18.0–48.8) | 70.0 (36.7–99.0) | 0.0013 |
| AUC insulin | 4,583 (3,625–7,218) | 8,350 (6,218–13,119) | 0.0001 |
| QUICKI | 0.410 (0.38–0.44) | 0.333 (0.32-0.35) | <0.0001 |
| HOMA | 0.92 (0.59–1.44) | 3.22 (2.14–4.57) | <0.0001 |
| ISI (per Gutt16) | 5.9 (5.0–8.0) | 4.1 (3.3–5.2) | <0.0001 |
| G0/I0 ratio | 19.4 (12.6–31.4) | 6.9 (5.0–10.1) | <0.0001 |
| G120/I120 ratio | 2.6 (1.7–5.3) | 1.7 (1.2–2.7) | 0.0049 |
| AUC G/I ratio | 3.0 (1.9–3.8) | 1.7 (1.2–2.4) | 0.0009 |
AUC, area under the curve; G120, glucose at time 120; HOMA, homeostatic model assessment; I120, insulin at time 120; ISI, insulin sensitivity index; QUICKI, quantitative insulin sensitivity check index.
Table 3.
Prevalence of Dichotomous Outcomes by Body Mass Index Strata in Women with Polycystic Ovary Syndrome (n = 78)
| BMI < 25 | BMI ≥ 25 (95% CI) | p value, test | |
|---|---|---|---|
| Insulin resistant (Io > 20) | n = 0 | n = 10, 21% (7-28) | 0.0049, Fisher |
| Elevated HOMA per Kaufmann19 (>3.8) | n = 0 | n = 19, 40% (10-33) | <0.0001, Fisher |
| Elevated HOMA per Matthews15 (>2.5) | n = 0 | n = 32, 68% (31-59) | <0.0001, Fisher |
| G0/I0 ratio < 7.2 | n = 1, 3% | n = 27, 57% (17-42) | <0.0001, Fisher |
| G120/I120 ratio ≤ 1.0 | n = 9, 16% (3-25) | n = 10, 21% (5-23) | 0.77, Fisher |
| Impaired fasting glucose (125 ≥ Go ≥ 100) | n = 0 | n = 2, 4% (1-14) | 0.51, Fisher |
| Impaired glucose tolerance using 2-hour values (140 ≤ G120 ≤ 199) | n = 1, 3% (1-16) | n = 10, 21% (12-35) | 0.0426, Fisher |
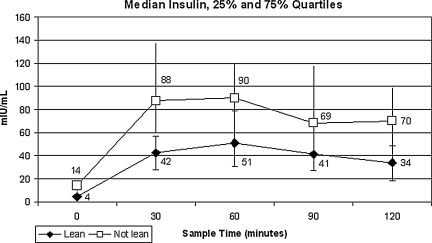
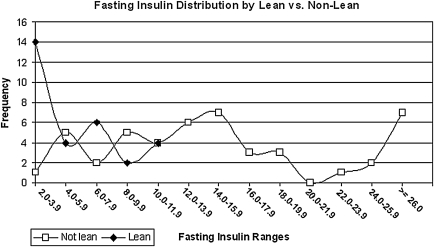
Figure 1 illustrates the difference in the mean insulin levels at 30-minute intervals of the OGTT by the two BMI strata. Note the increased yet parallel insulin response found in the nonlean BMI strata. The frequency distribution of I0 in 2-mIU/mL segments for lean and nonlean women is graphed in Figure 2. All the I0 levels for the lean PCOS women are under 8.0 mIU/mL, whereas the preponderance of the nonlean observations are >8.0 mIU/mL.
FIG. 1.
Median serum insulin levels in 30-minute intervals by lean vs. nonlean women with polycystic ovary syndrome (PCOS) (n = 78). p < 0.002 at all five time points.
FIG. 2.
Distribution of fasting serum insulin levels by lean vs. nonlean women with PCOS (n = 78).
After adjusting for age and race, body mass as a continuous variable was predictive of all log-transformed insulin and glucose measures analyzed (p < 0.008) (Table 4). Together, BMI, age and race accounted for 57-59 % of the variability in the I0, QUICK I, HOMA-IR and G0/I0 ratio results, as demonstrated by the R2 values. For all I and G measures, a lower BMI was associated with improved I and G values.
Table 4.
Linear Regression for Prediction of Natural Log-Transformed Glucose and Insulin Measurements Based on Body Mass Index in Women with Polycystic Ovary Syndrome
| Measure | BMI coefficient | p value | Global F test | R2 | Model p value |
|---|---|---|---|---|---|
| Fasting glucose | 0.01 | < 0.0001 | 7.2 | 0.23 | 0.0003 |
| AUC glucose | 0.01 | 0.0008 | 4.3 | 0.15 | 0.008 |
| Fasting insulin | 0.10 | < 0.0001 | 34.2 | 0.59 | < 0.0001 |
| AUC insulin | 0.06 | < 0.0001 | 12.3 | 0.34 | < 0.0001 |
| QUICKI | −0.02 | < 0.0001 | 35.7 | 0.59 | < 0.0001 |
| G0/I0 ratio | −0.09 | < 0.0001 | 32.0 | 0.57 | < 0.0001 |
| HOMA | 0.10 | < 0.0001 | 34.9 | 0.59 | < 0.0001 |
| G120 | 0.02 | 0.0004 | 4.8 | 0.16 | 0.004 |
| I120 | 0.07 | < 0.0001 | 8.1 | 0.26 | 0.0001 |
| G120/I120 ratio | −0.05 | 0.0002 | 6.4 | 0.22 | 0.0006 |
| ISI (per Gutt16) | −0.04 | < 0.0001 | 11.1 | 0.32 | < 0.0001. |
| AUCG/AUCI ratio | −0.05 | < 0.0001 | 10.5 | 0.31 | < 0.0001 |
All models controlled for age and race; age was not significant in any model.
Discussion
Women with PCOS are known to be at increased risk for IR, IGT, and type 2 diabetes mellitus. In general, women with PCOS, even those who are glucose intolerant, often have a normal FBG.22 Currently, guidelines from the Androgen Excess Society recommend that a 2-hour OGTT be performed on all obese women with PCOS.7 This recommendation is based on the significant prevalence of IGT in obese women with PCOS and their increased risk of developing diabetes.23 Because of a lack of sufficient data in lean women with PCOS, these guidelines do not give a recommendation in regard to the assessment of IGT in lean women. We found that in this population of women with PCOS, the incidence of IR, based on the methods we used, was very low in those who were lean (0 in our study population). Furthermore, only 1 of the 31 lean women with PCOS we studied had IGT based on a 2-hour OGTT, and none of the lean women with PCOS had IGT based on either their FBG level or HgbA1C levels.
There are several methods of assessing insulin sensitivity. The gold standard for assessment of insulin sensitivity is the hyperinsulinemic-euglycemic clamp technique. However, because this technique is both labor intensive and expensive, it is not suitable for daily clinical use. Other methods, such as fasting insulin levels,19 HOMA,24 G/I ratio,25 QUICKI,17 various calculations derived from the OGTT such as AUC,16 and 2-hour G/I ratios are easier and much less expensive to administer and have been shown to correlate well with clamp techniques.18,26,27 For these reasons, we chose to evaluate these testing methods in our study. It is important to understand that fasting insulin, AUC insulin, and G/I ratios, unlike HOMA and QUICKI, do not accurately reflect insulin sensitivity in women with IGT or type 2 diabetes mellitus. Furthermore, although we referenced the cutoff points used to diagnose IR from each test that we used in this study, the data available to determine the best cutoff point for IR in any population is somewhat limited.
Our findings in regard to IR in lean women with PCOS were different from those found in a recent study performed in Japan.28 Using AUCI, Takeuchi et al. found that 52.9% of lean Japanese women had an abnormal value. Using HOMA-IR, these investigators found that 19% of lean women with PCOS were IR. However, in regard to IGT, our findings were similar to those found by Wei et al.,29 who studied a cohort of lean infertile Chinese women with PCOS. They found that only 4.3% of women with PCOS and a BMI < 24 kg/m2 had IGT. Both Chang et al.30 and Dunaif et al.1 have reported on the presence of IR in women with PCOS that is independent of obesity. One possible reason for the differences between our findings and those of other clinical investigators may be the method by which the study population is defined. We used the NICHD definition for PCOS in our study. Investigators who use the criteria from the Rotterdam consensus conference or from a revision thereof as their entry criteria could be studying a very different population.31 The NICHD criteria include either hyperandrogenemia or hyperandrogenism as part of the diagnosis, whereas neither is required to make the diagnosis with the Rotterdam criteria. These different diagnostic criteria would certainly select different PCOS subpopulations that are likely to have different rates of IR. Furthermore, independent variables, such as smoking, which has been associated with increased insulin levels, and other as yet unidentified variables may play a role in IR.32 In our study, we did not find any differences in insulin levels or IR related to smoking (data not shown).
Differences in the incidence of IR in women with PCOS have been identified among various ethnic and racial groups. These differences between groups may be reflective of genetic defects, differences in physical activity, or dietary habits that are more common in one ethnic group vs. another.33 Furthermore, the cutoff point used to define IR appears to be different among various ethnic groups. Kauffman et al.19 found that in regard to diagnosing IR with a fasting insulin level, a cutoff point of ≥20 μU/mL was appropriate for white women, but in Mexican American women, a cutoff point of ≥23 μU/mL should be used. The same can be said for other tests of IR, including HOMA-IR, QUICKI, and AUCI. Furthermore, finding normal cutoff points for these tests is much more difficult, and they are more cumbersome to calculate. As our population was a combination of white, Mexican American, Asian, and black women, we used a fasting insulin level of ≥20 μU/mL to define IR. This lower fasting insulin cutoff point was specifically chosen to reduce the bias of our results toward a falsely low rate of IR.
Clearly, the results of our study can only be applied to the specific population of women we evaluated. In our study, the subjects were young, with an average age of 24.1 years. As age was identified as a confounder for BMI and BMI is related to IR, the young age of our study population may explain the low incidence of IR in our study. Furthermore, the majority of the women in the lean PCOS group were Caucasian, 64%. A greater mix of other racial and ethnic groups might have changed our overall results and conclusions. Furthermore, we did not assess the family history of the women in our study. If the family history of type 2 diabetes mellitus was lower in the lean women with PCOS in our study compared to a population mean of similar BMI, this may have biased our results.
We found several differences between the two BMI groups that we compared. Included were age, work status, and gravidity. Of these, only age seems to have biological plausibility in regard to a direct effect on glucose metabolism. Therefore, our linear regression model only controlled for age. The results, however, were striking. BMI was significantly associated with all the glucose levels, insulin levels, and IR testing models that we evaluated.
Our study had several strengths. First, we studied a treatment-naïve population. Our study groups were composed of community-dwelling individuals as opposed to a clinic cohort. Evaluating a clinic cohort may have increased the number of comorbidities and biased the severity of the disease state in our study population. We did not control for dietary differences or exercise (other than as a yes/no variable) in our study. Still, a lack of dietary restriction would have tended to increase and not decrease the rate of IGT or IR found in either group. Finally, our sample size was somewhat limited; a larger sample size might have produced different results.
The finding that obese women with PCOS are more likely to be IR as compared to a cohort of lean women with this syndrome is not novel. However, one question that needs to be more carefully addressed is: Are the cutoff points used to define underweight, normal weight, overweight, obesity, and morbid obesity the best cutoff points at which to begin IR or IGT testing in women with PCOS? Instead of using these BMI cutoff points to determine in whom IR or IGT testing is clinically indicated, it makes more sense to use a correlation coefficient to select the BMI at which these tests should be performed. Future studies with sufficient power could use R2 coefficients to select an appropriate cutoff point for testing of a given population that is appropriate in regard to sensitivity, specificity, and cost-effectiveness calculations.
Although the 3% incidence of IGT we found in lean women with PCOS is likely to be greater than that in the general population of the same age and BMI, we do not currently advocate routine testing for either IGT or for IR using a 2-hour OGTT in young lean women with PCOS. Larger population studies are needed to determine if there is a select population of lean women with PCOS, such as Japanese women, who are at a significantly increased risk for either IR or IGT who would warrant routine testing. Because of the difficulty associated with calculating and interpreting many of the tests of IR, such as HOMA-IR, QUICKI, and AUCI, it is not likely that these tests will be clinically useful to the practicing clinician. The type of long-term evaluation and follow-up for lean women with PCOS was not evaluated in this study. The answer to this question will require data from significantly powered longitudinal studies.
Acknowledgments
This publication was made possible by grant R21 AT002520 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health and grant M01RR000847 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, NCCAM, or the National Institutes of Health. We acknowledge the assistance of the research coordinator, Parchayi Dalal, and the staff of the UVA General Clinical Research Center.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Dunaif A. Segal KR. Futterweit W. Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R. Woods KS. Reyna R. Key TJ. Knochenhauer ES. Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Asuncion M. Calvo RM. San Millan JL. Sancho J. Avila S. Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 4.Zawadzki J. Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif AGJ, editor; Haseltine F, editor; Merriam GR, editor. The polycystic ovary syndrome. Cambridge: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 5.Dunaif A, editor; Givens JR, editor; Haseltine F, editor. The polycystic ovary syndrome. Cambridge: Blackwell Scientific; 1992. [Google Scholar]
- 6.Ovalle F. Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77:1095–1105. doi: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 7.Salley KE. Wickham EP. Cheang KI, et al. Glucose intolerance in polycystic ovary syndrome—A position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007;92:4546–4556. doi: 10.1210/jc.2007-1549. [DOI] [PubMed] [Google Scholar]
- 8.Essah PA. Wickham EP. Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obste Gynecol. 2007;50:205–225. doi: 10.1097/GRF.0b013e31802f3547. [DOI] [PubMed] [Google Scholar]
- 9.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2003;81:19–26. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen A. Verdonck L. Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 11.Rosner W. Errors in the measurement of plasma free testosterone. J Clin Endocrinol Metab. 1997;82:2014–2015. doi: 10.1210/jcem.82.6.9999. [DOI] [PubMed] [Google Scholar]
- 12.Södergard R. Bäckström T. Shanbhag V. Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17[beta] to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 13.ARUP Laboratories Lab Guide. www.aruplab.com/guides/clt/tests/clt_212b.htm. [Jun 11;2003 ]. www.aruplab.com/guides/clt/tests/clt_212b.htm
- 14.Lamar ME. Kuehl TJ. Cooney AT. Gayle LJ. Holleman S. Allen SR. Jelly beans as an alternative to a fifty-gram glucose beverage for gestational diabetes screening. Am J Obstet Gynecol. 1999;181:1154–1157. doi: 10.1016/s0002-9378(99)70099-2. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Gutt M. Davis CL. Spitzer SB, et al. Validation of the insulin sensitivity index (ISI(0,120)): Comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Katz A. Nambi SS. Mather K, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 18.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137:959–965. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman RP. Baker VM. Dimarino P, et al. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: A comparison of two distinct populations. Am J Obstet Gynecol. 2002;187:1362–1369. doi: 10.1067/mob.2002.126650. [DOI] [PubMed] [Google Scholar]
- 20.Radziuk J. Insulin sensitivity and its measurement: Structural commonalities among the methods. J Clin Endocrinol Metab. 2000;85:4426–4433. doi: 10.1210/jcem.85.12.7025. [DOI] [PubMed] [Google Scholar]
- 21.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Legro RS. Kunselman AR. Dodson WC. Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 23.Norman RJ. Masters L. Milner CR. Wang JX. Davies MJ. Relative risk of conversion from normoglycemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16:1995–1998. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- 24.Mather KJ. Hunt AE. Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: Implications for research applications. J Clin Endocrinol Metab. 2001;86:5457–5464. doi: 10.1210/jcem.86.11.7880. [DOI] [PubMed] [Google Scholar]
- 25.Ducluzeau PH. Cousin P. Malvoisin E, et al. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:3626–3631. doi: 10.1210/jc.2003-030219. [DOI] [PubMed] [Google Scholar]
- 26.Legro R. Finegood D. Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2694–2698. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 27.Quon MJ. Limitations of the fasting glucose to insulin ratio as an index of insulin sensitivity. J Clin Endocrinol Metab. 2001;86:4615–4617. doi: 10.1210/jcem.86.10.7952. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi T. Tsutsumi O. Taketani Y. Abnormal response of insulin to glucose loading and assessment of insulin resistance in non-obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:385–391. doi: 10.1080/09513590802173584. [DOI] [PubMed] [Google Scholar]
- 29.Wei H-J. Young R. Kuo IL. Liaw C-M. Chiang H-S. Yeh C-Y. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: A cross-sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864–1868. doi: 10.1016/j.fertnstert.2008.02.168. [DOI] [PubMed] [Google Scholar]
- 30.Chang RJ. Nakamura RM. Judd HL. Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–359. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 31.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 32.Cupisti S. Häberle L. Dittrich R, et al. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94:673–677. doi: 10.1016/j.fertnstert.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 33.Holte J. Polycystic ovary syndrome and insulin resistance: Thrifty genes struggling with over-feeding and sedentary life style? J Endocrinol Invest. 1998;21:589–601. doi: 10.1007/BF03350784. [DOI] [PubMed] [Google Scholar]