Abstract
Transcription factor GATA4 is expressed in Sertoli and Leydig cells and is required for proper development of the murine fetal testis. The role of GATA4 in adult testicular function, however, has remained unclear due to prenatal lethality of mice harboring homozygous mutations in Gata4. To characterize the function of GATA4 in the adult testis, we generated mice in which Gata4 was conditionally deleted in Sertoli cells using Cre-LoxP recombination with Amhr2-Cre. Conditional knockout (cKO) mice developed age-dependent testicular atrophy and loss of fertility, which coincided with decreases in the quantity and motility of sperm. Histological analysis demonstrated Sertoli cell vacuolation, impaired spermatogenesis, and increased permeability of the blood-testis barrier. RT-PCR analysis of cKO testes showed decreased expression of germ cell markers and increased expression of testicular injury markers. Our findings support the premise that GATA4 is a key transcriptional regulator of Sertoli cell function in adult mice.
Keywords: infertility, spermatogenesis, testis, transcription factor
1. Introduction
Transcription factor GATA4 has been implicated in the development and function of the mammalian testis (Viger et al., 2008). During fetal testicular development GATA4 is expressed in pre-Sertoli cells, Sertoli cells, fetal Leydig cells, fibroblast-like interstitial cells, and peritubular myoid cells (Bielinska et al., 2007; Viger et al., 1998). Postnatally, GATA4 is found mainly in Sertoli cells and adult Leydig cells (Ketola et al., 2002; Ketola et al., 1999; LaVoie et al., 2004; McCoard et al., 2001; Oreal et al., 2002). The expression level of GATA4 in postnatal Sertoli cells does not vary with the seminiferous epithelial cycle (Imai et al., 2004), in contrast to cycling GATA1 expression (Yomogida et al., 1994). Promoter studies have identified several groups of putative target genes for GATA4 in testis, including genes associated with early differentiation (Dmrt1, Sry, and Sox9) (Bouma et al., 2007; Lei and Heckert, 2004; Manuylov et al., 2007; Tevosian et al., 2002), cell signaling (Fshr, Lhcgr) (Hermann and Heckert, 2005; Rahman et al., 2004), growth factor and peptide hormone production (Inha, Inhb, Amh) (Feng et al., 2000), steroid biosynthesis (Star, Cyp11a1, Cyp17a1) (Hiroi et al., 2004; Sher et al., 2007; Tevosian et al., 2002; Tremblay et al., 2002; Tremblay and Viger, 2001), and cell-cell interactions (Clmp, Cldn11) (Lui et al., 2007; Sze et al., 2008).
Gata4 knockout mice die by embryonic day (E) 9.5 due to defects in ventral morphogenesis and heart development (Kuo et al., 1997; Molkentin et al., 1997), so the role of this transcription factor in postnatal gonadal function cannot be ascertained from these animals. Analysis of other genetically-engineered mice has shown that interactions between GATA4 and its cofactor, FOG2, are necessary for early testis development (Bouma et al., 2007; Manuylov et al., 2007; Tevosian et al., 2002). Fog2−/− mice and Gata4ki/ki mice, which bear a knock-in mutation that abrogates the interaction of GATA4 with FOG cofactors (Crispino et al., 2001), exhibit identical testicular phenotypes that include decreased testicular Sry expression and aberrant differentiation of early Sertoli and fetal Leydig cells (Manuylov et al., 2007; Tevosian et al., 2002). Consequently, Fog2−/− mice and Gata4ki/ki mice exhibit male-to-female sex reversal (Manuylov et al., 2007; Tevosian et al., 2002). C57Bl/6J (B6) XY mice develop testes if they are heterozygous for a Gata4ki allele, but if the B6 Y chromosome is replaced by the AKR Y chromosome, the mice develop ovaries or ovotestes (Bouma et al., 2007). Studies of chimeric mice derived from Gata4−/− embryonic stem cells indicate that GATA4 plays an integral role in the development of fetal Leydig cells (Bielinska et al., 2007).
Although GATA4 appears to be essential for proper fetal testis development, the role of this transcription factor in adult testicular function remains unclear, owing in part to the prenatal lethality of Gata4ki/ki mice and other homozygous Gata4 mutant mice (Kuo et al., 1997; Molkentin et al., 1997; Narita et al., 1997a; Narita et al., 1997b; Tevosian et al., 2002; Watt et al., 2004). Adult transgenic mice expressing a tetracycline-inducible small interfering RNA directed against Gata4 breed poorly even in the absence of tetracycline induction and have reduced testicular expression of GATA4 target genes, such as Amh and StAR (Thurisch et al., 2009). Here, we examine the impact of GATA4 deficiency on testicular physiology by conditionally deleting Gata4 in the Sertoli cells of adult mice.
2. Materials and Methods
2.1. Experimental mice
Procedures involving mice were approved by the institutional committee for laboratory animal care and were conducted in accordance with the National Research Council’s (NRC) publication Guide for Care and Use of Laboratory Animals. Gata4Fx/Fx mice (also termed Gata4tm1.1Sad/J), which are homozygous for a floxed allele of Gata4 (Oka et al., 2006; Watt et al., 2004), were purchased from The Jackson Laboratory (Bar Harbor, ME) and genotyped as described elsewhere (Oka et al., 2006; Watt et al., 2004). Amhr2Cre/+ mice (also termed B6;129S7-Amhr2tm3(cre)Bhr/Mmnc) were obtained from the MMRRC (Chapel Hill, NC) and genotyped as described elsewhere (Jamin et al., 2002; Jeyasuria et al., 2004). To generate conditional knockout (cKO) mice, Gata4Fx/Fx mice were mated with Amhr2Cre/+ mice, and the resultant Gata4Fx/+;Amhr2Cre/+ mice were mated with Gata4Fx/Fx mice to produce Gata4Fx/Fx;Amhr2Cre/+ mice. All mice had free access to water and standard rodent chow and were exposed to 12 h light/12 h dark photoperiods. At specified times mice were killed by CO2 asphyxiation.
2.2. Tissue isolation and histological analyses
Testes and epididymides were harvested, fixed overnight in Bouin s solution (for morphology) or 4% paraformaldehyde in PBS (for immunostaining), and embedded in paraffin for routine histology. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) reagent.
2.3. Electron microscopy
Mice were anesthetized with sodium pentobarbital (25 mg/100 g body weight i.p.; Abbott Laboratories, North Chicago, IL). Testes were perfused with modified Karnovsky fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer) by the vascular route. Perfused testes were postfixed overnight in the same fixative, rinsed, and fixed in 2% OsO4 for 1 h. The samples were then dehydrated and embedded in epon. Thick sections (1 μm) were stained with toluidine blue and examined by light microscopy to determine which blocks were to be thin-sectioned. Thin sections were stained with uranyl acetate and lead citrate and examined by electron microscopy using an Hitachi EM300 (Hitachi Instruments, Naperville, IL).
2.4. Immunostaining
Paraffin sections were processed for immunoperoxidase staining or immunofluorescence microscopy as described elsewhere (Bielinska et al, 2007). The following primary antibodies were employed: 1) goat anti-GATA4 (sc-1237, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:200 dilution; 2) goat anti-caspase-3 (sc-1225, Santa Cruz Biotechnology, Inc.) at a 1:200 dilution, 3) rabbit anti-GATA1 (sc-13053, Santa Cruz Biotechnology, Inc.) at a 1:200 dilution, and 3) rabbit anti-androgen receptor (AR) (sc-816, Santa Cruz Biotechnology, Inc.) at a 1:200 dilution. The secondary antibodies employed for immunoperoxidase staining were donkey anti-goat biotinylated IgG (Jackson Immunoresearch, West Grove, PA) at a 1:1000 dilution and goat anti-rabbit biotinylated IgG (NEF-813, NEN Life Science, Boston MA), 1:2000 dilution. The avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and diaminobenzidine (Sigma-Aldrich Corp., St. Louis, MO) were used to visualize the bound antibody; slides were then counterstained with toluidine blue. Secondary antibodies used for immunofluorescence microscopy were goat anti-rabbit rhodamine (Jackson Immunoresearch Lab) at a 1:800 dilution.. The slides were then mounted with DAPI fluorescent mounting media (Vector Laboratories, Burlingame, CA).
2.5. Histomorphometric analysis
The number of immunoreactive Sertoli cells per seminiferous tubule was determined by counting 15–20 tubules of each genotype at ages 2.5 mo, 4 mo, and 6 mo (Brehm et al., 2007). Only round tubules were counted. Previous studies have shown that GATA1 and AR are expressed in Sertoli cells in a seminiferous cycle-dependent fashion, mainly in stages VII, VIII, and IX (Yomogida et al., 1994; Zhou et al., 2002). Consequently, only tubules at these stages strongly expressing GATA1 and AR were scored in the analysis.
2.6. Fertility, mating behavior, and endocrine assays
Fertility was evaluated by housing male mice with wild-type B6 females for a minimum of 3 mo and measuring litter sizes. Mating behavior was evaluated by observation of males after introduction of a female into the cage. Cauda epididymides were harvested from 2.5- and 6-mo-old mice, and sperm was collected by placing each cauda into 250 μL of HTF Embryomax (Millipore, Billerica, MA). Media was overlayed with filtered light mineral oil and pre-incubated overnight in 5% CO2 at 37°C. Sperm was gently squeezed out of the cauda using watchmaker s forceps and allowed to capacitate for 15 minutes in 5% CO2 at 37°C. Sperm motility was then evaluated by Computer Assisted Sperm Analysis (Hamilton Thorne Biosciences IVOS, Beverly, MA) with parameters optimized for detection of mouse sperm (Slott et al., 1993). In order to compensate for the large quantitative differences in the number of sperm harvested from mutant and control epididymides, percentage changes in motility parameters were compared rather than absolute numbers (O’Bryan et al., 2008). Serum follicle stimulating hormone (FSH) and inhibin B measurements were made using enzyme immunoassay kits from Endocrine Technologies, Inc. (Newark, CA) and RayBiotech Inc, (Norcross, GA), respectively. Inhibin B enzyme immunoassay: Sensitivity, minimum detectable concentration is estimated to be 1 ng/mL; Intra-Assay: coefficient of variation (CV) < 10%; Inter-Assay: CV < 15%; FSH enzyme immunoassay: Sensitivity, minimum detectable concentration is estimated to be 1 ng/mL; Intra-Assay: CV < 7%; Inter-Assay: CV < 8%.
2.7. Blood-testis barrier permeability
The permeability of the blood-testis barrier was assayed using a surface biotinylation method, as described elsewhere (Komljenovic et al., 2009).
2.8. RT-PCR and genomic PCR analyses
2.8.1. RNA isolation and first strand cDNA synthesis
Total RNA was isolated from testes using TRIzol (Invitrogen, Carlsbad, CA). First strand cDNA was produced with the SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) using 500 ng of RNA as a template.
2.8.2. Qualitative RT-PCR for detection of Cre-mediated recombination in testes
First strand cDNA was subjected to RT-PCR using a forward primer from exon 2 of the Gata4 gene 5′-CCCTACCCAGCCTACATGG-3′ and a reverse primer from exon 7 of the gene 5′-GAGCTGGCCTGCGATGTCTGAGTG-3′ and the following reaction conditions: denaturation program (95°C for 3 min), amplification and quantification program repeated 35 times (95°C for 30 s, 55°C for 1 min, 72°C for 1 min) and a cooling step to 55°C. The intact Gata4Fx allele gave rise to a 782-bp band, whereas the recombined allele yielded a 401-bp band, reflecting deletion of exons 3–5.
2.8.3. Quantitative RT-PCR (qRT-PCR)
Conditions for all qRT-PCR reactions were optimized in a light cycler (Stratagene Mx3005, Agilent, CA) which was programmed as follows: denaturation program (95°C for 10 min), amplification and quantification program repeated 40 times (95°C for 30 s, 60°C for 1 min with single fluorescent measurement), melting program (60°C–95°C with a heating rate of 0.1°C per second and continuous fluorescence measurement) and a cooling step to 55°C. Melting curves did not reveal any significant contamination. The relative expression of target genes was calculated according to the delta-delta method developed by Perkin Elmer Applied Biosystems (Pfaffl, 2001), where amplification efficiencies of target and reference gene are presumed to equal 2. To compensate for variation among runs, the target gene expression was normalized to the expression of β-actin, L19 and Gapdh. Primer pairs used for qRT-PCR assays are listed in Table 1.
Table 1.
Primers for qRT-PCR
| Gene | Oligonucleotide sequence (5′ → 3′) | Size (bp) | Reference |
|---|---|---|---|
| ApoJ | F: AGCAGGAGGTCTCTGACAATG R: GGCTTCCTCTAAACTGTTGAGC |
164 | NM_013492.2 |
| β-actin | F: GCGTGACATCAAAGAGAAGC R: AGGATTCCATACCCAAGAAGG |
187 | NM_007393.2 |
| Cx30.2 | F: TCATGCTGATCTTCCGCATCC R: GAAGCGGTAGTGGGACACC |
149 | NM_178596.2 |
| Gapdh | GCTCACTGGCATGGCCTTCCGTG R: TGGAAGAGTGGGAGTTGCTGTTGA |
200 | NM_008084.2 |
| 17HSDb3 | F: AGGTTCTCGCAGCACCTTTTT R: CATCGCCTGCTCCGGTAATC |
100 | NM_008291.3 |
| L19 | F: GAAATCGCCAATGCCAACTC R: TCTTAGACCTGCGAGCCTCA |
405 | NM_005084 |
| Rhox5 | F: GCAACACCAGTCCCTGAACA R: CAAAATCTCGGTGTCGCAAA |
101 | NM_008818.2 |
| Stra8 | F: GAAGGTGCATGGTTCACCGTGG R: GCTCGATGGCGGGCCTCTG |
161 | NM_009292 |
| Spo11 | F: CGCGTGGCCTCTAGTTCTGAGG R: GGTATCATCCGAAGGCCGACAGAAT |
161 | NM_012046 |
| TNFα | F: CCCTCACACTCAGATCATCTTCT R: GCTACGACGTGGGCTACAG |
61 | NM_013693 |
| Tnp1 | F: GGCGATGATGCAAGTCGAA R: CCACTCTGATAGGATCTTTGGCTTTTGG |
162 | NM_009407 |
2.8.4. Genomic PCR for Sry
Genomic DNA was subjected to PCR using the forward primer 5′-TTGTCTAGAGAGCATGGAGGGCCATGTCAA-3′, the reverse primer 5′-CCACTCCTCTGTGACACTTTAGCCCTCCGA-3′, and the following reaction conditions: denaturation program (95°C for 3 min), amplification program repeated 35 times (95°C for 30 s, 55°C for 1 min, 72°C for 1 min) and a cooling step to 55°C. The PCR product was 273 bp.
2.9. Statistical methods
Testicular weights, seminal vesicle weights, litter sizes, sperm concentration, sperm motility, FSH/inhibin-B levels, Sertoli cell counts, and mRNA levels were analyzed by the Student s t-test. Statistical significance was set at P < 0.05.
3. Results
3.1. Conditional deletion of Gata4 in testicular somatic cells
The Cre/loxP recombination system was used to delete the Gata4 gene in testicular somatic cells. 129;B6 mice homozygous for a floxed allele of Gata4 (Oka et al., 2006; Watt et al., 2004) were crossed with B6 mice harboring an Amhr2-Cre “knock-in” allele (Jamin et al., 2002). Cre-mediated excision of the regions between exons 3 and 5 converts the floxed Gata4 gene into a recombined allele no longer capable of encoding a functional GATA4 protein (Oka et al., 2006; Watt et al., 2004). The Amhr2-Cre transgene, which is expressed in fetal Sertoli cells, postnatal Sertoli cells, and postnatal Leydig cells (Tanwar et al., 2010), has been used previously to target gene deletion in testicular somatic cells, although the extent of deletion in Sertoli vs. Leydig cells has varied depending on the gene studied (Archambeault and Yao, 2010; Boyer et al., 2008; Jamin et al., 2002; Jeyasuria et al., 2004; Pangas et al., 2008; Tanwar et al., 2010; Xu et al., 2007).
Intercrosses of Gata4Fx/Fx and Gata4Fx/+;Amhr2Cre/+ mice yielded Gata4Fx/Fx;Amhr2Cre/+ mice (hereafter referred to as cKO mice) and control Gata4Fx/+;Amhr2Cre/+ mice in the expected Medelian ratio of 1:1. Among cKO mice, the ratio of males to females was 1:1 (n = 50 total), suggesting that there was no male-to-female sex reversal. Underscoring the lack of sex reversal, 0 of 20 phenotypically female cKO mice had evidence of an occult Y chromosome based on genomic PCR for Sry.
3.2. Late-onset testicular atrophy and fertility loss in Gata4 cKO male mice
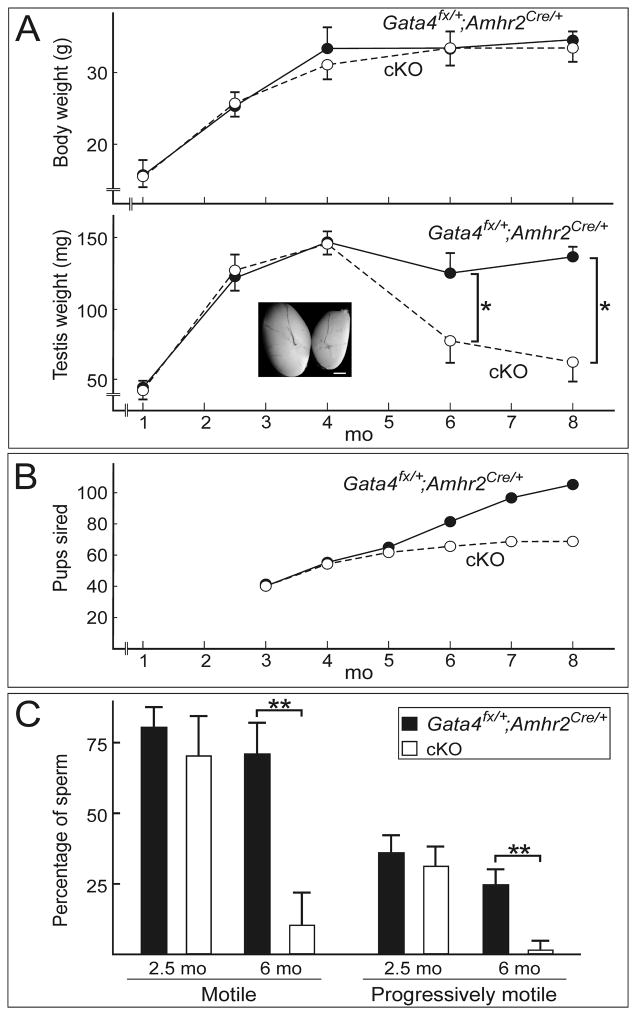
The growth of cKO mice was indistinguishable from that of Gata4Fx/+;Amhr2Cre/+ mice (Fig 1A). The external genitalia and testicular descent of cKO males appeared normal, but cKO mice developed age-dependent testicular atrophy (Fig 1A). By 6 mo of age, the average weight of a cKO testis was 59% of that of a Gata4Fx/+;Amhr2Cre/+ testis (P < 0.05), and by 8 mo of age it was 41% (P < 0.05).
Fig 1. Testicular atrophy and fertility defects in Gata4 cKO mice.
(A) Age-dependent changes in body weight and testicular weight in Gata4Fx/+;Amhr2Cre/+ and cKO mice. (*, P < 0.05). The inset shows testes from 6-mo-old Gata4Fx/+;Amhr2Cre/+ (left) and cKO (right) mice. Bar = 1 mm. (B) Cumulative number of pups sired by Gata4Fx/+;Amhr2Cre/+ (black circles) or cKO (white circles) males mated continuously with wild-type females (n = 5 per group). (C) Sperm motility parameters in 2.5- and 6-mo-old Gata4Fx/+;Amhr2Cre/+ (black bars) and cKO (white bars) mice. (n = 4–6 per group) (**, P < 0.001).
Testicular atrophy in the cKO mice was accompanied by a decline in fertility. Gata4Fx/+;Amhr2Cre/+ mice and cKO mice exhibited similar fertility rates for the first 5 mo of life, followed by an abrupt decrease in the fertility of the cKO animals (Fig 1B). In the eighth month of life, Gata4Fx/+;Amhr2Cre/+ males sired an average of 0.22 + 0.02 pups/day when housed with a wild-type female, whereas cKO males sired 0 + 0 pups/day (P < 0.001). Observation of the mating behavior of cKO males showed that they copulated with females at a rate comparable to wild-type males (data not shown), suggesting that behavioral factors were not the cause of reduced fertilty.
At 6 mo of age caudal epididymal sperm concentrations were significantly lower in the cKO animals than in the Gata4Fx/+;Amhr2Cre/+ mice (5.0 + 8.1 million/mL vs. 32.6 + 25 million/mL; n = 5 per group, P < 0.05). Computer assisted analysis of sperm from the cauda epididymides of cKO mice demonstrated age-dependent, statistically significant (P < 0.001) decreases in both the percentage of total motile sperm and the percentage of progressively motile sperm (Fig 1C).
cKO and Gata4Fx/+;Amhr2Cre/+ mice (ages 5–8 mo) had comparable circulating levels of FSH [0.57 ± 0.18 (n = 12) and 0.55 ± 0.17 ng/mL (n = 6), respectively], implying that the differences in testicular size were not due to reduced FSH secretion by the anterior pituitary gland. Likewise, cKO and Gata4Fx/+;Amhr2Cre/+ mice (ages 5–8 mo) had similar circulating levels of inhibin B, a hormone produced by Sertoli cells that inhibits FSH secretion by the pituitary [136 ± 61 (n = 6) and 146 ± 57 ng/mL (n = 7), respectively]. Seminal vesicle weights, a proxy for circulating androgen levels, did not differ between cKO and Gata4Fx/+;Amhr2Cre/+ at either 2.5 mo of age (9.2 ± 0.0 and 9.2 ± 0.4 mg/g body weight, respectively, n = 5 per group) or 6 mo of age (11.4 ± 2.8 and 11.6 ± 2.4 mg/g body weight, respectively, n = 5 per group). This finding suggests that testosterone production was not impaired in the cKO mice.
3.3. Histological and ultrastructural changes in the testes of Gata4 cKO mice
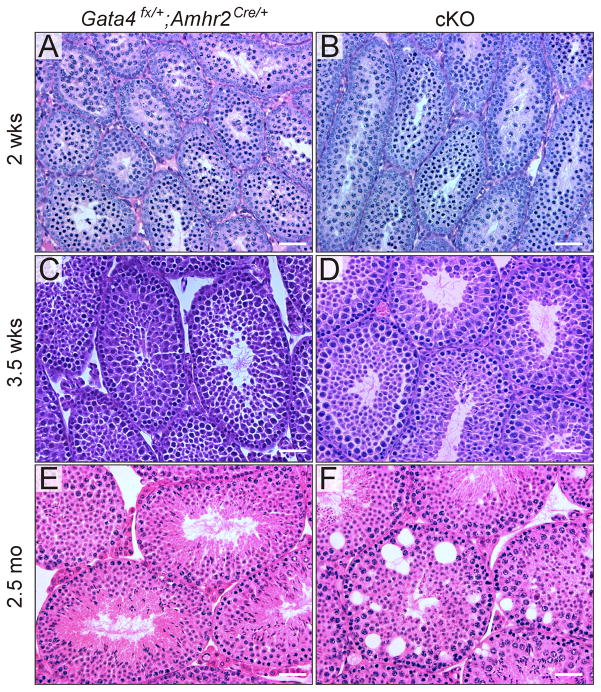
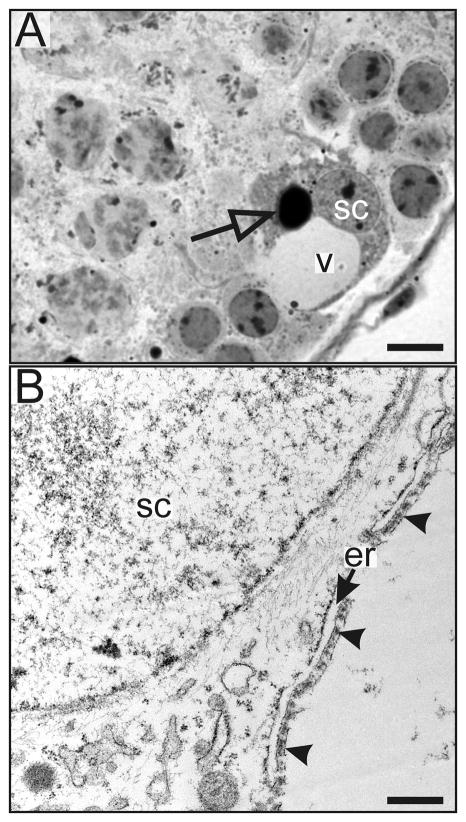
Light microscopic examination of the seminiferous epithelium of cKO mice revealed several age-dependent histological abnormalities (Fig 2). The most obvious of these was epithelial vacuolization, first evident in 2.5-mo-old cKO mice (Fig 2F). Vacuolar degeneration is a well known response of Sertoli cells to injury that has been linked to various types of experimental damage including targeted mutagenesis, toxicants, and cryptorchidism (Berthet et al., 2004; Firestein et al., 2002; Greenbaum et al., 2006; Hellsten et al., 2002; Papaioannou et al., 2009; Vigil and Bustos-Obregon, 1985; Weiss et al., 2003). Examination of semithin (Fig 3A) and ultrathin (Fig 3B) sections confirmed that the vacuoles were associated with Sertoli cells. The vacuoles were surrounded by membrane and sometimes contained multivesicular body-like structures inside (data not shown). The vacuolar membranes were often lined by actin filament bundles with endoplasmic reticulum cisternae located beneath them (Fig 3B); this structure is characteristic of the adhesion complexes that attach germ cells to the seminiferous epithelium and suggests that the vacuoles reflect germ cell loss (Hellsten et al., 2002; Hess and Franca, 2005). Another morphologic abnormality evident in the Sertoli cells of Gata4 cKO mice was the accumulation of large lipid droplets (Fig 3A), which has been reported to accompany germ cell destruction (Setchell and Breed, 2006).
Fig 2. Sertoli cell vacuolization in Gata4 cKO mice.
Testis from Gata4Fx/+;Amhr2Cre/+ (A,C,E) and Gata4 cKO (B,D,F) mice of the specified ages were fixed with Bouin s solution, and paraffin sections were stained with H&E. Note the presence of vacuoles in the seminiferous epithelium of 2.5-mo-old cKO mice (F). Bars = 50 μm.
Fig 3. Representative light and electron micrographs of testicular sections from Gata4 cKO mice.
A 2.5-mo-old cKO mouse was perfusion-fixed, and testis tissue was processed for light and electron microscopy. (A) Semithin section stained with toluidine blue. Note the presence of a large vacuole associated with the Sertoli cell. The open arrow highlights a lipid droplet. (B) Electron micrograph of a Sertoli cell. Note that the vacuolar membrane is lined by actin filament bundles (arrowheads) with endoplasmic reticulum cisternae (arrow) located beneath. Bars = 10 μm (A), 250 nm (B). Abbreviations: er, endoplasmic reticulum; sc, Sertoli cell nuclei; v, vacuole.
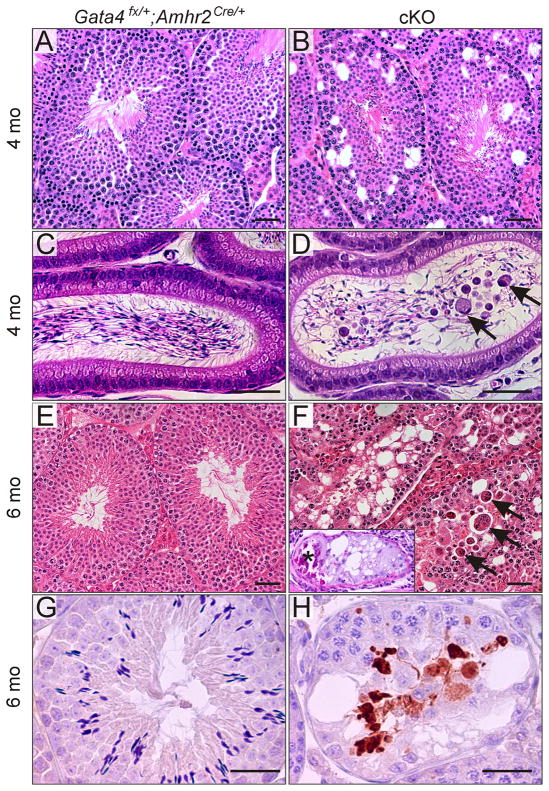
In 4-mo-old cKO mice, there was evidence of not only vacuolar degeneration (Fig 4A vs. B) but also disrupted spermatogenesis. Spermatocytes and spermatids were prematurely released from the seminiferous epithelium and sloughed into the epididymis (Fig 4C vs. D). By 6 mo of age, there was progressive atrophy of tubules at all stages of the seminiferous epithelial cycle (Fig 4E vs. F). Tubular atrophy reflected marked depletion of germ cells in the seminiferous tubules of cKO mice. The remaining germ cells were inappropriately positioned within the seminiferous epithelium. Multinucleated giant cells, syncytia of degenerating spermatids (Fotovati et al., 2006; Singh and Chakravarty, 2001; Yan et al., 2003), were present in the seminiferous tubules of cKO testis (Fig 4F, arrows). Dystrophic calcification was evident in severely degenerated tubules of the 6-mo-old cKO mice (Fig 4F, inset). Caspase-3 immunohistochemistry demonstrated increased apoptosis of germ cells in cKO mice, particularly germ cells in the later stages of development (Fig 4G vs. H). No overt histological abnormalities were seen in the testicular interstitium of cKO mice (Fig 4A vs. B).
Fig 4. Histological abnormalities in the seminiferous epithelium and epididymides of Gata4 cKO mice.
Testis (A,B,E-H) and epididymides (C, D) from 4-mo-old (A-D) and 6-mo-old (E-H) mice of the specified genotypes were fixed, and paraffin sections were stained with H&E (A-F), PAS (F, inset), or caspase-3 antibody (G,H). Compared to Gata4Fx/+;Amhr2Cre/+ mice, the seminiferous tubules of cKO mice exhibited vacuolization (B,F), premature release of germ cells from the seminiferous epithelium with sloughing into the epididymis (D, arrows), inappropriate positioning of germ cells within the seminiferous epithelium (F), a decreased number of elongated spermatids (F), multinucleated giant spermatogenic cells (F, arrows), dystrophic calcification (F, inset,*), and increased germ cell apoptosis based on caspase-3 immunostaining (H). Bars = 50 μm.
3.4. Age-dependent loss of Gata4 expression in cKO mice
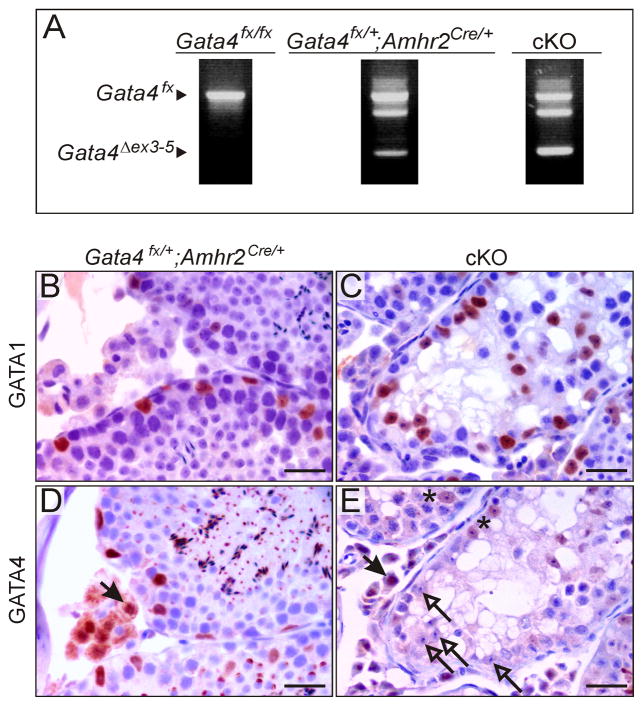
To verify deletion of Gata4 by Amhr2-Cre in the mouse testis, we employed an RT-PCR strategy that distinguishes transcripts derived from the intact (Gata4Fx) and recombined (Gata4Δex3–5) alleles. As shown in Fig 5A, RT-PCR analysis of control RNA from testes of Gata4Fx/Fx mice yielded a single 782 bp band corresponding to the intact Gata4Fx allele. In contrast, analysis of RNA from the testes of cKO and Gata4Fx/+;Amhr2Cre/+ mice (1-mo-old) yielded a 401 bp band indicative of the recombined allele.
Fig 5. Cre-LoxP mediated deletion of Gata4 in the mouse testis.
(A) Testis RNA was isolated from 1-mo-old mice of the specified genotypes was subjected to RT-PCR analysis with primers that distinguish the intact floxed Gata4 allele (Gata4Fx) from the recombined allele lacking exons 3 to 5 (Gata4Δex3–5). Note that a transcript derived from the recombined allele is seen in the Gata4Fx/+;Amhr2Cre/+ and cKO mice but not the control (Gata4Fx/Fx) mice. (B-E) Testis from 6-mo Gata4Fx/+;Amhr2Cre/+ or cKO mice were sectioned and subjected to immunoperoxidase staining to visualize the distribution of GATA1 and GATA4 within this tissue. Note that the number of GATA1 immunoreactive cells is similar in the Gata4Fx/+;Amhr2Cre/+ and cKO mice, whereas the number of GATA4-immunoreactive Sertoli cells is reduced in the testes of cKO mice. Closed arrows highlight GATA4 immunoreactive Leydig cells. Open arrows indicate GATA4-negative Sertoli cells. Asterisks highlight Sertoli cells that are weakly immunoreactive for GATA4, presumably reflecting recombination of a single floxed allele. The punctate perinuclear staining in germ cells reflects direct unspecific binding of the goat anti-mouse secondary antibody to acrosomal components (McClusky et al., 2009). Bar = 50 μm.
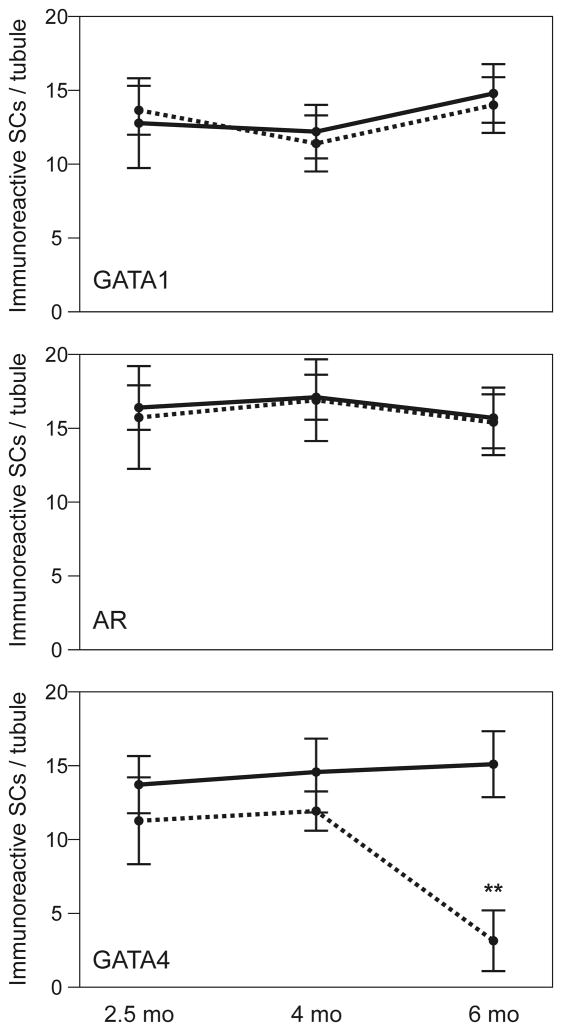
To show that the loss of GATA4 protein in Sertoli cells correlates temporally with changes in the testes of cKO mice, adjacent sections of testis from 2.5-, 4-, and 6-mo-old cKO and Gata4Fx/+;Amhr2Cre/+ mice were stained with antibody against GATA4, GATA1, or AR. Sample photomicrographs from the analysis are shown in Fig 5B–E, and age-dependent changes in Sertoli cell immunoreactivity are summarized in Fig 6. The number of GATA4-immunoreactive Sertoli cells in the testes of cKO mice declined dramatically between 4 and 6 mo of age (P < 0.0001), whereas the number of GATA1- and AR-immunoreactive Sertoli cells remained constant between 2.5 and 6 mo of age. Although the Sertoli cells of older cKO mice had reduced expression of GATA4, nearly all of the Leydig cells in these mice appeared to express GATA4 protein (Fig 5E). We conclude that the histological and functional changes in older cKO mice correlate with loss of GATA4 protein in Sertoli but not in Leydig cells.
Fig 6. Age-dependent loss of GATA4 immunoreactivity in the Sertoli cells of cKO mice.
Adjacent sections of testis from 2.5-, 4-, and 6-mo-old cKO (dashed lines) and Gata4Fx/+;Amhr2Cre/+ (solid lines) mice were stained with antibody against GATA4, GATA1, or AR. The number of immunoreactive Sertoli cells was quantified as described in Methods. Note that the number of GATA4 immunoreactive cells declines dramatically between 4 and 6 mo of age, whereas the number of GATA1- and AR-immunoreactive Sertoli cells remains constant.
3.5. Increased permeability of the blood-testis barrier in Gata4 cKO mice
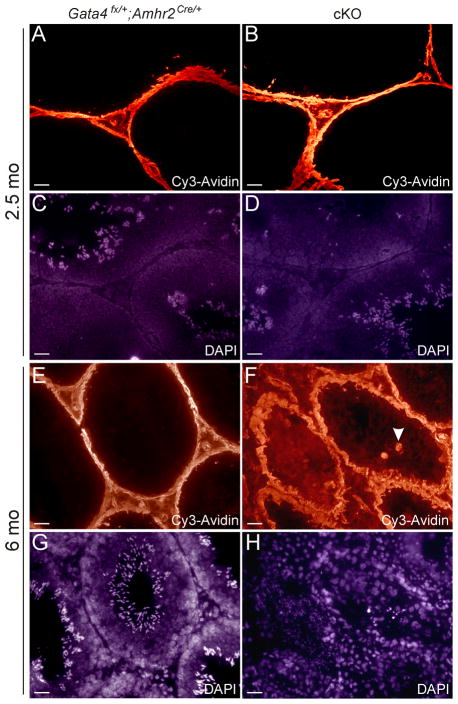
One of the functions of Sertoli cells is to maintain the blood-testis barrier (BTB). We therefore hypothesized that loss of GATA4 expression in Sertoli cells might disrupt the integrity of the BTB. The permeability of the BTB was assessed by injecting a surface biotinylation reagent into the testicular interstitium and detecting its penetration into the seminiferous tubules with fluorescently-labeled streptavidin (Fig 7A–H). The BTB appeared intact mice in 2.5-mo-old Gata4Fx/+;Amhr2Cre/+ and cKO mice; biotinylation was restricted to testicular interstitium and the basal compartment of the seminiferous tubules. In 6-mo-old cKO mice, however, biotinylation was detected along the Sertoli cell plasma membranes from the basement membrane to the tubular lumen (Fig 7F). Furthermore, biotinylated germ cells, including multinuclear giant cells (Fig 7F, arrowhead), were evident in seminiferous tubules of 6-mo-old cKO mice. In contrast, the BTB of 6-mo-old Gata4Fx/+;Amhr2Cre/+ mice appeared intact (Fig 7E). We conclude that older cKO mice develop increased permeability of the BTB with advancing age.
Fig 7. Increased permeability of the BTB in the testes of Gata4 cKO mice.
Testes of anesthetized 2.5-mo-old (A-D) or 6-mo-old (E-H) mice of the specified genotypes were injected with a surface biotinylation reagent and then harvested 30 min later. Cryosections were stained with Cy3-strepavidin (A,B,E,F) and DAPI (C,D,G,H) and then subjected to fluorescence microscopy. Biotinylation was restricted to the interstitium and the basal compartment in 2.5-mo-old Gata4Fx/+;Amhr2Cre/+ mice (A), 2.5-mo-old cKO mice (B), and 6-mo-old Gata4Fx/+;Amhr2Cre/+ mice (E). In contrast, surface biotin labelling was seen in cells within the seminiferous tubules of the 6-mo-old cKO mice (F), including multinucleated giant cells (arrowhead). Note also the disorganized cytoarchitecture of the 6-mo-old cKO testis (H) compared to the Gata4Fx/+;Amhr2Cre/+ control (G). Bars = 50 μm.
3.6. Changes in gene expression in the testis of Gata4 cKO mice
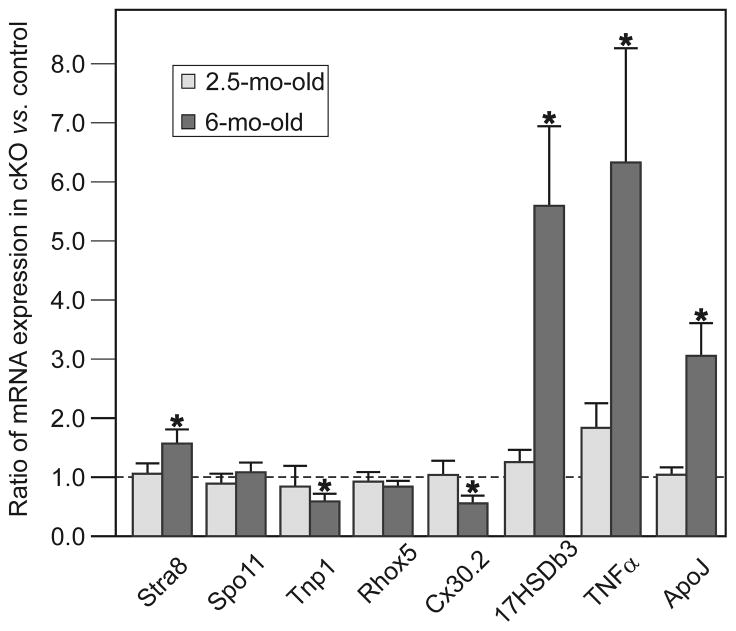
To determine the impact of GATA4 deficiency on gene expression, testes from 2.5- and 6-mo-old cKO mice and Gata4Fx/+;Amhr2Cre/+ mice were subjected to qRT-PCR analysis. Each bar in Fig 8 depicts the ratio of mRNA levels in cKO vs. Gata4Fx/+;Amhr2Cre/+ mice, normalized to the housekeeping gene β-actin. Comparable results were obtained when the data was normalized to two other housekeeping genes, ribosomal protein L19 and Gapdh (data not shown). In keeping with the histological and BTB analyses, differences in relative gene expression between cKO mice and Gata4Fx/+;Amhr2Cre/+ mice were more pronounced in testes from 6-mo-old mice than 2.5-mo-old mice.
Fig 8. Relative mRNA expression in testis from cKO versus Gata4Fx/+;Amhr2Cre/+ mice.
Testes from 2.5-mo-old and 6-mo-old were subjected to qRT-PCR analysis. Each bar depicts the ratio of mRNA expression in cKO vs. Gata4Fx/+;Amhr2Cre/+ testes (mean + SEM, n = 4–6 ) normalized to β-actin. A ratio of 1 (shown by the dotted line) indicates equivalent expression in the two genotypes. (*, P < 0.05 ).
First, we examined a series of markers corresponding to different stages of germ cell development. Expression of Stra8 mRNA, which encodes a premeiotic germ cell-specific cytoplasmic protein found mainly in spermatogonia (Yelick et al., 1989), was increased in 6-mo-old cKO mice, suggesting enrichment of this early progenitor population. Levels of Spo11 mRNA, a marker of spermatocytes (Oulad-Abdelghani et al., 1996), were comparable in the cKO mice and Gata4Fx/+;Amhr2Cre/+ mice. Spo11 encodes a protein that creates double-strand DNA breaks during the early stages of meiosis. Tnp1 mRNA, which encodes a basic protein expressed during the brief period when histones are being replaced by protamines in the haploid stage of spermatogenesis (Shannon et al., 1999), was significantly reduced in the 6-mo-old cKO mice. These results, coupled with the aforementioned histological findings, suggest that testicular atrophy in the cKO mice reflects depletion of germ cells in the later stages of development, mainly in spermiogenesis.
Expression of Rhox5, an androgen-dependent homeobox gene expressed in Sertoli cells that promotes germ cell survival (Bailey et al., 2002), was slightly reduced in the cKO mice, although this did not reach statistical significance. Previous studies have shown that Rhox5 is transcriptionally activated by GATA factors including GATA4 (Bhardwaj et al., 2008). Expression of connexin 30.2 (Cx30.2), a known target of GATA4 in the heart (Munshi et al., 2009), was significantly decreased in the cKO testis. Immunolocalization studies (Nielsen and Kumar, 2003) suggest that Cx30.2 may mediate interactions between Sertoli and germ cells.
Expression of the Leydig cell marker 17HSDb3, the enzyme that converts androstenedione into testosterone, was increased in the cKO testis, consistent with Leydig cell enrichment in the cKO testis. Expression of ApoJ (clusterin), a glycoprotein secreted by Sertoli cells in response to injury (Bailey et al., 2002), was increased in the cKO mice. It has been proposed that ApoJ functions in part as a “biological detergent” that aids in the degradation of hydrophobic molecules that accumulate during tissue damage (Bailey and Griswold, 1999). Another injury marker, TNFα was significantly upregulated in the cKO testis. In the testis TNFα is produced mainly by spermatocytes and spermatids (Siu et al., 2003). At the low concentrations found in normal testis, TNFα functions as a survival factor (Suominen et al., 2004); however, this cytokine is thought to act as a proapoptotic factor in inflammatory and genetic disorders affecting the testis (Guazzone et al., 2009). The increase of TNFα expression in the cKO mice could be due to increased expression of this cytokine in the testicular macrophages, as part of an inflammatory response to the damage occurring within the seminiferous epithelium and the disruption of the BTB.
4.1. Discussion
Sertoli cells provide a structural and biochemical microenviroment that facilitates the efficient and autonomous development of germ cells into spermatozoa (Griswold, 2005). Mutagenesis of genes associated with Sertoli cell function generally leads to lowered sperm counts but does not totally block spermatogenesis; examples include the genes encoding FSH-β (Layman, 2000), FSH-R, (Layman, 2000), ApoJ (Bailey et al., 2002), fyn (Maekawa et al., 2002), Sox8 (O’Bryan et al., 2008), and Bclw (Russell et al., 2001). The data presented here show that GATA4 is another factor that controls adult Sertoli cell function. Young adult Gata4 cKO mice produced motile sperm and sired pups. By 6 mo of age, however, cKO mice developed testicular atrophy and loss of fertility. Sperm counts were reduced, and those sperm that reached the epididymides had significantly reduced motility. Sertoli cell vacuolization and progressive tubule destruction were noted at all stages of the seminiferous epithelial cycle with attendant germ cell loss and compromised integrity of the BTB. Collectively, these findings indicate that GATA4 is a critical transcriptional regulator of adult Sertoli cell function and is required for their proper physical and paracrine interactions with germ cells.
Previous studies of mice harboring mutant Gata4 alleles suggested that GATA4 is required for Sry expression, testicular cord formation, and all subsequent steps in testicular development (Bouma et al., 2007; Manuylov et al., 2007; Tevosian et al., 2002). Consequently, male-to-female sex reversal is a hallmark of such mutants. Although Amhr2-Cre is expressed in prenatal Sertoli cells (Tanwar et al., 2010), we did not observe an overt defect in embryonic or early postnatal testicular development in Gata4 cKO mice. The absence of a prenatal testicular phenotype has been observed with other conditional knockout mice generated with Amhr2-Cre (Boyer et al., 2008; Tanwar et al., 2010) and is presumed to reflect delayed or inefficient Amhr2-Cre mediated recombination in the embryonic and fetal testes. Alternatively, there might be selective pressure against progenitor cells that undergo Cre-mediated deletion of Gata4 during early gonadogenesis.
Since Amhr2-Cre is expressed in adult Leydig cells (Boyer et al., 2008; Jeyasuria et al., 2004; Tanwar et al., 2010), the finding of normal-appearing, GATA4-immunoreactive Leydig cells in the testis of cKO mice was unexpected. One possible explanation is inefficient Cre-mediated recombination of the Gata4Fx gene in Leydig cells. Another possibility is that Leydig cells in which GATA4 expression is silenced through Cre-mediated recombination are replaced through proliferation and differentiation of interstitial progenitor cells with at least one intact Gata4Fx allele (Boyer et al., 2008).
The phenotype of Gata4 cKO mice resembles that of mice with sustained activation of Wnt/β-catenin signaling in Sertoli cells through conditional mutagenesis of the β-catenin gene by Amhr2-Cre (Boyer et al., 2008; Tanwar et al., 2010). Common phenotypic features include age-dependent testicular atrophy, impaired fertility, Sertoli cell vacuolation, and impaired spermatogenesis with sloughing of spermatocytes and spermatids into the seminiferous tubule lumen, formation of multinucleated giant cells, and dystrophic calcification. This raises the possibility that conditional mutagenesis of Gata4 in Sertoli cells leads to aberrant Wnt/β-catenin signaling. In embryonic female mice, germline Gata4 loss-of-function mutations have been linked to excess expression of Dkk1, a secreted inhibitor of the canonical Wnt/β-catenin pathway, a signaling cascade critical for female gonadogenesis (Manuylov et al., 2008; Tevosian and Manuylov, 2008). Perhaps in adult male mice, conditional loss-of-function mutations in Gata4 elicit the opposite effect (i.e., increased signaling through the Wnt/β-catenin pathway). Future studies will explore this possibility and attempt to delineate the mechanisms underlying germ cell loss in the Gata4 cKO mice. It will also be of interest to determine whether GATA4 mutations underlie hypospermatogenesis phenotypes seen in men.
5. Conclusions
Conditional deletion of Gata4 mediated by Amhr2-Cre causes Sertoli cell dysfunction and impaired fertility in adult male mice. Our findings, together with those of other investigators, support the notion that GATA4 is a key transcriptional regulator of Sertoli cell function in both fetal and adult mice.
Acknowledgments
Grant Support: NIH DK52574 and DK75618; Academy of Finland; and the Sigrid Juselius Foundation.
We thank members of the DDRCC Histology Core and Karen Green for their assistance.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest that may be perceived as prejudicing the impartiality of the research reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archambeault DR, Yao HH. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci U S A. 2010;107:10526–10531. doi: 10.1073/pnas.1000318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R, Griswold MD. Clusterin in the male reproductive system: localization and possible function. Mol Cell Endocrinol. 1999;151:17–23. doi: 10.1016/s0303-7207(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- Berthet C, Morera AM, Asensio MJ, Chauvin MA, Morel AP, Dijoud F, Magaud JP, Durand P, Rouault JP. CCR4-associated factor CAF1 is an essential factor for spermatogenesis. Mol Cell Biol. 2004;24:5808–5820. doi: 10.1128/MCB.24.13.5808-5820.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A, Rao MK, Kaur R, Buttigieg MR, Wilkinson MF. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol Cell Biol. 2008;28:2138–2153. doi: 10.1128/MCB.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007;236:203–213. doi: 10.1002/dvdy.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Washburn LL, Albrecht KH, Eicher EM. Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci U S A. 2007;104:14994–14999. doi: 10.1073/pnas.0701677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D. Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biol Reprod. 2008;79:475–485. doi: 10.1095/biolreprod.108.068627. [DOI] [PubMed] [Google Scholar]
- Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lecureuil C, Steger K, et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839–844. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZM, Wu AZ, Zhang Z, Chen CL. GATA-1 and GATA-4 transactivate inhibin/activin beta-B-subunit gene transcription in testicular cells. MolEndocrinol. 2000;14:1820–1835. doi: 10.1210/mend.14.11.0549. [DOI] [PubMed] [Google Scholar]
- Firestein R, Nagy PL, Daly M, Huie P, Conti M, Cleary ML. Male infertility, impaired spermatogenesis, and azoospermia in mice deficient for the pseudophosphatase Sbf1. J Clin Invest. 2002;109:1165–1172. doi: 10.1172/JCI12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotovati A, Nakayama K, Nakayama KI. Impaired germ cell development due to compromised cell cycle progression in Skp2-deficient mice. Cell Div. 2006;1:4. doi: 10.1186/1747-1028-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD. Perspective on the function of Sertoli cells. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. San Diego: Elsevier; 2005. pp. 15–40. [Google Scholar]
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech. 2009;72:620–628. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- Hellsten E, Bernard DJ, Owens JW, Eckhaus M, Suchy SF, Nussbaum RL. Sertoli cell vacuolization and abnormal germ cell adhesion in mice deficient in an inositol polyphosphate 5-phosphatase. Biol Reprod. 2002;66:1522–1530. doi: 10.1095/biolreprod66.5.1522. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Heckert LL. Silencing of Fshr occurs through a conserved, hypersensitive site in the first intron. Mol Endocrinol. 2005;19:2112–2131. doi: 10.1210/me.2004-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Franca LR. Structure of Sertoli Cells. In: Skinner MK, Griswold MD, editors. Sertoli cell biology. Amsterdam; Boston: Elsevier Academic Press; 2005. pp. 19–40. [Google Scholar]
- Hiroi H, Christenson LK, Strauss JF., III Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: temporal and spatial changes in transcription factor binding and histone modification. Mol Cell Endocrinol. 2004;215:119–126. doi: 10.1016/j.mce.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Imai T, Kawai Y, Tadokoro Y, Yamamoto M, Nishimune Y, Yomogida K. In vivo and in vitro constant expression of GATA-4 in mouse postnatal Sertoli cells. Mol Cell Endocrinol. 2004;214:107–115. doi: 10.1016/j.mce.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, de Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- Ketola I, Anttonen M, Vaskivuo T, Tapanainen JS, Toppari J, Heikinheimo M. Developmental expression and spermatogenic stage specificity of transcription factors GATA-1 and GATA-4 and their cofactors FOG-1 and FOG-2 in the mouse testis. Eur J Endocrinol. 2002;147:397–406. doi: 10.1530/eje.0.1470397. [DOI] [PubMed] [Google Scholar]
- Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999;140:1470–1480. doi: 10.1210/endo.140.3.6587. [DOI] [PubMed] [Google Scholar]
- Komljenovic D, Sandhoff R, Teigler A, Heid H, Just WW, Gorgas K. Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice. Cell Tissue Res. 2009;337:281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anadappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- LaVoie HA, McCoy GL, Blake CA. Expression of the GATA-4 and GATA-6 transcription factors in the fetal rat gonad and in the ovary during postnatal development and pregnancy. Mol Cell Endocrinol. 2004;227:31–40. doi: 10.1016/j.mce.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Layman LC. Mutations in the follicle-stimulating hormone-beta (FSH beta) and FSH receptor genes in mice and humans. SeminReprodMed. 2000;18:5–10. doi: 10.1055/s-2000-13470. [DOI] [PubMed] [Google Scholar]
- Lei N, Heckert LL. Gata4 regulates testis expression of Dmrt1. Mol Cell Biol. 2004;24:377–388. doi: 10.1128/MCB.24.1.377-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Wong EW, Guan Y, Lee WM. Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A. J Cell Physiol. 2007;211:638–648. doi: 10.1002/jcp.20970. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Toyama Y, Yasuda M, Yagi T, Yuasa S. Fyn tyrosine kinase in Sertoli cells is involved in mouse spermatogenesis. Biol Reprod. 2002;66:211–221. doi: 10.1095/biolreprod66.1.211. [DOI] [PubMed] [Google Scholar]
- Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. The regulation of Sox9 gene expression by the GATA4/FOG2 transcriptional complex in dominant XX sex reversal mouse models. Dev Biol. 2007;307:356–367. doi: 10.1016/j.ydbio.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Devel. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- McClusky LM, Patrick S, Barnhoorn IE, van Dyk JC, de Jager C, Bornman MS. Immunohistochemical study of nuclear changes associated with male germ cell death and spermiogenesis. J Mol Histol. 2009;40:287–299. doi: 10.1007/s10735-009-9240-3. [DOI] [PubMed] [Google Scholar]
- McCoard SA, Wise TH, Fahrenkrug SC, Ford JJ. Temporal and spatial localization patterns of Gata4 during porcine gonadogenesis. Biol Reprod. 2001;65:366–374. doi: 10.1095/biolreprod65.2.366. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Munshi NV, McAnally J, Bezprozvannaya S, Berry JM, Richardson JA, Hill JA, Olson EN. Cx30.2 enhancer analysis identifies Gata4 as a novel regulator of atrioventricular delay. Development. 2009;136:2665–2674. doi: 10.1242/dev.038562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson D. Cardiomyocyte differentiation by GATA-4 deficient embryonic stem cells. Devel. 1997a;122:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Developmental Biology. 1997b;189:270–274. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 2003;540:151–156. doi: 10.1016/s0014-5793(03)00252-7. [DOI] [PubMed] [Google Scholar]
- O’Bryan MK, Takada S, Kennedy CL, Scott G, Harada S, Ray MK, Dai Q, Wilhelm D, de Kretser DM, Eddy EM, et al. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol. 2008;316:359–370. doi: 10.1016/j.ydbio.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circulation Research. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- Oreal E, Mazaud S, Picard JY, Magre S, Carre-Eusebe D. Different patterns of anti-Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn. 2002;225:221–232. doi: 10.1002/dvdy.10153. [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, Schaad O, Vejnar CE, Kuhne F, Descombes P, Zdobnov EM, et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol. 2009;326:250–259. doi: 10.1016/j.ydbio.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman NA, Kiiveri S, Rivero-Muller A, Levallet J, Vierre S, Kero J, Wilson DB, Heikinheimo M, Huhtaniemi I. Adrenocortical tumorigenesis in transgenic mice expressing the inhibin à-subunit promoter/SV40 virus T-antigen transgene: Relationship between ectopic expression of luteinizing hormone receptor and transcription factor GATA-4. Mol Endocrinol. 2004;18:2553–2569. doi: 10.1210/me.2002-0282. [DOI] [PubMed] [Google Scholar]
- Russell LD, Warren J, Debeljuk L, Richardson LL, Mahar PL, Waymire KG, Amy SP, Ross AJ, MacGregor GR. Spermatogenesis in Bclw-deficient mice. Biol Reprod. 2001;65:318–332. doi: 10.1095/biolreprod65.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP, Breed WG. Anatomy, vasculature, and innervation of the male reproductive tract. In: Neill JD, Challis JRG, de Kretser DM, Pfaff DW, Richards JS, Plant TM, Wassarman PM, editors. Knobil and Neill’s Physiology of Reproduction. St. Louis: Elsevier; 2006. pp. 771–826. [Google Scholar]
- Shannon M, Richardson L, Christian A, Handel MA, Thelen MP. Differential gene expression of mammalian SPO11/TOP6A homologs during meiosis. FEBS Lett. 1999;462:329–334. doi: 10.1016/s0014-5793(99)01546-x. [DOI] [PubMed] [Google Scholar]
- Sher N, Yivgi-Ohana N, Orly J. Transcriptional regulation of the cholesterol side chain cleavage cytochrome P450 gene (CYP11A1) revisited: binding of GATA, cyclic adenosine 3′,5′-monophosphate response element-binding protein and activating protein (AP)-1 proteins to a distal novel cluster of cis-regulatory elements potentiates AP-2 and steroidogenic factor-1-dependent gene expression in the rodent placenta and ovary. Mol Endocrinol. 2007;21:948–962. doi: 10.1210/me.2006-0226. [DOI] [PubMed] [Google Scholar]
- Singh SK, Chakravarty S. Effect of nitrofurazone on the reproductive organs in adult male mice. Asian J Androl. 2001;3:39–44. [PubMed] [Google Scholar]
- Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Slott VL, Suarez JD, Poss PM, Linder RE, Strader LF, Perreault SD. Optimization of the Hamilton-Thorn computerized sperm motility analysis system for use with rat spermatozoa in toxicological studies. Fundam Appl Toxicol. 1993;21:298–307. doi: 10.1006/faat.1993.1102. [DOI] [PubMed] [Google Scholar]
- Suominen JS, Wang Y, Kaipia A, Toppari J. Tumor necrosis factor-alpha (TNF-alpha) promotes cell survival during spermatogenesis, and this effect can be blocked by infliximab, a TNF-alpha antagonist. Eur J Endocrinol. 2004;151:629–640. doi: 10.1530/eje.0.1510629. [DOI] [PubMed] [Google Scholar]
- Sze KL, Lee WM, Lui WY. Expression of CLMP, a novel tight junction protein, is mediated via the interaction of GATA with the Kruppel family proteins, KLF4 and Sp1, in mouse TM4 Sertoli cells. J Cell Physiol. 2008;214:334–344. doi: 10.1002/jcp.21201. [DOI] [PubMed] [Google Scholar]
- Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–432. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Devel. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To beta or not to beta: Canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurisch B, Liang S, Sarioglu N, Schomburg L, Bungert J, Dame C. Transgenic mice expressing small interfering RNA against Gata4 point to a crucial role of Gata4 in the heart and gonads. J Mol Endocrinol. 2009;43:157–169. doi: 10.1677/JME-09-0030. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Hamel F, Viger RS. Protein kinase A-dependent cooperation between GATA and CCAAT/enhancer-binding protein transcription factors regulates steroidogenic acute regulatory protein promoter activity. Endocrinology. 2002;143:3935–3945. doi: 10.1210/en.2002-220413. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142:977–986. doi: 10.1210/endo.142.3.7995. [DOI] [PubMed] [Google Scholar]
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Devel. 1998;125:2665–2755. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- Vigil P, Bustos-Obregon E. Alkylating agents and mouse spermatogenesis: effects of a single dose of cyclophosphamide. Andrologia. 1985;17:276–282. doi: 10.1111/j.1439-0272.1985.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. ProcNatlAcadSci USA. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol. 2003;23:8084–8091. doi: 10.1128/MCB.23.22.8084-8091.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine. 2007;32:96–106. doi: 10.1007/s12020-007-9015-0. [DOI] [PubMed] [Google Scholar]
- Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelick PC, Kwon YH, Flynn JF, Borzorgzadeh A, Kleene KC, Hecht NB. Mouse transition protein 1 is translationally regulated during the postmeiotic stages of spermatogenesis. Mol Reprod Dev. 1989;1:193–200. doi: 10.1002/mrd.1080010307. [DOI] [PubMed] [Google Scholar]
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Devel. 1994;120:1759–1766. doi: 10.1242/dev.120.7.1759. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]