Abstract
Production of clinical-grade gammaretroviral vectors for ex vivo gene delivery requires a scalable process that can rapidly generate large amounts of vector supernatant, clear large numbers of residual packaging cells with minimal decreases in vector titer, and satisfy all current regulatory guidelines regarding product biosafety. To that end, we have developed a simplified method that is compliant with current good manufacturing practices for the production of clinical-grade gammaretroviral vectors in a clinical research environment. We validated a large-scale production platform utilizing 1,700-cm2 expanded surface roller bottles and a “modified” step-filtration process consisting of a 40/150-μm dual-screen filter for aggregate removal followed by a Sepacell 500II leukocyte reduction filter for removal of residual packaging cells. This clarification process can clear at least 2 × 109 viable producer cells using a single filter set-up without any significant loss of titer post-filtration. This platform typically generates 18 liters of vector supernatant to support small-scale clinical trials, but can easily be scaled up to 70 liters during a single manufacturing run. To date, this platform has generated five clinical-grade gammaretroviral vector products, four of which are now being used in adoptive cell therapy clinical trials for the treatment of a variety of solid cancers.
Feldman and colleagues describe a simplified scalable process for the production of gammaretroviral vectors. According to the authors, this process is compliant with current good manufacturing practices and has been used to generate five clinical-grade gammaretroviral vector products, four of which are now being used in adoptive cell therapy clinical trials for the treatment of a variety of solid cancers.
Introduction
Since the first clinical trial using adoptive cell therapy (ACT) of gene-modified cells in 1990 (Rosenberg et al., 1990), gene therapy has become a major focus for the treatment of a variety of genetic disorders, infectious diseases, and cancer. The Surgery Branch of the National Cancer Institute (NCI) has used two adoptive cell transfer strategies for the treatment of patients with Stage IV metastatic melanoma. The first strategy relies on the harvest and expansion of autologous tumor-infiltrating lymphocytes (TILs) derived from resected tumor (reviewed in Rosenberg and Dudley, 2009). However, for those patients for whom TILs cannot be generated, an alternative strategy exists whereby peripheral blood lymphocytes (PBLs) can be genetically modified to express a tumor-reactive T-cell receptor (TCR) directed against such antigens as melanoma antigen recognized by T cells 1 (MART-1), gp100, or other tumor antigens (Morgan et al., 2006). Genetically modified PBLs can mediate tumor regressions and may have a significant advantage over TILs in that PBLs can be redirected against a variety of cancers based on the availability of tumor-specific TCRs or chimeric antigen receptors (CARs).
For Surgery Branch, NCI clinical trials utilizing gene-modified T cells for ACT, gammaretroviral vectors derived from PG13 packaging cells have been the primary gene delivery vehicle. Several groups have developed gammaretroviral clinical production strategies based on flat-stock tissue culture vessels such as standard roller bottles and cell factories (Reeves and Cornetta, 2000; Reeves et al., 2000; Cornetta et al., 2005; Przybylowski et al., 2006) or suspension cultures (Pizzato et al., 2001; Wu et al., 2002; Ghani et al., 2007, 2009). However, regardless of the cell culture platform, the clarification process must remove viable residual packaging cells from the bulk harvest without significant loss of vector titer. Classical methods for clarification include centrifugation, depth, and membrane filtration. These methods either cannot adequately clear viable residual packaging cells (i.e., centrifugation) or require a significant process development effort to minimize product loss during large-scale vector production. Reeves and Cornetta (2000) developed a process known as step-filtration for the removal of viable residual packaging cells from retroviral vector supernatants. Step-filtration consists of three blood filters in series: (1) a 40/150-μm dual-screen aggregate filter, (2) a 20-μm aggregate filter, and (3) a Sepacell 500II leukocyte reduction filter (Baxter, Deerfield, IL) for the removal of residual packaging cells. This process can efficiently clear greater than 2 × 109 residual packaging cells while improving vector recovery by nearly 50% compared with membrane filtration (Reeves and Cornetta, 2000). Gammaretroviral vectors generated using step-filtration have been used in a variety of clinical trials (Reeves and Cornetta, 2000; Cornetta et al., 2005; Johnson et al., 2006, 2009; Morgan et al., 2006; Przybylowski et al., 2006).
In order to rapidly generate clinical-grade gammaretroviral vectors to support a variety of ACT human gene therapy clinical trials for the treatment of patients with melanoma and other cancer histologies, we developed and validated a simple and efficient production platform in expanded surface roller bottles and a “modified” step-filtration process, which, because of the discontinuation of the 20-μm filter, consists of a 40/150-μm dual-screen filter followed by a Sepacell 500II leukocyte reduction filter. To date, we have manufactured five current good manufacturing practices (cGMP)-quality gammaretroviral vector products. This article provides a summary of our experience of establishing a vector production facility in a clinical research institute and developing a process for the rapid generation of cGMP-quality gammaretroviral vector in a clinical research environment.
Materials and Methods
Patient peripheral blood mononuclear cells and cell lines
PG13 packaging clones were generated using the PG13 gibbon ape leukemia virus packaging cell line (ATCC CRL-10686) and the human ecotropic packaging cell line Phoenix ECO (kindly provided by Dr. Gary Nolan, Stanford University, Stanford, CA). All cells were cultured in D10 medium consisting of high-glucose (4.5 g/liter), Dulbecco's modified essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), and 6 mM (final concentration) glutamine (Invitrogen). Cells were maintained at 37°C and 5% CO2. Melanoma cell lines mel526 and mel624 (HLA-A2+/MART-1+) and mel888 and mel938 (HLA-A2−) were isolated from surgically resected metastases as previously described (Topalian et al., 1989) and were cultured in R10 medium consisting of RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum. All peripheral blood mononuclear cells and lymphocytes used for transduction were obtained from aphereses of participants on Institutional Review Board-approved Surgery Branch protocols and cultured in AIM-V medium (Invitrogen) supplemented with 5% human AB serum (Valley Biomedical, Winchester, VA), 0.05 mM β-mercaptoethanol, 0.1 mM non-essential amino acids, 25 mM HEPES, and 2 mM l-glutamine (Invitrogen).
Generation of PG13 packaging clones
For a given TCR or CAR, a PG13 retroviral packaging cell clone was generated as described previously (Hughes et al., 2005) with the following changes. Phoenix ECO cells were transfected with 9 μg of plasmid DNA (pMSGV1-TCR or -CAR) using Lipofectamine 2000 transfection reagent (Invitrogen). After 48 hr supernatant was harvested and used to transduce the retroviral packaging cell line PG13. For the transduction, non–tissue culture–treated six-well plates were coated with 10 μg/ml recombinant fibronectin fragment (RetroNectin) as described by the manufacturer (Takara Bio, Otsu, Japan). Retroviral vector supernatant (4 ml) was added to each well followed by centrifugation (2,000 × g) at 32°C. After 2 hr, supernatant was removed, and 5 × 105 PG13 cells were added to the well and centrifuged (1,000 × g) for 10 min at 32°C. Two rounds of transduction were performed, and then PG13 packaging clones were generated by limiting dilution cloning. Because of the lack of a selectable marker, high-titer clones were identified by RNA dot blot as described previously (Onodera et al., 1997; Hughes et al., 2005). Gammaretroviral vector from the six highest-titer packaging clones was generated as described previously (Kochenderfer et al., 2009). In brief, 175-cm2 tissue culture flasks (Nunc, Cole-Parmer, Vernon Hills, IL) were seeded at 4 × 104 cells/cm2, followed by a medium exchange (30 ml) on day 3. Supernatant was harvested 24 hr later, aliquoted, and stored at −80°C until further use. Supernatant from each clone was evaluated for the ability to efficiently transduce human PBLs and elicit interferon-γ (IFNγ) in a cytokine release assay. The clone conferring the highest transduction efficiency and IFNγ release following PBL transduction was selected for production of a master cell bank (MCB) and subsequent cGMP-quality gammaretroviral vector supernatant.
Generation of GMP retroviral vector supernatant by “modified” step-filtration
A total of 26 1,700-cm2 Cellbind expanded surface roller bottles (Corning, Acton, MA) were seeded on day 0 with a specific PG13 cell clone at a cell density of 4 × 104 cells/cm2 in 200 ml of D10 medium. On day 3, the medium was exchanged and replaced with 120 ml of D10 medium. Medium containing the gammaretroviral vector was then harvested daily with roller bottles being refed with 120 ml of medium. Glucose levels were monitored daily using the Accu-chek system from Roche (Basal, Switzerland). If glucose levels dropped below 2 g/liter, the volume of the medium exchange was doubled to 240 ml per roller bottle. The roller bottles were divided into two sets of 13 bottles for all subsequent harvests. Each harvest consisted of 3 liters of bulk vector supernatant, which was clarified using a “modified” step-filtration process consisting of a 40/150-μm dual-screen aggregate filter upstream of a Sepacell 500II filter for removal of residual packaging cells. Following “modified” step-filtration, all harvests were aliquoted and stored at −80°C until further use. An aliquot from each harvest was tested for transduction efficiency and cytokine release as described previously. The cGMP-quality MCB and gammaretroviral vector supernatant was subjected to an extensive biosafety testing program in accordance with current regulatory guidelines of the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration (Points to Consider in the Production and Testing of New Drugs and Biologicals Produced by Recombinant DNA Technology, 1985; Points to Consider in the Use of Cell Lines to Produce Biologicals, 1993; Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy, 1998; Guidance for Industry, INDs—Approaches to Complying with cGMP During Phase I, 2006) (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/hum).
PBL transductions
For PBL transductions and determination of virus titer, PBLs (2 × 106/ml) were stimulated with interleukin-2 (300 IU/ml, Chiron Corp., Emeryville, CA) and OKT3 (50 ng/ml, Ortho, Bridgewater, NJ) on day 0. Non–tissue culture–treated 6-well plates were coated with RetroNectin at 10 μg/ml (2 ml/well) on day 1 and stored overnight at 4°C. Because our vectors lack a selectable marker and because of the variability associated with gammaretroviral vector transduction and receptor expression in different patient PBLs, we used the following transduction scheme for determination of transduction efficiency and titer. In brief, vector supernatants were diluted 1:2 in D10 (4 ml/well) and applied to plates on day 2 followed by centrifugation at 2,000 × g for 2 hr at 32°C. Half the volume was aspirated, and PBLs were applied (0.25 × 106/ml, 4 ml/well), centrifuged for 10 min at 1,000 × g, and then incubated at 37°C and 5% CO2. PBLs from three patients were transduced for each assessment, and vector titers were calculated as follows: (percentage tetramer+ or CAR+ cells) × (total cells) × (dilution factor)/supernatant volume.
Clarification of gammaretroviral vector supernatants
Vector supernatant produced from PG13-F5mA2aB (1G12), encoding a murinized MART-1-reactive TCR (Johnson et al., 2006), was prepared as described above. In order to assess the effect of filter matrix and pore size on vector recovery, 250 ml of vector supernatant was applied to 500 ml 0.45-μm and 0.2-μm (pore size) polyvinylidene difluoride (PVDF) or 0.2-μm polyethersulfone (PES) Stericup bottle top filters, respectively (Millipore, Billerica, MA). Vector supernatant was vacuum-filtered, and time (min) was recorded at 50-ml intervals until filtration was complete or the filter clogged. Viable residual cells were detected by the residual cell detection assay (RCDA) (Przybylowski et al., 2006). In brief, the post-clarification filtrate was centrifuged at 1,000 × g for 10 min in 250-ml bottles (Corning). The upper portion of the filtrate was aspirated, and the remaining 10 mL was triturated and plated in a 10-cm2 tissue culture dish (Becton-Dickinson, Franklin Lakes, NJ). After 7–10 days, the medium was removed, and colonies were then fixed and stained with crystal violet before being enumerated. For the clarification by centrifugation, 250 ml of unprocessed vector supernatant was centrifuged for 10 min at 1,000 × g and then tested by RCDA. For “modified” step-filtration, 250 ml of vector supernatant was applied to a 40/150-μm dual-screen filter followed by a Sepacell 500II filter in series and then tested by RCDA. Vector recovery was determined as described above. Vector recovery following filtration was calculated as the percentage of CD3+/MART-1 tetramer+ cells following PBL transduction. For all samples, the vector titers were normalized to the pre-clarification titer to calculate the percent recovery.
Fluorescence-activated cell sorting analysis
Analysis of the expression of cell surface markers was carried out using fluorescein isothiocyanate– or phycoerythrin-conjugated antibodies directed against CD3 or CD8 (BD Biosciences, San Jose, CA). Fluorescent peptide (MART-127–35)/HLA-A*02 tetramers were purchased from Beckman-Coulter (Fullerton, CA). The relative log fluorescence of live cells was determined using a FACSCanto flow cytometer (BD Biosciences). Analysis was performed using Flowjo software (Treestar Inc., Ashland, OR).
Cytokine release assays
Cytokine release was measured following the incubation of 105 transduced T lymphocytes with 105 tumor target cells in 200 μl for 18 hr at 37°C. Melanoma cell lines HLA-A2+/MART-1+ (mel526 and mel624) and HLA-A2- (mel888 and mel938) were cultured in R10 medium consisting of RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum. Dilutions of culture supernatant were then tested for IFNγ by enzyme-linked immunosorbent assay (Pierce, Rockford, IL).
Statistical analysis
Where appropriate, results were compared using a using a one-way analysis of variance followed by Tukey's multiple comparison analysis between groups.
Results and Discussion
We developed a production process based, in part, on a technology transfer from the Indiana University Vector Production Facility (IUVPF) for the manufacture of cGMP-quality retroviral vectors. The IUVPF vector production process utilizes 50 standard 850-cm2 roller bottles (60 ml of D10 medium per bottle) for culturing PG13 or other packaging cell clones. To allow for greater scalability and improved product yield, without making a significant change to the type of cell culture vessel, we validated 1,700-cm2 expanded surface roller bottles for cell culture and vector production. By using expanded surface roller bottles we could reduce the number of roller bottles required for production by half or double our product yield using similar numbers of bottles. We compared the growth of PG13 cells in standard (850-cm2) or expanded (1,700-cm2) roller bottles cultured in 60 or 120 mL, respectively. Neither glucose consumption (Supplementary Fig. S1A) nor vector transduction efficiency (Supplementary Fig. S1B) at each harvest was significantly different following the cell culture scale-up of PG13 packaging clones into 1,700-cm2 roller bottles. During the harvest period, similar to the standard roller bottle cultures, glucose concentration dropped below 2 g/liter by day 2, requiring the feed volume to be doubled (240 ml of D10 medium). Subsequent harvests had similar titers and twice the volume, compared with earlier harvest using 120 ml per bottle, which resulted in a 50% increase in the vector yield (Supplementary Fig. S1B).
Initial efforts to establish a downstream clarification process to remove residual packaging cells and minimize vector loss were focused on clarification by centrifugation, membrane filtration, or “modified” step-filtration. The “modified” step-filtration setup consisted of a 40/150-μm dual-screen aggregate filter followed by the Sepacell 500II blood filter. The 20-μm filter, previously described in the original step-filtration process, was omitted because it is no longer manufactured. Initial efforts focused on vector recovery following membrane filtration showed that for PVDF membranes, filtration rate (Supplementary Fig. S2A) and vector recovery (Supplementary Fig. S2B) were directly proportional to membrane pore size, with the 0.45-μm pore size being superior to the 0.2-μm pore size (50% and 17% vector recovery, respectively). These data corroborate a previously published report demonstrating a positive correlation between filtration rate through a 0.45-μm (pore size) PVDF filter and vector recovery (Reeves and Cornetta, 2000). Interestingly, when an aliquot of the same vector supernatant was clarified using a 0.2-μm PES filter we observed the fastest flow rate (>250 ml/min) but the lowest level of vector recovery, only 13% (Supplementary Fig. S2A and B). Given that the only difference between these experiments was the physical composition of the membrane (PVDF vs. PES), possible explanations for this discrepancy are that PES is less compatible for clarification of gammaretroviral vectors or the faster flow rate resulted in increased shear forces leading to decreased vector recovery. It is possible that with significant process optimization vector recovery could be improved following 0.2-μm (pore size) PES membrane filtration. However, as our goal was to minimize process optimization, we next compared the membrane filter with the highest percentage of vector recovery (0.45-μm [pore size] PVDF) to clarification by centrifugation and our “modified” step-filtration process. Results showed that “modified” step-filtration provided greater than 60% recovery compared with less than 50% recovery for the 0.45-μm (pore size) PVDF filter. Centrifugation yielded greater than 80% vector recovery (Supplementary Fig. S2C); however, centrifugation is difficult to perform with large volumes of medium and therefore is not considered feasible at large scale. In addition, centrifugation, unlike membrane filtration and “modified” step-filtration, failed to clear all residual packaging cells as measured using the RCDA (Przybylowski et al., 2006) (see Supplementary Table S2 for RCDA validation). Following centrifugation (1 × 106 PG13 cells in 250 ml), greater than 2 × 103 viable PG13 cells were detected in the clarified medium (data not shown). Therefore, subsequent experiments focused on the continued development of the “modified” step-filtration process.
As an estimate of the number of viable cells required to be removed during clarification, viable residual packaging cells were enumerated from individual 850-cm2 roller bottles over 4 successive days of harvest (equivalent to the duration of a production run), and the average cell number per bottle was then used to calculate the estimated number of residual cells for a 50-roller bottle production run. The calculated number of residual packaging cells by day 4 corresponded to approximately 1.3 ± 0.2 × 108 cells that would minimally need to be removed by the “modified” step-filtration process (Supplementary Table S3). Next, we validated the “modified” step-filtration process for removal of residual viable packaging cells and compared it to cell removal data from previously published reports (Table 1). Four sequential 250-ml challenges (cell densities ranged from 1 × 104 to 1 × 107 cells/ml) were applied to individual “modified” step-filtration units (40/150-μm [pore size] and Sepacell 500II filters), which demonstrated the abililty to remove up to 2.78 × 109 PG13 cells. In addition, following a single bulk cell challenge of 2 × 109 PG13 or 293T cells to individual filters, respectively, no viable residual cells were detected post-filtration. We were also able to demonstrate an additional 3 logs of cell removal following freeze/thaw of vector supernatants spiked with viable packaging cells (Supplementary Table S4). Thus, “modified” step-filtration is comparable to step filtration for the removal of residual viable packaging cells from bulk harvest supernatant. Additionally, in combination with a single freeze/thaw cycle, this process, similar to step-filtration, provides up to 12 logs of residual cell removal (Reeves and Cornetta, 2000; Przybylowski et al., 2006).
Table 1.
Validation of Cell Removal by Step-Filtration and Modified Step-Filtration
| Facility | Cell line | Volume assayed (ml) | Total cell challengea | Colonies detected post-filtrationb | LRVc | Ref. |
|---|---|---|---|---|---|---|
| IUVPF | PG13 | 2,100 | 2.78 × 109d | 0/4 | 9.4 | Reeves and Cornetta (2000) |
| Sloan-Kettering Gene | PG13 | 2,600 | (1.02–1.18) × 109e | 0/3 | 8.57 ± 0.50 | Przybylowski et al. (2006) |
| Transfer and Somatic | (1.30–2.22) × 109e | 0/3 | 8.66 ± 0.55 | |||
| Cell Therapy Facility | (1.08–1.16) × 109e | 0/3 | 8.57 ± 0.49 | |||
| SBVPF | PG13 | 1,000 | 2.0 × 109f | 0/3g | 9.3 | |
| 1,000 | 2.78 × 109f | 0/3g | 9.4 | |||
| 293T | 1,000 | 2.0 × 109f | 0/3 | 9.3 |
PG13 or 293T packaging cells were expanded, and cell suspensions were prepared in D10 medium.
Following filtration, the filtrate was divided into aliquots and subjected to the centrifugation RCDA. Cells were allowed to grow for 7–14 days before being inspected visually for colonies or fixed with methanol and stained with crystal violet before being scored.
Log-removal value; inverse log10 of the total cells removed during filtration. Colony detection was based on the detection assay sensitivity of three to five cells.
Individual filter units were sequentially challenged with 250 ml of D10 medium containing increasing concentration of cells ranging from 104 to 107 cells/ml (total cell challenge per filter was 2.78 × 109).
Individual filters were challenged with the indicated number of PG13 cells in D10 medium.
Three separate filters were challenged with PG13 or 293T cells at a concentration of 2 × 106 cell/mL in 1,000 ml of D10 medium.
For each series of experiments, cell challenges were carried out using three individual filter setups (n = 3). Aliquots (250 ml) for each post-filtration spiked medium were tested by RCDA. Results are presented as total colonies detected per filter unit.
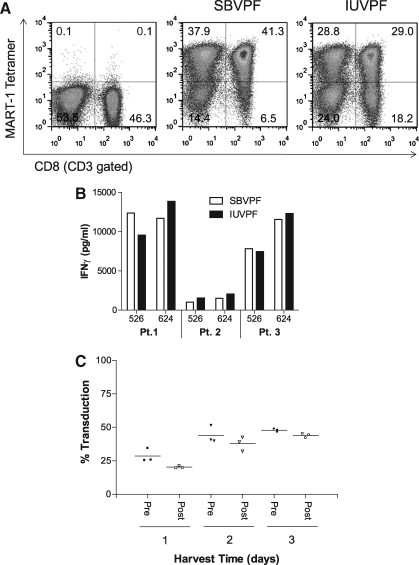
To test our vector production process at scale, a pilot production run utilizing expanded surface roller bottles and “modified” step-filtration was carried in the newly established Surgery Branch Vector Production Facility (SBVPF). The pilot compared SBVPF-generated vector supernatant encoding an HLA-A2-restricted MART-1-reactive TCR (Johnson et al., 2006), referred here as SBVPF DMF5, to a previously manufactured cGMP-quality lot of the same vector produced at IUVPF, referred here as IUVPF DMF5. The same MCB was used to generate both SBVPF and IUVPF vector supernatants. PBLs were transduced with the respective vector supernatants according to our clinical transduction protocol (Fig. 1A). SBVPF DMF5 was able to transduce PBLs to significantly higher levels compared with the IUVPF DMF5 vector supernatant (79% and 58% CD3+/MART-1 tetramer+ cells, respectively). This difference is does not appear to be a result of decreased transduction efficiency due to vector instability, as the IUVPF and SBVPF vectors were able to efficiently transduce PBLs following storage at −80°C for 18–36 months (Supplementary Fig. S3). PBLs transduced with SBVPF and IUVPF DMF5 vector were functionally comparable as measured by IFNγ release following overnight culture with HLA-A2+/MART-1+ tumor targets (Fig. 1B). Vector recovery, as measured by the percentage of MART-1 tetramer+ T cells of the CD3+ population after a single PBL transduction, both pre- and post-modified step-filtration, indicated that there was minimal loss of vector using the modified step-filtration process (Fig. 1C).
FIG. 1.
Pilot vector production run using expanded surface roller bottles and modified step-filtration. For determination of tetramer-positive cells, PBLs were subjected to fluorescence-activated cell sorting. Analysis of the expression of cell surface markers was carried out using fluorescein isothiocyanate– and allophycocyanin-conjugated antibodies directed against CD3 or CD8 (BD Biosciences). Phycoerythrin-conjugated peptide (MART-127–35)/HLA-A*02 tetramers were purchased from Beckman-Coulter. Vector titers were calculated as follows: (percentage tetramer-positive cells) × (total cells) × (dilution factor)/supernatant volume. (A) Representative fluorescence-activated cell sorting plot of PBLs transduced using the clinical protocol and DMF5 vector produced at both SBVPF and IUVPF. The TCR-transduced PBLs were gated on CD3+/CD8+/tetramer+ cells. (B) Representative data from three patients measuring cytokine release following the incubation of 1 × 105 transduced T lymphocytes with 1 × 105 tumor target cells (HLA-A2+/MART-1+, 526 and 624; HLA-A2-, 888 and 938) for 18 hr. Dilutions of supernatant were then tested for IFNγ by enzyme-linked immunosorbent assay (Thermo Scientific). HLA-A2- tumor lines did not elicit any INFγ secretion (data not shown) (C) Vector recovery following clarification by modified step-filtration and single PBL transductions using the SBVPF DMF5 vector supernatants. Post-clarification SBVPF DMF5 supernatants showed no significant loss of vector titer following modified step-filtration (one-way analysis of variance, p ≥ 0.05). Data presented were from three independent patient transductions.
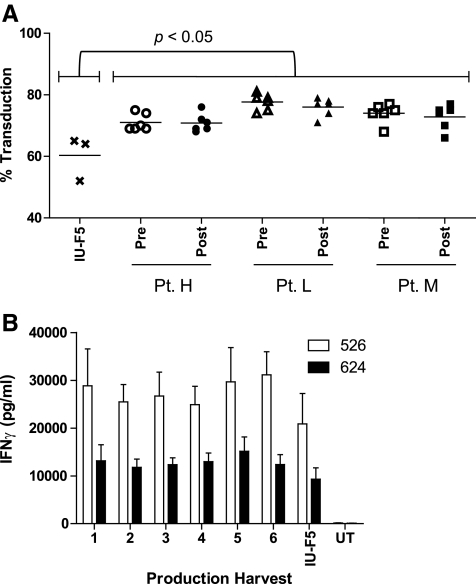
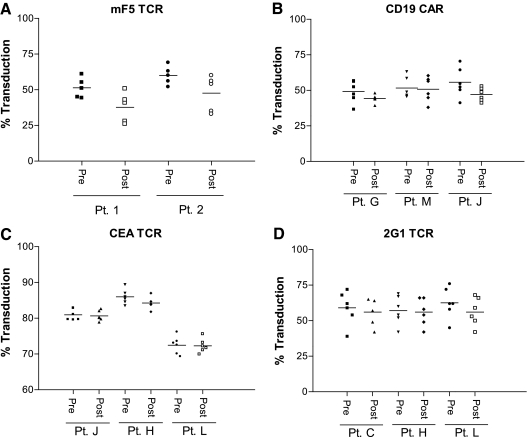
The first clinical production run performed in the SBVPF was for the manufacture of cGMP-quality DMF5 gammaretroviral vector supernatant (Johnson et al., 2006, 2009). As shown in Fig. 2A, the level of SBVPF DMF5 PBL transduction pre- or post-filtration ranged between 66% and 81%, respectively. There was no significant loss of vector product following clarification by “modified” step-filtration for any of the harvests. In addition, although we did not directly test this, others have shown that successive harvests over a 3-day period can be stored at 4°C, pooled, and processed as a single bulk presumably with minimal loss of vector titer (Przybylowski et al., 2006). When tested in the same assay against the IUVPF DMF5 vector product, the SBVPF DMF5 product exhibited significantly higher transduction efficiency (Fig. 2A, p < 0.05); although in a co-culture assay the IUVPF DMF5-transduced PBLs released less IFNγ, the difference was not significant (Fig. 2B) and may suggest that once a threshold level of TCR expression is achieved, the functional benefit of additional TCRs on the cell surface is minimal. It should be pointed out here that we could not directly compare step-filtration to “modified” step-filtration because of the unavailability of the 20-μm (pore size) filter. Therefore, differences in product recovery and transduction efficiency cannot be solely attributed to “modified” step-filtration. To date, we have manufactured four additional clinical-grade gammaretroviral vector products encoding a murine–human hybrid TCR directed against MART-1 (mF5) and TCR directed against carcinoembryonic antigen (CEA) and renal cell carcinoma (2G1), as well as a CAR targeting CD19 (Cohen et al., 2006; Johnson et al., 2006; Wang et al., 2008; Kochenderfer et al., 2009; Parkhurst et al., 2009). Each product was evaluated for transduction efficiency, vector recovery, and viable residual packaging cells following “modified” step-filtration. Following a single transduction of patient PBLs with each of the clinical vectors, no significant decrease in transduction efficiency was observed (Fig. 3). Based on these data, pre- and post-clarification titers were used to determine product recovery, which ranged from a low of 76.8% to a high of 98.8% for the mF5 and CEA TCRs, respectively (Table 2). In addition, each post-clarification production harvest was tested using the RCDA with no evidence of viable residual packaging cells in any of the harvests tested (Table 2).
FIG. 2.
Production of cGMP-quality DMF5 gammaretroviral vector. The cGMP MCB encoding the DMF5 TCR [PG13-F5Af2aB (C162D1)] MCB was used to seed 26 1,700-cm2 expanded surface roller bottles for vector production. Samples of vector supernatant were collected pre- and post-filtration following each harvest. (A) Transduction efficiency for each of the six harvests was determined in PBL of three patients following a single transduction as the percentage of CD3+/MART-1 tetramer+ T cells pre- and post-modified step-filtration with no significant reduction in vector titer. As a control, cGMP-quality DMF5 vector supernatant manufactured at IUVPF using step-filtration was used to transduce the same PBLs (one-way analysis of variance, p ≤ 0.05). (B) PBLs from three individual patients were transduced with each of the six vector harvests and tested in duplicate for IFNγ release following co-culture with HLA-A2+/MART-1+ tumor targets. Transduced PBLs were also tested against HLA-A2− tumor targets and showed no specific IFNγ release (data not shown). Errors bars represent ± SEM (p ≥ 0.05).
FIG. 3.
Gammaretroviral vector recovery following cGMP manufacturing using modified step-filtration. PG13 MCBs encoding the mF5 MART-1-specific TCR (mF5), CD19-specific CAR (CD19 CAR), CEA TCR, and renal cell carcinoma-specific (2G1) TCR were produced and used to generate cGMP-quality gammaretroviral vector supernatant as described. Transduction efficiency pre- and post-modified step-filtration was assessed for each vector. Each harvest was tested against patient PBLs with the percentage transduction (of the CD3+ T-cell population) shown for each harvest. No significant differences were detected between the pre- and post-filtration vector products when analyzed by one-way analysis of variance (p ≥ 0.05). The horizontal line represents the mean. (A) mF5 transduction efficiency was measured as the percentage of CD3+/MART-1 tetramer+ cells. (B) CD19 CAR detection was determined using a biotin-labeled polyclonal goat anti-mouse F(ab)2 (Jackson Immunoresearch, West Grove, PA) followed by staining with streptavidin-phycoerythrin (BD Pharmingen, San Diego, CA). CD19 CAR transduction efficiency was determined as the percentage of CD3+/CD19 CAR+ cells. (C) CEA TCR transduction efficiency was measured as the percentage of CD3+/murine β chain constant region (eBiosciences, San Diego)-positive cells. (D) 2G1 TCR expression was measured as the percentage of CD3+/anti-Vβ2 antibody-positive cells (Beckman Coulter).
Table 2.
Summary of SBVPF Clinical Gammaretroviral Vector Products
| |
|
|
|
Titer (TU/ml, × 105)a |
|
|
|
|---|---|---|---|---|---|---|---|
| Clinical vector | Target antigen | No. of harvests | Production volume (L) | Pre-filtration | Post-filtration | Recovery (%) | Colonies detectedb |
| mF5TCR | MART-1 | 5 | 15 | 5.6 ± 0.6c | 4.3 ± 1.0c | 76.8 | 0/5 |
| F5TCR | MART-1 | 6 | 18 | 7.4 ± 0.2d | 7.3 ± 0.1d | 98.6 | 0/6 |
| CD 19 CAR | CD 19 | 6 | 18 | 5.2 ± 0.6d | 4.7 ± 0.3d | 90.4 | 0/6 |
| CEA TCR | CEA | 6 | 18 | 8.0 ± 0.1d | 7.9 ± 0.1d | 98.8 | 0/6 |
| 2G1 TCR | Renal cell carcinoma | 6 | 18 | 6.0 ± 0.4d | 5.8 ± 0.4d | 96.7 | 0/6 |
Transducing units (TU)/ml = (% tetramer+)(total cells) (dilution factor)/supernatant volume. TCR or CAR expression was determined as described in Materials and Methods.
A 100-ml bag from each harvest was thawed and tested for residual viable packaging cells using the centrifugation RCDA as described in Materials and Methods.
Average titer of all harvests for a given product ± SEM for two separate patient PBLs.
Average titer of all harvests for a given product ± SEM for three separate patient PBLs.
A major issue facing any group interested in retroviral-mediated gene delivery is the time and cost associated with generating cGMP-quality vector, which include generating a packaging cell clone and MCB, vector production, and the subsequent product biosafety testing. In our experience, outsourcing these efforts can take up to a year or more for final product release at a cost of greater than $250,000. To meet an increasing clinical research demand, to better manage cost, and to reduce the time-to-clinic, we established the SBVPF. We validated a retroviral production process, utilizing expanded surface (1,700-cm2) roller bottles and a “modified” step-filtration process for the rapid production of cGMP-quality retroviral vector products. The modifications described herein can decrease the number of cell culture vessels by half or allow for double the product yield if greater quantities of vector supernatant are required. In addition, by using a 40/150-μm (pore size) dual-screen filter and a Sepacell 500II leukocyte reduction filter, we have improved the process by eliminating one of the three filters required for step-filtration with increased vector recovery and levels of cell clearance comparable to those previously reported for clarification by step-filtration. “Modified” step-filtration can also be extended to lentiviral vector production, as this process was shown to clear over 2 × 109 293T cells, typically used during the transient production of lentiviral vectors. In less than 6 months, expanded surface roller bottles and “modified” step-filtration can easily generate 18–70 liters of clarified gammaretroviral vector supernatant for use in the genetic modification of lymphocytes or other cells ex vivo. If product concentration or purification is required, additional process optimization, as well as evaluation of alternative packaging lines, may also be required. Efforts are currently underway to develop a closed system and further improve the cell culture process by moving to a microcarrier-based or suspension culture system in combination with “modified” step-filtration. To date, five clinical-grade products, all of which have undergone rigorous biosafety testing in compliance with current regulatory guidelines, have been generated in the SBVPF, four of which are currently being used in ongoing Phase I/2 clinical trials for patients with metastatic cancer.
Supplementary Material
Acknowledgments
The authors would like to thank Arnold Mixon and Shawn Farid for assistance with flow cytometry.
Author Disclosure Statement
No competing financial interests exist.
References
- Center for Biologics Evaluation and Research. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; Rockville, MD: 1998. [Google Scholar]
- Center for Biologics Evaluation and Research. Guidance for Industry: INDs—Approaches to Complying with CGMP During Phase 1: Draft Guidance. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; Rockville, MD: 2006. [Google Scholar]
- Cohen C.J. Zhao Y. Zheng Z. Rosenberg S.A. Morgan R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K. Matheson L. Ballas C. Retroviral vector production in the National Gene Vector Laboratory at Indiana University. Gene Ther. 2005;12(Suppl. 1):S28–S35. doi: 10.1038/sj.gt.3302613. [DOI] [PubMed] [Google Scholar]
- Ghani K. Cottin S. Kamen A. Caruso M. Generation of a high-titer packaging cell line for the production of retroviral vectors in suspension and serum-free media. Gene Ther. 2007;14:1705–1711. doi: 10.1038/sj.gt.3303039. [DOI] [PubMed] [Google Scholar]
- Ghani K. Wang X. de Campos-Lima P.O. Olszewska M. Kamen A. Rivière I. Caruso M. Efficient human hematopoietic cell transduction using RD114- and GALV-pseudotyped retroviral vectors produced in suspension and serum-free media. Hum. Gene Ther. 2009;20:966–974. doi: 10.1089/hum.2009.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.S. Yu Y.Y.L. Dudley M.E. Zheng Z. Robbins P.F. Li Y. Wunderlich J. Hawley R.G. Moayeri M. Rosenberg S.A. Morgan R.A. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum. Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A. Heemskerk B. Powell D.J., Jr. Cohen C.J. Morgan R.A. Dudley M.E. Robbins P.F. Rosenberg S.A. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A. Morgan R.A. Dudley M.E. Cassard L. Yang J.C. Hughes M.S. Kammula U.S. Royal R.E. Sherry R.M. Wunderlich J.R. Lee C.C. Restifo N.P. Schwarz S.L. Cogdill A.P. Bishop R.J. Kim H. Brewer C.C. Rudy S.F. VanWaes C. Davis J.L. Mathur A. Ripley R.T. Nathan D.A. Laurencot C.M. Rosenberg S.A. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N. Feldman S.A. Zhao Y. Xu H. Black M.A. Morgan R.A. Wilson W.H. Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R. Hughes M.S. Yang J.C. Sherry R.M. Royal R.E. Topalian S.L. Kammula U.S. Restifo N.P. Zheng Z. Nahvi A. de Vries C.R. Rogers-Freezer L.J. Mavroukakis S.A. Rosenberg S.A. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Biologics Research and Review, Center for Biologics Evaluation and Research. Points to Consider in the Production and Testing of New Drugs and Biologicals Produced by Recombinant DNA Technology. U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; Rockville, MD: 1985. [Google Scholar]
- Office of Biologics Research and Review, Center for Biologics Evaluation and Research. Points to Consider in the Use of Cell Lines to Produce Biologicals. U.S. Department of Health and Human Services, Food and Drug Admininstration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; Rockville, MD: 1993. [Google Scholar]
- Onodera M. Yachie A. Nelson D.M. Welchlin H. Morgan R.A. Blaese R.M. A simple and reliable method for screening retroviral producer clones without selectable markers. Hum. Gene Ther. 1997;8:1189–1194. doi: 10.1089/hum.1997.8.10-1189. [DOI] [PubMed] [Google Scholar]
- Parkhurst M.R. Joo J. Riley J.P. Yu Z. Li Y. Robbins P.F. Rosenberg S.A. Characterization of genetically modified T-cell receptors that recognize the CEA:691–699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin. Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzato M. Merten O.W. Blair E.D. Takeuchi Y. Development of a suspension packaging cell line for production of high titre, serum-resistant murine leukemia virus vectors. Gene Ther. 2001;8:737–745. doi: 10.1038/sj.gt.3301457. [DOI] [PubMed] [Google Scholar]
- Przybylowski M. Hakakha A. Stefanski J. Hodges J. Sadelain M. Riviere I. Production scale-up and validation of packaging cell clearance of clinical-grade retroviral vector stocks produced in cell factories. Gene Ther. 2006;13:95–100. doi: 10.1038/sj.gt.3302648. [DOI] [PubMed] [Google Scholar]
- Reeves L. Cornetta K. Clinical retroviral vector production: Step filtration using clinically approved filters improves titers. Gene Ther. 2000;7:1993–1998. doi: 10.1038/sj.gt.3301328. [DOI] [PubMed] [Google Scholar]
- Reeves L. Smucker P. Cornetta K. Packaging cell line characteristics and optimizing retroviral vector titer: The National Gene Vector Laboratory experience. Hum. Gene Ther. 2000;11:2093–2103. doi: 10.1089/104303400750001408. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Aebersold P. Cornetta K. Kasid A. Morgan R.A. Moen R. Karson E.M. Lotze M.T. Yang J.C. Topalian S.L. Merino M.J. Culver K. Miller A.D. Blaese R.M. Anderson W.F. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Dudley M.E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S. Solomon D. Rosenberg S. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J. Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- Wang Q.J. Hanada K.-i. Yang J.C. Characterization of a novel nonclassical T cell clone with broad reactivity against human renal cell carcinomas. J. Immunol. 2008;181:3769–3776. doi: 10.4049/jimmunol.181.6.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C. Huang G.Y. Liu J.H. Production of retrovirus and adenovirus vectors for gene therapy: A comparative study using microcarrier and stationary cell culture. Biotechnol. Prog. 2002;18:617–622. doi: 10.1021/bp020026p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.