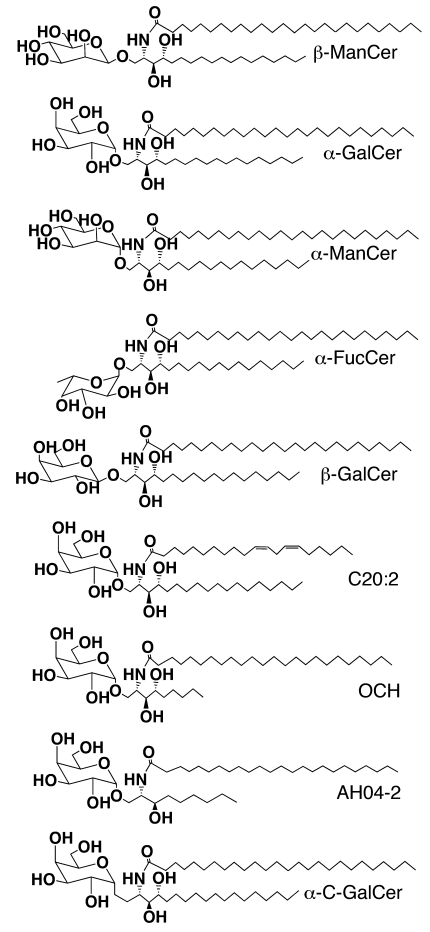
Abstract
Type 1 or invariant NKT (iNKT) cell agonists, epitomized by α-galactosylceramide, protect against cancer largely by IFN-γ–dependent mechanisms. Here we describe what we believe to be a novel IFN-γ–independent mechanism induced by β-mannosylceramide, which also defines a potentially new class of iNKT cell agonist, with an unusual β-linked sugar. Like α-galactosylceramide, β-mannosylceramide directly activates iNKT cells from both mice and humans. In contrast to α-galactosylceramide, protection by β-mannosylceramide was completely dependent on NOS and TNF-α, neither of which was required to achieve protection with α-galactosylceramide. Moreover, at doses too low for either alone to protect, β-mannosylceramide synergized with α-galactosylceramide to protect mice against tumors. These results suggest that treatment with β-mannosylceramide provides a distinct mechanism of tumor protection that may allow efficacy where other agonists have failed. Furthermore, the ability of β-mannosylceramide to synergize with α-galactosylceramide suggests treatment with this class of iNKT agonist may provide protection against tumors in humans.
Introduction
NKT cells are a unique lymphocyte population that expresses a TCR as well as NK lineage markers and possesses functional properties of both T and NK cells (1–3). Type 1 NKT cells, often referred to as invariant NKT (iNKT) cells, express a semi-invariant TCRα chain composed of a Vα14-Jα18 chain rearrangement in mice (Vα24-Jα18 in humans) that pairs preferentially with Vβ8.2, Vβ7, and Vβ2 (Vβ11 in humans). NKT cells are defined functionally by their ability to recognize glycolipid antigens presented in the context of the nonclassical MHC-like molecule CD1d, as opposed to conventional T cell recognition of peptide antigens presented by classical MHC molecules. NKT cells bridge the gap between the innate and adaptive immune systems and are equipped to rapidly respond to stimuli to elicit an immune response (4, 5).
α-Galactosylceramide (α-GalCer), a glycolipid originally isolated from a marine sponge, is the most extensively studied ligand for CD1d and is well established to be a potent stimulator of iNKT cells in both mice and humans (6–8). When iNKT cells are activated, they can rapidly produce large amounts of cytokines, including IFN-γ, IL-4, and IL-13, and the cytokine profile differs depending on the stimulus. Following activation with α-GalCer, high levels of IFN-γ produced by iNKT cells promote immunity against tumors as well as infectious pathogens (4, 9–11). A number of studies in murine tumor models have shown the necessity of IFN-γ in iNKT cell–induced antitumor immunity, with or without α-GalCer stimulation (2, 11–15). The ability of α-GalCer to induce elimination of tumors is completely iNKT cell dependent, as no protection occurs in Jα18–/– mice, which lack iNKT cells (12).
Previous work has demonstrated that alterations of the lipid structure of α-GalCer elicit different patterns of cytokine production by stimulated iNKT cells (16–20). Schmieg et al. observed that the C-glycoside analog of α-GalCer (α-C-GalCer) induced an immune response more skewed toward Th1 (IFN-γ) cytokines and was even more effective against B16 melanoma metastasis, further supporting the widely held belief that IFN-γ induction is a key factor for generating antitumor immunity (18). Other studies have characterized the functional activity of analogs of α-GalCer that induce more Th2 cytokine production, such as C20:2 and OCH (15, 16, 21, 22).
Modifications of the sugar moiety of glycosylceramides also alter their ability to stimulate iNKT cells. Kawano et al. reported that α-GalCer is more potent than α-glucosylceramide, while α-mannosylceramide (α-ManCer) and β-galactosylceramide (β-GalCer) failed to induce proliferation, as measured by 3H-thymidine incorporation (6). In a subsequent study, differences in proliferation induced by these glycosylceramides were attributed to the affinity of the glycolipid/CD1d complex for the TCR (23). Far fewer studies have characterized β-linked glycosylceramides. It has been shown that β-galactosylceramide is able to bind the TCR on iNKT cells, although the binding affinity is much lower than that of α-GalCer (24). β-Linked glycosylceramides only weakly induced cytokine production and have not been shown to induce significant antitumor immune responses, although some activity against autoimmune disease has been reported (24–27). The lysosomal glycolipid isoglobotrihexosylceramide is also β-linked and has been shown to be a weak agonist for iNKT cells; however, it has not been shown to have antitumor activity (28). Other antigens for iNKT cells have been described, such as lysophospholipids, which can activate iNKT cells to produce cytokines, and non-glycosidic antigens, which stimulate iNKT cells to mature DCs and prime antigen-specific T and B cells (29, 30). A variety of microbial glycolipid antigens for iNKT cells have also been described, suggesting a role for iNKT cells in responding to infection (31). The effect of these agonists in the context of tumor immunity has not been reported.
Here, we report that we discovered what we believe to be a novel mechanism of iNKT cell–dependent antitumor immunity, which was nitric oxide and TNF-α dependent and largely independent of IFN-γ that was induced by β-mannosylceramide (β-ManCer), defining a potentially new class of antitumor iNKT cell agonists and a potentially new mechanism by which they can protect against cancer that suggests promising clinical applications. In contrast, all the α-GalCer analogs were completely dependent on IFN-γ for tumor protection and independent of nitric oxide and TNF-α. To our knowledge, this is the first report of a β-linked glycosylceramide possessing the ability to induce significant antitumor immunity, and its distinctive mechanism of protection, which we believe to be novel, may define a new class of iNKT cell agonists to be tested in the setting of human tumors, with the potential to allow selective induction of different and synergistic mechanisms of protection.
Results
β-ManCer, but not α-ManCer, α-fucosylceramide, or β-GalCer, induces strong protection against CT26 lung metastasis in an iNKT cell–dependent manner.
In this study, we examined the antitumor activity of a panel of synthetic glycosylceramides (Figure 1) in a lung metastasis model of the CT26 colon carcinoma. This panel included β-ManCer, which contains the same ceramide tails as the prototypical iNKT cell antigen, α-GalCer, α-ManCer, α-fucosylceramide (α-FucCer), and β-GalCer. The activity of these compounds was compared with that of α-GalCer.
Figure 1. Structures of a panel of glycolipids used in this study.
This panel included β-ManCer, α-GalCer, α-ManCer, α-FucCer, β-GalCer, C20:2, OCH, AH04-2, and α-C-GalCer.
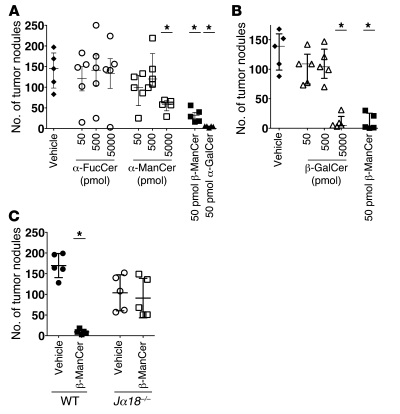
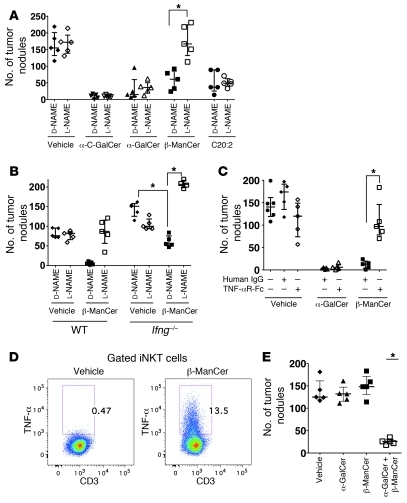
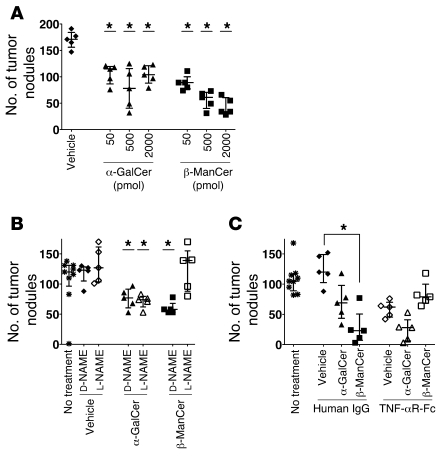
In order to examine the ability of these glycolipids to induce antitumor immunity, BALB/c mice were injected i.v. with the syngeneic colon carcinoma CT26 cells, followed by glycolipid administration. Surprisingly, we observed strong protection induced by β-ManCer at a low dose of 50 pmoles (Figure 2A), which was similar to protection after treatment with α-GalCer. α-FucCer failed to induce any tumor protection, even at a dose of 5,000 pmoles (Figure 2A). β-ManCer was 100-fold more potent than α-ManCer and β-GalCer, as 5,000 pmoles of α-ManCer or β-GalCer induced protection comparable to that induced by 50 pmoles of β-ManCer (Figure 2, A and B).
Figure 2. β-ManCer, but not α-ManCer or α-FucCer, induced strong protection against CT26 lung metastasis in an iNKT cell–dependent manner.
CT26 cells (5 × 105 cells) were injected i.v. into the tail vein of BALB/c mice (5 mice per group), and glycolipids were administered within 1 hour after tumor challenge. Mice were sacrificed 14–16 days after tumor challenge, and lung metastases were enumerated. (A) Mice were treated with vehicle (black diamonds), α-FucCer (white circles), α-ManCer (white squares), 50 pmoles β-ManCer (black squares), or 50 pmoles α-GalCer (black triangles). (B) Mice were treated with vehicle (black diamonds), β-GalCer (white triangles), or 50 pmoles β-ManCer (black squares). (C) WT BALB/c (black symbols) or Jα18–/– (white symbols) mice (5 mice per group) were treated with vehicle (circles) or 50 pmoles β-ManCer (squares). *P < 0.05 compared with vehicle control. Representative experiments of at least 2 repeats are shown. Vertical bars indicate the interquartile range, and horizontal bars indicate the median value. Each symbol represents an individual mouse.
In order to rule out the possibility that β-ManCer was inducing tumor protection by a mechanism independent of iNKT cells, Jα18–/– mice, which lack only iNKT cells, were challenged with CT26 cells and treated with β-ManCer. All protection was lost in Jα18–/– mice, confirming that iNKT cells are necessary for β-ManCer–induced protection (Figure 2C).
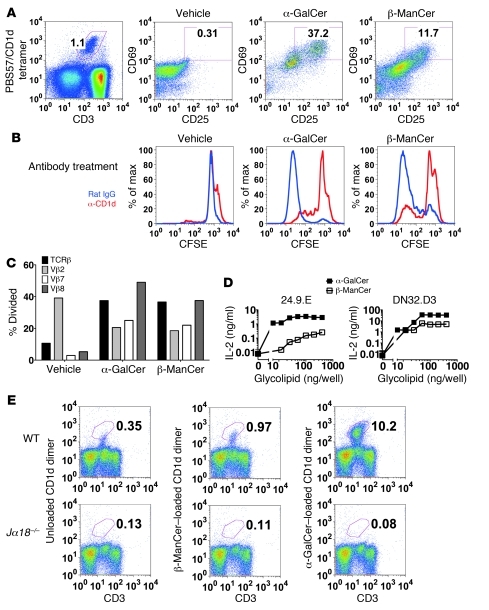
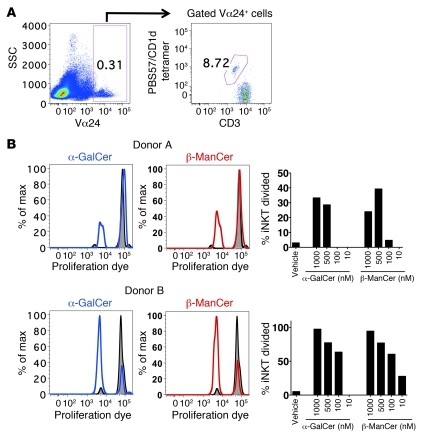
Although we demonstrated that the tumor protection induced by β-ManCer was dependent on iNKT cells, we next characterized the ability of β-ManCer to activate iNKT cells. Mouse splenocytes were stimulated overnight with α-GalCer or β-ManCer, and iNKT cell activation was measured by upregulation of activation markers CD25 and CD69. iNKT cells express low levels of CD69, and this expression increases upon activation (32). β-ManCer induced upregulation of CD25 and CD69 on iNKT cells, albeit not to the same extent as that after α-GalCer stimulation (Figure 3A). β-ManCer also induced proliferation of 40% of iNKT cells, as measured by CFSE dilution after a 3.5-day stimulation, comparable to α-GalCer, which induced proliferation of 44% of iNKT cells, and this proliferation was inhibited to 5.4% (86% inhibition) with a CD1d-blocking antibody, confirming that iNKT cells recognize β-ManCer in the context of CD1d (Figure 3B). Taken together, these data demonstrate that β-ManCer presented by CD1d is able to stimulate iNKT cells. Because we observed less activation of iNKT cells upon β-ManCer stimulation compared with that upon α-GalCer stimulation, we wanted to determine whether β-ManCer stimulated only a subset of iNKT cells. While iNKT cells use the semi-invariant TCRα chain, this can pair with multiple Vβ chains (Vβ2, Vβ7, and Vβ8 in mice). We characterized the proliferation of different Vβ+ iNKT cell subsets by CFSE dilution after a 3.5-day stimulation. β-ManCer and α-GalCer induced similar proliferation of the different Vβ subsets of iNKT cells (Figure 3C). Vβ2+ iNKT cells had the highest background proliferation, but no additional proliferation was observed after stimulation. We consistently observed an unexplained decrease in proliferation of the Vβ2 subset after antigen stimulation. Vβ8+ iNKT cells proliferated the most, followed by Vβ7+ iNKT cells. This suggests that β-ManCer stimulates NKT cells with a similar Vβ repertoire as α-GalCer.
Figure 3. β-ManCer activates mouse iNKT cells.
(A) Mouse splenocytes were stimulated with vehicle, α-GalCer, or β-ManCer for 18 hours, and activation was measured by FACS analysis. iNKT cells were identified as CD3intermediatePBS57/CD1d tetramer+, and CD69 and CD25 expression was determined on the gated cells. Numbers in plots represent the percentage of cells in gated population. (B) Mouse splenocytes were labeled with CFSE and stimulated with vehicle, α-GalCer, or β-ManCer for 3.5 days in the presence of anti-CD1d antibody 20H2 (10 μg/ml) or rat IgG control. Proliferation of iNKT cells, gated as in A on CD1d-tetramer+ and CD3-intermediate cells, was measured by CFSE dilution. (C) The proliferation of iNKT cells positive for different Vβ chains was measured by CFSE dilution. % Divided, percentage of Vβ+ CD1d tetramer+ iNKT cells which divided. (D) 24.9.E and DN32.D3 hybridoma cells were stimulated with α-GalCer–loaded (black squares) or β-ManCer–loaded (white squares) mCD1d dimers for 24 hours. IL-2 in the supernatants was determined by ELISA. Each point represents mean ± SD of triplicates, although the SDs are too small for error bars to be visible relative to the size of the symbols. Representatives of at least 2 independent experiments are shown. (E) Liver lymphocytes were stained with α-GalCer– or β-ManCer–loaded or unloaded mCD1d dimers. The top panels illustrate staining of liver lymphocytes from WT mice, and the bottom panels illustrate liver lymphocytes from Jα18–/– mice. Representative plots from 6 experiments with reproducible results are shown. Numbers in plots represent the percentage of cells in gated population
In order to further demonstrate that β-ManCer directly activates iNKT cells, we tested the ability of β-ManCer to stimulate the 24.9.E and DN32.D3 NKT cell hybridomas, which express the iNKT cell TCR Vα14Jα18 (33, 34). In this system, β-ManCer or α-GalCer were loaded onto mouse CD1d (mCD1d) dimers, which were coated onto 96-well plates in the absence of any additional cell types, including APCs. β-ManCer loaded dimers were able to induce IL-2 production by both the 24.9.E and DN32.D3 NKT cell lines (Figure 3D). Unloaded CD1d (0 ng/well) or soluble β-ManCer in the absence of CD1d (data not shown) failed to induce IL-2 production above background. While β-ManCer induces less IL-2 production than α-GalCer (10- to 50-fold difference), this is not surprising, since almost all assays comparing α-GalCer and β-ManCer demonstrate that β-ManCer is not as potent a stimulator of iNKT cells as α-GalCer. However, the increase in IL-2 production after β-ManCer stimulation is 25- and 506-fold higher than that induced by unloaded mCD1d for the 24.9.E and DN32.D3 hybridomas, respectively, indicating a substantial and unequivocal increase in IL-2 production. This demonstrates that β-ManCer, presented by CD1d, directly activates iNKT cells in the absence of any other cells.
We next visualized β-ManCer–reactive iNKT cells by flow cytometry. mCD1d dimers loaded with β-ManCer stained approximately 1% of liver lymphocytes (Figure 3E), which was consistently 2- to 3-fold higher than background staining with unloaded mCD1d dimers. This staining was not observed for liver lymphocytes from Jα18–/– mice, indicating that the staining with β-ManCer–loaded mCD1d dimers was iNKT cell specific.
The activity and mechanism of action of β-ManCer were then compared with those of α-GalCer as well as those of 2 previously described α-GalCer analogs, C20:2 and OCH, which are known to activate iNKT cells but induce different cytokine profiles more skewed toward a Th2 response (16, 20). Also included in this study was AH04-2, the aminodiol analog of OCH, which has been shown to have a similar cytokine profile to OCH (35). A rank order of tumor protection was established at a dose of 50 pmoles (Figure 4A), although all glycolipids tested elicited protection with a high dose of 500 pmol (data not shown). α-GalCer induced the greatest protection, followed by β-ManCer and C20:2, which were similarly protective. AH04-2 and OCH induced substantially less tumor protection. This 50 pmole dose was used in subsequent in vivo experiments to investigate correlates of tumor protection. The results at 5 pmoles indicate that α-GalCer is still about a log more potent than β-ManCer.
Figure 4. In vitro and in vivo cytokine production induced by glycolipid treatment.
(A) CT26 cells (5 × 105 cells) were injected i.v. into the tail veins of BALB/c mice. Mice (5 mice per group) were treated with 50 pmoles (black symbols) or 5 pmoles (white symbols) α-GalCer (triangles), β-ManCer (squares), C20:2 (circles), AH04-2 (diamonds), or OCH (inverted triangles) within 1 hour after tumor challenge. Mice were sacrificed 14–16 days after tumor challenge, and lung metastases were enumerated. *P < 0.05 compared with vehicle control. Vertical bars indicate the interquartile range, and horizontal bars indicate the median value. Each symbol represents an individual mouse. (B) BALB/c splenocytes were stimulated with various concentrations of glycolipid or vehicle control for 48 hours, and the concentrations of IFN-γ, IL-4, IL-13, and TNF-α in supernatant were determined by ELISA. Each data point represents mean ± SD of triplicates. (C) BALB/c mice (5 mice per group) were challenged with CT26 (5 × 105) i.v., followed by 50 pmoles glycolipid or vehicle control i.p. at time 0. Mice were bled retro-orbitally at 0, 3, 6, 12, and 24 hours, and the amount of IFN-γ, IL-4, IL-13, IL-12(p70), and TNF-α in plasma was determined by Bio-plex. Each data point represents mean ± SD of triplicates. Representative experiments of at least 2 repeats are shown. Due to substantial overlap of data points corresponding to little or no detectable cytokine, some data points are not visible, but all compounds shown in legend were tested.
β-ManCer induces a low level of cytokines.
In order to identify potential correlates of tumor protection, we characterized the cytokine production induced by the glycolipid panel. IFN-γ, IL-13, and IL-4 production were determined in vitro by treating splenocytes from naive BALB/c mice for 48 hours with the various lipids over a 10,000-fold range of concentrations (Figure 4B). α-GalCer induced the greatest IFN-γ production, while C20:2, OCH, and AH04-2 induced a Th2-skewed cytokine profile, with a lower IFN-γ level and higher amounts of IL-4 and IL-13. The difference in the potency of α-GalCer and β-ManCer to induce cytokine production is consistent with the difference observed with iNKT cell hybridomas (Figure 3D) as well as with the difference in frequency of iNKT cells stained with CD1d dimers (Figure 3E). Interestingly, β-ManCer induced the lowest levels of cytokine production, with only TNF-α detected at concentrations of less than 30 nM.
In vivo cytokine production in plasma by tumor-challenged mice in response to these glycolipids was also assessed at the 50 pmole dose at which a rank order of protection was established (Figure 4C). Similar to the in vitro results, α-GalCer induced the most IFN-γ, followed by C20:2. Again, C20:2 produced a cytokine profile more skewed toward Th2, with increased IL-4 and IL-13 production. C20:2 and α-GalCer induced similar levels of IL-12 and TNF-α. Little cytokine production was detected after OCH and AH04-2 administration, which is to be expected because these analogs do not induce significant protection at this dose. The lack of detectable cytokine production after in vitro stimulation with β-ManCer was also confirmed in vivo, as there was no substantial increase in IFN-γ, IL-4, IL-13, or TNF-α levels after treatment with 50 pmoles β-ManCer and only a modest increase in IL-12, which was still lower than that observed with α-GalCer or C20:2. From these data, it can be concluded that, with the α-galactosyl glycosylceramides tested here, the amount of IFN-γ correlates with tumor protection. However, β-ManCer is a potent stimulator of antitumor immunity, despite its paradoxical failure to induce substantial levels of IFN-γ, IL-4, IL-12, IL-13, or TNF-α that can be detected systemically in the circulation.
β-ManCer–induced protection was only partially reversed in IFN-γ knockout mice, whereas α-GalCer–induced protection was completely reversed.
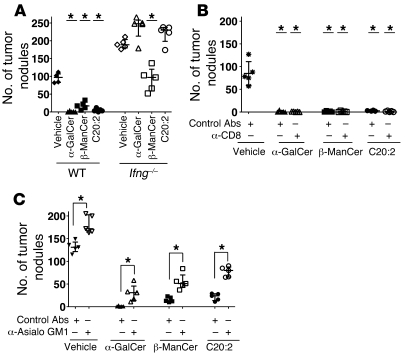
It has been reported that IFN-γ is necessary for iNKT cell–mediated antitumor immunity (2). To determine the requirement of IFN-γ for the function of these glycolipids, the ability of β-ManCer, α-GalCer, and C20:2 to prevent tumor formation in IFN-γ knockout mice was examined. Consistent with previous reports, α-GalCer and C20:2 completely failed to induce any tumor protection in IFN-γ knockout mice, whereas β-ManCer treatment still resulted in 50% fewer lung nodules compared with that in control mice (P = 0.0079) (Figure 5A). In contrast to the α-galactosyl ceramides, α-GalCer and C20:2, whose protection is completely dependent on IFN-γ, β-ManCer–induced protection has a substantial IFN-γ–independent component.
Figure 5. Tumor protection induced by α-GalCer and C20:2, but not β-ManCer, is dependent on IFN-γ.
CT26 cells (5 × 105 cells) were injected i.v. into the tail vein of BALB/c WT or Ifng–/– mice on day 0, and glycolipids (50 pmoles) were administered within 1 hour after tumor challenge. Mice (5 mice per group) were sacrificed 14–16 days after tumor challenge, and lung metastases were enumerated. (A) WT (black symbols) or Ifng–/– (white symbols) were used. *P < 0.05 compared with respective vehicle control. (B) WT BALB/c mice were treated with 200 μg anti-CD8 antibodies (white symbols) or rat IgG control antibodies (black symbols) on days –1, 0, 5, and 10. *P < 0.05 compared with vehicle control. (C) BALB/c mice were treated 25 μl anti-asialo GM1 antibodies (white symbols) or control rabbit serum (black symbols) on days –1, 0, 5, and 10. *P < 0.05 compared with respective groups. Representative experiments of at least 2 repeats are shown. Vertical bars indicate the interquartile range, and horizontal bars indicate the median value. Each symbol represents an individual mouse.
In order to further investigate the mechanism(s) by which these glycolipids induce tumor protection, the involvement of effector cells, which can lead to tumor cell lysis, was examined. Both CD8+ T and NK cells were depleted with antibodies prior to tumor challenge and glycolipid administration. CD8 depletion had no effect on the number of lung nodules compared with that in mice treated with control antibody (Figure 5B). Depletion of NK cells with anti-asialo GM1 antibody resulted in a slight but significant (P = 0.0079) increase in numbers of tumor nodules, which was similar in all groups, including vehicle-treated mice (Figure 5C). Thus, although NK cells play some innate role in limiting tumor growth, as seen in the vehicle control, the ability of these glycolipids to prevent tumor formation, at least in this tumor model, is not dependent on CD8+ T cells and largely independent of NK cells.
β-ManCer–induced tumor elimination is NOS and TNF-α dependent.
Having ruled out CD8+ T cells and NK cells as the major mediators of protection, we examined the role of nitric oxide, by which macrophages may protect against tumors. Mice were treated with N-nitro-l-arginine-methyl ester (l-NAME), which is reported to inhibit NOS in vivo (36). NOS inhibition had no effect on tumor formation in vehicle-, α-GalCer–, or C20:2-treated mice, as there was no difference between mice treated with l-NAME or its inactive enantiomer, N-nitro-d-arginine-methyl ester (d-NAME) (Figure 6A). However, administration of l-NAME significantly abrogated protection induced by β-ManCer (P = 0.0027), such that there was no significant difference between β-ManCer–treated and vehicle-treated control mice. These data also suggest distinct mechanisms of tumor protection between β-ManCer and the α-galactosylceramides.
Figure 6. Tumor protection induced by β-ManCer is NOS and TNF-α dependent.
CT26 cells (5 × 105 cells) were injected i.v. into the tail veins of BALB/c mice (5 mice per group) on day 0, and glycolipids were administered within 1 hour after tumor challenge. Mice were sacrificed 14–16 days after tumor challenge, and lung metastases were enumerated. (A) WT or (B) WT Ifng–/– BALB/c mice were treated with l-NAME (white symbols) or the inactive stereoisomer d-NAME (black symbols) twice on days 0 and 1 and once daily for days 2–14. (C) WT BALB/c mice were treated with etanercept (TNF-αR-Fc) (white symbols) or human IgG (black symbols) every other day, beginning the day of tumor challenge. (D) WT BALB/c splenocytes were incubated with 100 nM β-ManCer or vehicle overnight, harvested, and analyzed by FACS analysis for intracellular TNF-α. iNKT cells were defined as CD3intermediatePBS57/CD1d tetramer+ as shown in Figure 3 and analyzed for intracellular TNF-α. Representative FACS plots are shown. Numbers in plots represent the percentages of TNF-α+ iNKT cells. (E) Vehicle (black diamonds), 0.125 pmoles α-GalCer (black triangles), 3 pmoles β-ManCer (black squares), or 0.125 pmoles α-GalCer and 3 pmoles β-ManCer (white squares) were administered i.p. within 1 hour after tumor challenge in BALB/c mice. Mice were sacrificed 14–16 days after tumor challenge, and lung metastases were enumerated. *P < 0.05 compared with vehicle control. Representative experiments of 2 repeats are shown. Vertical bars indicate the interquartile range, and horizontal bars indicate the median value. Each symbol represents an individual mouse.
Previously, it has been reported that α-C-GalCer, the C-glycoside analog of α-GalCer in which a CH2 group replaces the glycosidic oxygen, is more potent than α-GalCer against lung metastasis in the B16 melanoma transplantable tumor model (18). This analog induces a cytokine response more skewed toward Th1 cytokines than does α-GalCer due to markedly reduced production of Th2 cytokines. Because α-C-GalCer and α-GalCer have been classified differently based on the cytokine profile they induce and the mechanism of induction (18, 37), we wanted to determine whether α-C-GalCer induced antitumor immunity through a mechanism similar to that of β-ManCer. At the 50 pmole dose, mice treated with α-C-GalCer and α-GalCer developed a similar number of lung nodules (Figure 6A). NOS inhibition had no effect on the activity of α-C-GalCer against lung tumors, similar to the results obtained with the other α-GalCer analogs. These data suggest that β-ManCer does not fit into any of the previous classifications of iNKT agonists.
We showed that blockade of NOS, but not knockout of IFN-γ, completely reversed β-ManCer–induced protection. Thus, we wanted to determine whether the protection induced by β-ManCer in Ifng–/– mice could also be reversed by inhibiting NOS. Indeed, protection induced by β-ManCer was completely lost in Ifng–/– mice when NOS was inhibited (Figure 6B), suggesting a surprising IFN-γ–independent mechanism for NOS induction. We also noted that l-NAME treatment reduced the number of lung metastases in Ifng–/– mice in the absence of any additional treatment, suggesting that the increased susceptibility of Ifng–/– mice to CT26 lung metastasis may be due in part to nitric oxide. However, this effect is small compared with the clear reversal of β-ManCer–induced protection by NOS inhibition. Because NOS can also be induced by TNF-α, we next wanted to determine whether β-ManCer was inducing NOS through TNF-α. Indeed, blockade of TNF-α by soluble TNF-αR-Fc fusion protein completely reversed the protection induced by β-ManCer but had no effect on protection induced by α-GalCer (Figure 6C). Additionally, when splenocytes were incubated overnight with β-ManCer, iNKT cells could make TNF-α, as measured by intracellular cytokine staining (Figure 6D). Taken together, these data confirm that β-ManCer and α-GalCer induce tumor immunity through distinct mechanisms.
Simultaneous treatment with α-GalCer and β-ManCer induces synergistic antitumor activity.
This study provides multiple lines of evidence that β-ManCer and the α-galactosyl glycosylceramides induce tumor protection through distinct mechanisms. Thus, we hypothesized that simultaneous treatment with these glycolipids would induce synergistic protection against tumor formation. To address this hypothesis, mice were treated with low doses of each glycolipid separately as well as in combination. Neither 0.125 pmoles α-GalCer nor 3 pmoles β-ManCer alone induced any reduction in lung metastases (Figure 6E). However, the combination of these 2 antigens at these subtherapeutic doses resulted in a significant (P = 0.0119) 79% reduction of the median number of tumor nodules, suggesting that α-GalCer and β-ManCer can work synergistically to eliminate/prevent CT26 lung metastases.
β-ManCer also induces protection against B16F10 melanoma by NOS- and TNF-α–dependent mechanisms.
We wanted to confirm that the protection induced by β-ManCer was not unique to this specific tumor cell line or strain of mice. We compared the ability of α-GalCer and β-ManCer to protect in a model of B16F10 melanoma metastasis to lungs of C57BL/6 mice by titrating both in vivo. In fact, β-ManCer was at least as potent an inducer of tumor protection as α-GalCer in this model (Figure 7A). The mechanism(s) of tumor protection is also the same in this tumor model, as inhibiting NOS or TNF-α reversed the protection induced by β-ManCer but not that by α-GalCer (Figure 7, B and C).
Figure 7. β-ManCer also protects against B16F10 melanoma metastases in C57BL/6 mice.
B16F10 cells (5 × 105 cells) were injected i.v. into the tail veins of C57BL/6 mice (5 mice per group). (A) The indicated doses of glycolipid were administered within 1 hour after tumor challenge. (B) Mice were treated with l-NAME (white symbols) or the inactive stereoisomer d-NAME (black symbols) twice on days 0 and 1 and once daily for days 2–12. After NAME injections, 500 pmoles α-GalCer or 50 pmoles β-ManCer was administered. (C) WT mice were treated with etanercept (TNF-αR-Fc) (white symbols) or human IgG (black symbols) every other day, beginning immediately after tumor challenge. After antibody injections, 500 pmoles α-GalCer or 50 pmoles β-ManCer was administered. Mice were sacrificed 12 days after tumor challenge, and lung metastases were enumerated. *P < 0.05 compared with respective vehicle control. Representative experiments of 2 repeats are shown. Vertical bars indicate the interquartile range, and horizontal bars indicate the median value. Each symbol represents an individual mouse.
β-ManCer activates human iNKT cells.
Finally, we wanted to determine whether β-ManCer could activate human iNKT cells. Human PBMCs were stimulated with β-ManCer or α-GalCer for 4 days. iNKT cells were defined as Vα24+CD3intermediatePBS57/CD1d tetramer+ (Figure 8A), and proliferation was measured by dilution of CellTrace Violet Dye. β-ManCer induced proliferation of human iNKT cells similar to that induced by α-GalCer (Figure 8B). This suggests that β-ManCer has the potential for use in human patients.
Figure 8. β-ManCer activates human iNKT cells.
Human PBMCs were labeled with CellTrace Violet Dye and stimulated with vehicle, α-GalCer, or β-ManCer for 4 days. (A) iNKT cells were identified by FACS as Vα24+CD3intermediatePBS57/CD1d tetramer+. One representative donor is shown. Numbers in plots represent the percentages of Vα24+ cells and CD3+PBS57/CD1d tetramer+ cells, respectively. (B) Proliferation was measured by the percentage of cells that had diluted the proliferation dye. PBMCs from 5 donors were tested, and data from 2 representative donors are shown. Representative histograms of dilution of proliferation dye by iNKT cells from Donors A and B after stimulation with 500 nM α-GalCer (blue line), β-ManCer (red line), or vehicle (filled gray area) are shown. The bar graphs show the quantification of the percentage of iNKT cells from each donor that diluted the proliferation dye after stimulation over a range of glycolipid concentrations.
Discussion
These studies identify what we believe to be a new mechanism of protection against cancer mediated by NKT cells and identify what we believe to be a new class of NKT cell agonist, which activates both mouse and human iNKT cells. Our results demonstrate that, when stimulated with β-ManCer, iNKT cells induce antitumor immunity through an IFN-γ–independent mechanism that we believe to be novel, with potency almost as high as that of the well-characterized iNKT cell antigen α-GalCer, despite its poor ability to induce cytokines and its independence of IFN-γ. To our knowledge, this is the first study to show significant activity of a β-linked glycosylceramide in inducing an antitumor immune response through iNKT cell activation. In the past, it was suggested that any activity of β-anomers was due to contamination of the α-anomer in the glycolipid preparation, but this cannot be the case here, since α-ManCer is significantly less active than β-ManCer. Also the fact that α-ManCer did not induce strong protection suggests that the action of β-ManCer is not through mannose receptors on certain types of cells.
We have demonstrated by a variety of assays that β-ManCer can activate iNKT cells in a CD1d-dependent manner. While β-ManCer is consistently less potent than α-GalCer, the activation of iNKT cells by β-ManCer is substantial and unequivocal, and, most importantly, it is able to induce iNKT-dependent antitumor immune responses that are of interest because of their distinctive mechanism. We also were able to visualize β-ManCer–reactive iNKT cells by flow cytometric staining with β-ManCer–loaded mCD1d dimers. β-ManCer–loaded mCD1d dimers stained approximately 10-times fewer mouse liver lymphocytes than α-GalCer–loaded dimers, but this staining was consistently 2- to 3-fold higher than background staining with unloaded dimers and was completely absent in iNKT-deficient Jα18–/– mice used as a negative control. The difference in the frequency of β-ManCer–loaded dimer-staining cells explains the difference in the potency of the 2 glycolipids to induce cytokine production by spleen cells and IL-2 production by iNKT cell hybridomas. Weak staining with β-ManCer–loaded dimers is most likely due to a weaker binding affinity of β-ManCer to CD1d or of the β-ManCer/CD1d complex with the iNKT TCR, but this is still well within the range of that seen with many known physiologic iNKT cell antigens (38–41). Thus, despite the difference in activity levels, these data clearly demonstrate that β-ManCer directly activates iNKT cells through presentation by CD1d and is recognized by the canonical semi-invariant TCR present on the iNKT hybridomas lines and absent in Jα18–/– mice.
Based on previous studies, it is widely accepted in the field that IFN-γ is necessary for antitumor responses initiated by iNKT cells (2, 4, 10). Consistent with those previous reports, the antitumor response elicited by α-GalCer analogs was completely abrogated in Ifng–/– mice. While other studies have clearly demonstrated a requirement for NK cells in tumor killing induced by α-GalCer (2, 42), we observed a minimal effect of NK cell depletion in this tumor model, suggesting that the effector cells involved in iNKT-mediated tumor immunity may differ among tumor models. In contrast, β-ManCer–induced protection was only partially dependent on IFN-γ and was completely abrogated by NOS inhibition or TNF-α blockade, which did not have any impact on α-GalCer analog-induced protection. These results suggest the involvement of cells that can produce nitric oxides, such as macrophages. Therefore, this study also demonstrates that β-ManCer represents what we believe to be a new class of iNKT cell agonistic glycolipids that elicits effector mechanisms distinct from those of α-GalCer to cause tumor cell lysis.
In contrast to the reported IFN-γ requirement for tumor protection with α-GalCer (2), confirmed in the CT26 lung metastasis model in this study, we demonstrated that β-ManCer is still able to induce substantial IFN-γ–independent protection in Ifng–/– mice. Because Ifng–/– mice were inherently more susceptible than WT mice to tumor formation in this model, it is difficult to quantitatively compare the degree of protection of β-ManCer in the 2 different strains to determine how much, if any, of the protection is lost in the absence of IFN-γ. It is clear that β-ManCer–induced protection is completely dependent on NOS, since all protection induced by β-ManCer is lost in WT or Ifng–/– mice when NOS is inhibited. It is still possible that the NOS involved in the protection is induced by multiple pathways that require TNF-α, one of which also requires IFN-γ, and this accounts for any protection lost in Ifng–/– mice. At this dose of β-ManCer, we did not detect any IFN-γ in the plasma of treated mice, suggesting that any IFN-γ–dependent protection is due to IFN-γ produced in the local microenvironment. None of the other glycolipids tested could protect in the absence of IFN-γ. Even at the high dose at which β-GalCer and α-ManCer induced some protection, this protection was completely dependent on IFN-γ (data not shown). This finding, which we believe to be novel, that iNKT-mediated antitumor immunity can be induced in the absence of IFN-γ suggests that, while both α-GalCer and β-ManCer signal through the same TCRα chain and use a similar Vβ repertoire, in a CD1d-dependent manner (Figure 3), the response to TCR stimulation is quite different, analogous to what has been seen with altered peptide ligands that induce distinct T cell responses in conventional T cells (43–49). The ability to protect in Ifng–/– mice is consistent with another unique property of β-ManCer, namely its ability to induce an antitumor response similar to that of α-GalCer, despite inducing very little detectable cytokine production from in vitro–stimulated splenocytes or in the blood of in vivo–treated mice. β-ManCer and α-GalCer induce more similar levels of proliferation and activation of iNKT cells than they do cytokine production, as β-ManCer induces detectable, but significantly less, cytokine production, at least in vitro. This may be a reflection of the different signal strength required for expression of different genes thought to be activation markers of T cells. Since tumor protection is also similar between these 2 glycolipids, these data suggest that, at least for β-ManCer, tumor protection can occur in the absence of the massive cytokine production induced by α-GalCer. Higher concentrations of some cytokines like TNF-α might be achieved in the local environment. None of the other glycosylceramides tested depended on NOS or TNF-α for protection, which was also unique to β-ManCer. On the other hand, β-ManCer was completely dependent on NOS activation and TNF-α to induce tumor protection in both WT and Ifng–/– mice, suggesting that NOS, induced primarily by TNF-α rather than IFN-γ, was the major mediator involved in its protection for both the IFN-γ–independent pathway and any residual IFN-γ–dependent pathway. The complete dependence on NOS and TNF-α for β-ManCer–induced protection suggests that β-ManCer induces TNF-α production that results in upregulation of NOS and tumor elimination. Because there was not a significant increase in TNF-α in the blood of mice treated with β-ManCer, the critical TNF-α is most likely produced in the local microenvironment at levels that do not impact levels in the circulation. Thus, we believe this study identifies a new mechanism by which iNKT cells can protect against cancer with potential new clinical applications.
Previously, it has been reported that iNKT cells can control or suppress myeloid-derived suppressor cell (MDSC) expansion during influenza virus infection, and α-GalCer–activated iNKT cells suppressed the production of nitric oxide and arginase by MDSCs (50). In our study, NOS inhibition had no effect on protection induced by α-GalCer, suggesting that this mechanism is not involved in this system. Whereas NOS activity by MDSCs is immunosuppressive, we have shown here that NOS production induced by β-ManCer stimulation induces immune activation against tumor cells. These data highlight the dual role of nitric oxide, which can be both immunosuppressive as well as tumor inhibitory, depending on the context in which it is produced.
Both α-GalCer and β-ManCer are able to activate iNKT cells to induce an antitumor immune response, whereas β-GalCer and α-ManCer are at least 100-fold less potent. Because the ceramide structure is the same for α-ManCer, β-ManCer, and α-GalCer, this suggests that the at least 100-fold difference in potency of β-GalCer and α-ManCer compared with that of α-GalCer and β-ManCer is due to the orientation of the sugar head group protruding from the CD1d/glycolipid complex. Previously, it appeared that the α-linkage between sugars and lipids was critical for TCR recognition and, ultimately, the biological effect of CD1d-restricted glycolipids (24, 27). Data from the current study suggest that α-linkage versus β-linkage is not as important as the overall structure of the sugar head group protruding from CD1d in determining the TCR interaction, since α-ManCer and β-GalCer (24) are far less potent than β-ManCer and α-GalCer. α-ManCer and β-GalCer are 1,2-trans-O-glycosides, while β-ManCer and α-GalCer are 1,2-cis-O-glycosides (glycosides in which 1′- and 2′-hydroxyl groups of the carbohydrate are on the same side of the sugar ring). It has been suggested that the 2′-hydroxyl group on the galactose of α-GalCer is important for hydrogen bonding with the TCR (51, 52). The orientation of this hydroxyl group on β-ManCer in the CD1d/TCR complex may be similar to that on α-GalCer, whereas this hydrogen bonding may be lost with α-ManCer and β-GalCer, resulting in much weaker affinity for the TCR. This demonstrates the ability of the TCR of iNKT cells to discriminate among structural changes of glycolipid antigens.
A fraction of CD1d on the plasma membrane is localized to detergent insoluble microdomains called lipid rafts, and this localization was suggested to be important for activation of NKT cells (53–55). It has recently been reported that while α-GalCer presentation to the TCR was dependent upon association with lipid rafts, Th2-biased analogs of α-GalCer, such as C20:2, did not require lipid rafts (56). Whether or not these antigens were presented on CD1d in lipid rafts depended on the kinetics of loading onto CD1d, with quick binding to CD1d resulting in lipid raft independence. Th2-biased analogs were loaded directly onto CD1d at the cell surface very quickly, while analogs, such as α-GalCer, that induce both Th1 and Th2 cytokines required loading onto CD1d in the endosome and took substantially longer for presentation at the cell surface.
β-ManCer has the same ceramide tails as α-GalCer, which are a major component determining the affinity and site of glycolipid loading for CD1d, so it should also be dependent on endosomal trafficking for loading into the CD1d grove and probably undergoes lipid raft–dependent presentation. However, although its affinity for the TCR may be lower than that of α-GalCer, it appears to be in a distinct class of its own, because its mechanism of protection differs from that of all the others, including α-C-GalCer. The nature of the iNKT cell response to different glycolipid antigens is influenced by a variety of factors, including affinity for CD1d, affinity for TCR, dependence on lipid rafts for presentation to TCR, and stability of the glycolipid. Better understanding of how these factors shape the immune response to different glycolipid antigens will allow for more rational design of new lipids that induce the optimal cytokine profile and activate the most effective mechanisms to treat specific diseases.
The synergy we observed between α-GalCer and β-ManCer further confirms our conclusion that these 2 glycolipids use distinct mechanisms to induce tumor elimination. This finding is important, because it suggests that combination therapies of β-ManCer and α-GalCer or α-GalCer analogs could also cause synergistic tumor elimination in patients, and strong effects could be elicited without administration of high doses of any one lipid. This study also suggests that β-ManCer may have a different potential from that of α-GalCer for clinical use. We have demonstrated that β-ManCer has similar potency as α-GalCer for inducing an antitumor immune response. Because β-ManCer does not induce nearly as much cytokine production in vivo, higher doses could be used with less chance of inducing a cytokine storm.
Although α-GalCer is a very potent inducer of antitumor response in mouse tumor models, this reagent has not yet achieved much success in human patients (57). There are multiple potential explanations for the unsuccessful results of α-GalCer treatment in cancer patients. The first potential explanation is the induction of anergy, which is a serious problem, at least in mice, for multiple injections of α-GalCer. NKT cells are unable to respond to restimulation with α-GalCer for over a month after α-GalCer treatment (58), indicating that administration of multiple doses may not give increased therapeutic benefit. Conversely, we observed that cells stimulated with β-ManCer did not exhibit as strong an anergic phenotype as those stimulated with α-GalCer (data not shown; unpublished observations). The second potential explanation is that it has also been demonstrated that repeated doses of α-GalCer skews the cytokine profile toward Th2 (59), which would not be beneficial in the tumor setting, since IFN-γ mediates protection by α-GalCer. In contrast, patients could be treated with multiple doses of β-ManCer over time to increase the efficacy without concern about loss of IFN-γ production or shift in cytokine profile, since the tumor protection induced by β-ManCer does not require high levels of cytokine production and still occurs in the absence of IFN-γ. The third potential explanation is the existence of antibodies against α-linked sugars in humans in which α-linkages do not naturally occur, whereas mice lack such antibodies (60). This may be a contributing factor to the lack of clinical success of α-GalCer and would not be a problem for β-ManCer.
We demonstrated that α-GalCer and β-ManCer induce antitumor responses through different mechanisms and synergize when used together. We also observed stronger tumor protection for β-ManCer compared with that for α-GalCer in the B16F10 model in a different mouse strain, demonstrating that depending on the tumor or type of cancer in patients, β-ManCer has the potential to work better than α-GalCer. The mechanism of protection by β-ManCer is the same in both tumor models. Importantly, we also demonstrated that β-ManCer activates human iNKT cells. Taken together, these data strongly suggest the high potential of β-ManCer for clinical use.
In conclusion, we present here what we believe to be a completely new mechanism by which iNKT cells can protect against cancer, induced by an agonist, β-ManCer, that represents a potentially new class of iNKT cell agonists, which can protect on their own or synergistically enhance the efficacy of α-GalCer. This study suggests that β-ManCer may work well at inducing immune responses in humans, either alone or in combinatorial therapies with α-GalCer, to selectively induce desired types of immune responses in different settings.
Methods
Mice.
Female BALB/c mice were purchased from Animal Production Colonies, Frederick Cancer Research Facility, National Cancer Institute, or The Jackson Laboratory. Female C57BL/6 mice were purchased from Animal Production Colonies, Frederick Cancer Research Facility, National Cancer Institute. BALB/c Jα18–/– mice (provided by Masaru Taniguchi [RIKEN Institute, Yokohama, Kanagawa, Japan] and Dale Umetsu [Harvard Medical School, Boston, Massachusetts, USA]) were bred at the National Cancer Institute under pathogen-free conditions. BALB/c Ifng–/– mice were purchased from The Jackson Laboratory. Female mice (older than 6 weeks of age) were used for all experiments. All experimental protocols were approved by and performed under the guidelines of the National Cancer Institute’s animal care and use committee.
Reagents.
Purified rat anti-mouse CD8 monoclonal antibody (clone 2.43) was obtained from Harlan Laboratories. Rabbit anti-asialo GM1 antibody was purchased from Wako Chemical Company. Rat IgG control antibodies were purchased from Sigma-Aldrich, and control rabbit serum was obtained from Cedarlane Laboratories Ltd. l-NAME and d-NAME were purchased from Sigma-Aldrich. TNF-αR-Fc (etanercept), a fusion protein of the human TNF-α receptor with the Fc portion of human IgG1, was purchased from Amgen. Human IgG1 control antibodies were purchased from Invitrogen.
Glycolipid synthesis.
α-GalCer (KRN7000) was purchased from Alexis Biochemicals or Funakoshi Co. Ltd. α-C-GalCer was obtained from the NIH Tetramer Core Facility at Emory University (Atlanta, Georgia, USA). C20:2, OCH, and AH04-2 were synthesized and solubilized for in vitro or in vivo use as previously described (20, 21, 35).
The detailed method of chemical synthesis of β-ManCer, α-ManCer, and β-GalCer is described in the Supplemental Methods (supplemental material available online; doi: 10.1172/JCI42314DS1). Briefly, for the synthesis of β-ManCer we used “glycosylation via locked anomeric configuration” (61). This glycosylation method is based on displacing the triflyl group on glycosyl acceptor by 1,2-O-cis-stannylene acetal of mannose with formation of β-mannosides. Mannopyranosyl trichloroacetimidate (62) was used for α-ManCer synthesis. α-FucCer was synthesized by the stereospecific α-l-fucosylation method (63). Trimethylsilylation of l-fucose, followed by reaction with TMS-I, yields in situ per-O-trimethylsilyl-α-l-fucopyranosyl iodide, which reacts with lipid acceptor in the absence of promoter. All new compounds have been characterized by 1H, 13C, 2-dimensional 1H-13C NMR spectrometry, and ES mass spectrometry. The compounds were more than 99.9% pure, as the spectra showed only 1 peak, corresponding to the synthesized compound.
All glycolipids were dissolved to 500 μM in DMSO for in vitro use and 0.5% Tween20 in PBS for in vivo use. All glycolipids were soluble in these solvents after heating to 80°C and sonicating at 60°C.
Cell lines.
The CT26 colon carcinoma and B16F10 melanoma cell lines were maintained in RPMI 1640 and DMEM medium, respectively, supplemented with 10% FCS, l-glutamine, sodium pyruvate, nonessential amino acids, and 2-mercaptoethanol (5 × 10–5 M). iNKT hybridoma cell lines 24.9.E and DN32.D3 were obtained from Samuel Behar (Harvard Medical School) and Albert Bendelac (University of Chicago, Chicago, Illinois, USA) and were cultured in RPMI 1640 medium containing the supplements listed above. Cells were cultured in an atmosphere of 37°C and 5% CO2.
In vivo lung metastasis assay.
CT26 cells (5 × 105 cells) in 0.2 ml PBS were injected i.v. into the tail vein. Glycolipid or vehicle control (0.00025% Tween 20) was injected i.p. (in 0.2 ml PBS) within 1 hour after tumor challenge. Mice were sacrificed 12–16 days after tumor challenge. Lungs were stained and fixed, and metastases were enumerated as previously described (64). The same protocol was used for the B16F10 cell line, except that lungs were perfused with PBS instead of ink before removal.
For CD8+ T and NK cell depletion, mice were treated i.p. with anti-CD8 (200 μg/injection), anti-asialo GM1 (25 μl/injection), or control antibodies 1 day prior to tumor challenge, the day of tumor challenge, and 5 and 10 days after tumor challenge. CD8+ T and NK cell depletion of more than 90% and preservation of iNKT cells was confirmed by flow cytometric staining for CD8 (clone 53-6.7, BD Biosciences) and pan-NK cell marker (clone DX5, eBioscience) at the time of tumor challenge and at the conclusion of the experiment. It was also confirmed that NK cell depletion had no effect on iNKT cells by staining with PBS57-loaded CD1d tetramer (NIH Tetramer Facility) and anti-CD3 (clone 145-2C11, Biolegend). When indicated, mice received i.p. 0.2 mg l-NAME or d-NAME twice per day on the day of tumor challenge and the day after tumor challenge and once daily for 2 weeks after tumor injection. Blockade of TNF-α was achieved by i.p. administration of 100 μg etanercept every other day, beginning immediately after tumor challenge (65).
In vitro iNKT activation.
Splenocytes were harvested from mice (n = 3), and erythrocytes were depleted with ACK Lysis Buffer (Lonza). Cells were labeled with 0.1 μM CFSE (Invitrogen) for 15 minutes at room temperature. Labeled cells (4 × 106 cells/well of 24-well plate) were stimulated for 3.5 days with glycolipid or vehicle control. At the end of the culture, cells were harvested and stained with PBS57-loaded CD1d tetramer (NIH Tetramer Facility) and anti-CD3 (clone 145-2C11, Biolegend). The fluorescence of stained cells was measured by FACSCalibur (BD Biosciences), and data were analyzed by Flowjo (Tree Star).
In vitro TNF-α production by iNKT cells.
Splenocytes were harvested from mice (n = 3), and erythrocytes were depleted with ACK Lysis Buffer (Lonza). Cells (4 × 106 cells/well of 24-well plate) were stimulated overnight with 100 nM β-ManCer or vehicle control. At the end of the culture, cells were harvested and stained with PBS57-loaded CD1d tetramer (NIH Tetramer Facility) and anti-CD3 (clone 145-2C11, Biolegend). Cells were fixed and permeabilized with the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) and stained for intracellular TNF-α (clone MP6-XT22, BD Biosciences). The fluorescence of stained cells was measured by FACSCalibur (BD Biosciences), and data were analyzed by Flowjo (Tree Star).
Plate-bound mCD1d hybridoma stimulation assay.
Protocol was modified from that of Gumperz et al. (33). mCD1d dimer/Ig fusion protein (BD Biosciences) was incubated with the indicated concentrations of glycolipid in pH 5 sodium acetate buffer containing 0.01% Tween20 and 45 μg/ml saposin C (provided by Nico Tjandra and Motoshi Suzuki [both from National Heart, Lung, and Blood Institute, NIH, Bethesda, Maryland, USA]) overnight at 37°C. The mCD1d dimers loaded with glycolipid were loaded onto 96-well Protein G Plates (Pierce) and incubated for 48 hours at 37°C. The plates were washed with PBS and tissue culture media to remove anything not coated on the plates. 1 × 105 24.9.E or DN32.D3 iNKT hybridoma cells were added to each well and incubated at 37°C 5% CO2 for 24 hours. Supernatants were collected and analyzed for IL-2 by ELISA.
Visualizing liver iNKT cells with glycolipid-loaded CD1d dimers.
mCD1d/Ig fusion protein (CD1d dimers; BD Biosciences) was loaded with glycolipid at 37°C overnight. PE–anti-mouse IgG antibodies were added and incubated for 1 hour at room temperature, and mouse IgG isotype control was added for an additional 30 minutes at room temperature to saturate unbound excess anti-IgG antibodies. Livers were perfused with Liver Perfusion Medium (Invitrogen), and a single cell suspension was prepared in Liver Digest Medium (Invitrogen) and incubated at 37°C for 20 minutes. Hepatocytes were removed from the suspension by centrifugation (30 g for 1 minute), and liver lymphocytes were then purified from the cell suspension by a 40%/80% gradient of Percoll (Sigma-Aldrich). Liver lymphocytes were stained with CD1d dimers for 1 hour at 4°C, followed by staining with anti-CD3 (clone 145-2C11, Biolegend). The fluorescence of stained cells was measured by FACSCalibur (BD Biosciences), and data were analyzed by Flowjo (Tree Star).
In vitro cytokine assay.
Splenocytes (8 × 105 splenocytes/well of 96-well plate) from BALB/c mice were stimulated with glycolipid or vehicle control for 48 hours. Supernatants were collected, and the concentration of IFN-γ, IL-4, IL-13, or TNF-α was determined by ELISA (eBioscience).
In vivo cytokine assay.
The concentration of IFN-γ, IL-4, IL-13, IL-12 (p70), and TNF-α in plasma samples was determined by MILLIPLEX cytokine multiplex immunoassay kit (Millipore), using a Bio-Plex system (Bio-Rad) according to manufacturer’s instructions.
Human iNKT cell activation.
Human PBMCs from anonymous blood bank buffy coats (obtained with NIH approval) were separated by density centrifugation over a Ficoll-Paque gradient (GE Healthcare). Cells were labeled with 1 μM CellTrace Violet Cell Proliferation Dye (Invitrogen) for 15 minutes at room temperature. Labeled cells (4 × 106 cells/well of 24 well plate) were stimulated for 4 days with glycolipid or vehicle control. At the end of the culture, cells were harvested and stained with PBS57-loaded CD1d tetramer-APC (NIH Tetramer Facility), anti-Vα24-PE (clone C15, Beckman Coulter), anti-CD3-PE-Cy7 (clone UCHT1, BioLegend), and yellow LIVE/DEAD Fixable Dead Cell Stain (Invitrogen). The fluorescence of stained cells was measured by LSRII (BD Biosciences), and data were analyzed by FlowJo (Tree Star). iNKT cells were identified by gating on lymphocytes and live cells, followed by Vα24+CD3intermediatePBS57/CD1d tetramer+ cells. The percentage of iNKT cells that divided was determined by the percentage that had diluted the proliferation dye.
Statistics.
The data were analyzed using the nonparametric Mann-Whitney test using GraphPad Prism software (version 5; GraphPad software). The data were considered significant at P < 0.05. All experiments were repeated at least twice to confirm reproducibility of results.
Supplementary Material
Acknowledgments
The authors thank Satomi Takao, Karen Muindi, Susan Sharrow, and Tony Adams for their assistance in conducting these experiments; David Margulies and Howard Young for critical reading of the manuscript; Albert Bendelac for providing DN32.D3 iNKT cell hybridoma; Samuel Behar for providing iNKT cell hybridoma 24.9E; Nico Tjandra and Motoshi Suzuki for providing saposin C; and the NIH Tetramer Core Facility for supplying the PBS-57/CD1d tetramers. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by NIH grant AI45889 (to S.A. Porcelli). G.S. Besra is a former Lister Institute-Jenner Research Fellow and received support from the Medical Research Council (UK), the Wellcome Trust (084923/B/08/7), a Royal Society Wolfson Research Merit Award, and the James Bardrick Research Chair.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(2):683–694. doi:10.1172/JCI42314.
References
- 1. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2. Smyth MJ, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99(4):1259–1266. doi: 10.1182/blood.V99.4.1259. [DOI] [PubMed] [Google Scholar]
- 3. Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 4. Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180(6):3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 5. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6. Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(10–11):529–534. [PubMed] [Google Scholar]
- 8. Motoki K, et al. Immunostimulatory and antitumor activities of monoglycosylceramides having various sugar moieties. Biol Pharm Bull. 1995;18(11):1487–1491. doi: 10.1248/bpb.18.1487. [DOI] [PubMed] [Google Scholar]
- 9. Behar SM, Porcelli SA. CD1–restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 10. Smyth MJ, Godfrey DI. NKT cells and tumor immunity--a double-edged sword. Nat Immunol. 2000;1(6):459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 11. Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawano T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95(10):5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayakawa Y, et al. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31(6):1720–1727. doi: 10.1002/1521-4141(200106)31:6<1720::AID-IMMU1720>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14. Fuji N, Ueda Y, Fujiwara H, Toh T, Yoshimura T, Yamagishi H. Antitumor effect of alpha-galactosylceramide (KRN7000) on spontaneous hepatic metastases requires endogenous interleukin 12 in the liver. Clin Cancer Res. 2000;6(8):3380–3387. [PubMed] [Google Scholar]
- 15. Ambrosino E, et al. Cross–regulation between type I and type II NKT cells in regulating tumor immunity: A new immunoregulatory axis. J Immunol. 2007;179(8):5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 16. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 17. McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204(5):1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198(11):1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126(42):13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 20. Yu KO, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102(9):3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forestier C, et al. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J Immunol. 2007;178(3):1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 22. Silk JD, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169(3):1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 24. Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172(2):943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 25. Zigmond E, et al. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut. 2007;56(1):82–89. doi: 10.1136/gut.2006.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zigmond E, et al. Beta–glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am J Physiol Endocrinol Metab. 2009;296(1):E72–E78. doi: 10.1152/ajpendo.90634.2008. [DOI] [PubMed] [Google Scholar]
- 27. Parekh VV, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173(6):3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 28. Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 29. Fox LM, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7(10):e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silk JD, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180(10):6452–6456. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 31. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5(6):405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 32. Kitamura H, et al. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199(1):37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 33. Gumperz JE, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12(2):211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 34. Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 35. Ndonye RM, et al. Synthesis and evaluation of sphinganine analogues of KRN7000 and OCH. . J Org Chem. 2005;70(25):10260–10270. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]
- 36. Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WM. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J Exp Med. 1998;188(9):1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujii S, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103(30):11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198(1):173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 40. Kinjo Y, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15(7):654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer K, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101(29):10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13(4):459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 43. Alexander J, et al. Functional consequences of engagement of the T cell receptor by low affinity ligands. J Immunol. 1993;150(1):1–7. [PubMed] [Google Scholar]
- 44. Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181(4):1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaturvedi P, Yu Q, Southwood S, Sette A, Singh B. Peptide analogs with different affinities for MHC alter the cytokine profile of T helper cells. Int Immunol. 1996;8(5):745–755. doi: 10.1093/intimm/8.5.745. [DOI] [PubMed] [Google Scholar]
- 46. Sloan-Lancaster J, Steinberg TH, Allen PM. Selective activation of the calcium signaling pathway by altered peptide ligands. J Exp Med. 1996;184(4):1525–1530. doi: 10.1084/jem.184.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabinowitz JD, et al. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5(2):125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 48. Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide–major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand–related differences in T cell receptor–dependent intracellular signaling. J Exp Med. 1993;177(4):1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. z phosphorylation without ZAP–70 activation induced by TCR antagonists or partial agonists. Science. 1995;267(5197):515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 50. De Santo C, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 52. Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6(8):810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of alpha-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112(3):386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem Biophys Res Commun. 2005;327(4):1143–1154. doi: 10.1016/j.bbrc.2004.12.121. [DOI] [PubMed] [Google Scholar]
- 55. Peng W, Martaresche C, Escande–Beillard N, Cedile O, Reynier–Vigouroux A, Boucraut J. Influence of lipid rafts on CD1d presentation by dendritic cells. Mol Membr Biol. 2007;24(5–6):475–484. doi: 10.1080/09687680701359408. [DOI] [PubMed] [Google Scholar]
- 56. Im JS, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30(6):888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Motohashi S, Nakayama T. Natural killer T cell–mediated immunotherapy for malignant diseases. Front Biosci (Schol Ed). 2009;1:108–116. doi: 10.2741/s10. [DOI] [PubMed] [Google Scholar]
- 58. Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115(9):2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29(6):2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 60. Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17762. [PubMed] [Google Scholar]
- 61. Hodosi G, Kovac P. A fundamentally new, simple, stereospecific synthesis of oligosaccharides containing the β-mannopyranosyl and β-rhamnopyranosyl linkage. J Am Chem Soc. 1997;119(9):2335–2336. doi: 10.1021/ja964021y. [DOI] [Google Scholar]
- 62. Bien F, Ziegler T. Chemoenzymatic synthesis of glycosylated enantiomerically pure 4-pentene 1,2- and 1,3-diol derivatives. Tetrahedron: Asymmetry. 1998;9(5):781–790. [Google Scholar]
- 63. Uchiyama T, Hindsgaul O. Per-o-trimethylsilyl-α-l-fucopyranosyl iodide: a novel glycosylating agent for terminal α-l-fucosylation. Cheminform. 1996;27(42):499–501. [Google Scholar]
- 64. Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2004;114(1):80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 65. Fichtner-Feigl S, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68(9):3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.