Abstract
Th17 cells are a subset of CD4+ T cells with an important role in clearing certain bacterial and fungal pathogens. However, they have also been implicated in autoimmune diseases such as multiple sclerosis. Exposure of naive CD4+ T cells to IL-6 and TGF-β leads to Th17 cell differentiation through a process in which many proteins have been implicated. We report here that ectopic expression of liver X receptor (LXR) inhibits Th17 polarization of mouse CD4+ T cells, while LXR deficiency promotes Th17 differentiation in vitro. LXR activation in mice ameliorated disease in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, whereas LXR deficiency exacerbated disease. Further analysis revealed that Srebp-1, which is encoded by an LXR target gene, mediated the suppression of Th17 differentiation by binding to the E-box element on the Il17 promoter, physically interacting with aryl hydrocarbon receptor (Ahr) and inhibiting Ahr-controlled Il17 transcription. The putative active site (PAS) domain of Ahr and the N-terminal acidic region of Srebp-1 were essential for this interaction. Additional analyses suggested that similar LXR-dependent mechanisms were operational during human Th17 differentiation in vitro. This study reports what we believe to be a novel signaling pathway underlying LXR-mediated regulation of Th17 cell differentiation and autoimmunity.
Introduction
Th17 cells, a subset of CD4+ T cells characterized by the secretion of high levels of IL-17A, IL-17F, and IL-22, play an important role in both the immune response to invading pathogens and autoimmunity (1, 2). Naive CD4+ T cells differentiate into Th17 cells in the presence of TGF-β, IL-6, and antibodies against IL-4 and IFN-γ (3), with Stat3 (4), the nuclear receptor RAR-related orphan receptor RORγt (5), and RORα (6) implicated in this process. Besides these transcription factors, a host of other proteins have been reported to positively or negatively regulate Th17 differentiation. For example, aryl hydrocarbon receptor (Ahr), a cellular sensor of environmental toxins, promotes Th17 polarization through suppression of STAT1 phosphorylation (7–9). Runx1 (10), IRF4 (11), BATF (12), NLRP3 (13), and microRNA miR-326 (14) are reported to positively regulate Th17 cell differentiation, while Foxp3 (15), STAT5 (16), Ets-1 (17), IRF-4–binding protein (18), NR2F6 (19), Gfi1 (20), PPARγ (21), PKB/Akt (22), SOCS3 (23), and retinoic acid (24, 25) constrain Th17 generation.
Liver X receptor (LXR) is an orphan nuclear receptor with two isoforms: LXRα, which is specifically expressed in liver, fat, and macrophages; and LXRβ, ubiquitously expressed in various tissues (26–30). Walcher et al. reported that both isoforms are expressed in CD4+ T cells, implying that LXR may have a role in T cell function and effector T cell generation (31). LXR binds to DNA as a heterodimer with the retinoid X receptor (RXR) and acts as an important modulator of cholesterol homeostasis by regulating certain genes involved in cholesterol and fatty acid metabolism, such as ABCA1, ABCG1, SREBP1C, and fatty acid synthase (32). Recently, the role of LXR has been extended to the modulation of the immune response, with negative or positive effects under different circumstances. It has been reported that LXR is essential for macrophage survival and clearance of invading bacteria in protective immune responses (33, 34), whereas LXR activation also inhibited lymphocyte proliferation (35) and prevented the exposure of self-reactive antigens to T cells by regulating apoptotic cell clearance (36). Animal studies also showed LXR to be a double-edged sword in autoimmune disorders, as activation of LXR relieved EAE in one experimental model, but exacerbated articular inflammation and cartilage destruction in a collagen-induced arthritis model (37, 38). A recent study showed that an agonist of LXR, T0901317, suppressed EAE by inhibiting the expression of IL-23 receptor and other Th17 cytokines (39). However, the underlying mechanism remains elusive, because T0901317 has also been reported to be an farnesoid X receptor (FXR) agonist (40).
In the present study, using LXR-deficient mice and another well-defined agonist of LXR, GW3965, in addition to T0901317, we demonstrated that T0901317 and GW3965 ameliorated EAE in an LXR-dependent manner. More importantly, our results suggest that the LXR protein itself is a negative regulator of the initiation and perpetuation of EAE, as LXR deficiency caused exacerbation of EAE. The mechanism underlying LXR-mediated suppression of Th17 differentiation was investigated. We found that LXR overexpression significantly decreased in vitro cytokine-driven Th17 differentiation, while LXR deficiency promoted Th17 polarization. We further showed that Srebp-1 was recruited to the E-box element on the Il17 promoter upon LXR activation and interacted with Ahr to inhibit Il17 transcriptional activity. LXR activation also suppressed human Th17 differentiation, promoted SREBP-1 expression, and decreased AHR expression. Our study revealed what we believe to be a previously unreported pathway of Srebp-1 antagonism of Ahr in suppressing Th17 generation and autoimmunity.
Results
LXR is a negative regulator of EAE development and in vivo Th17 differentiation.
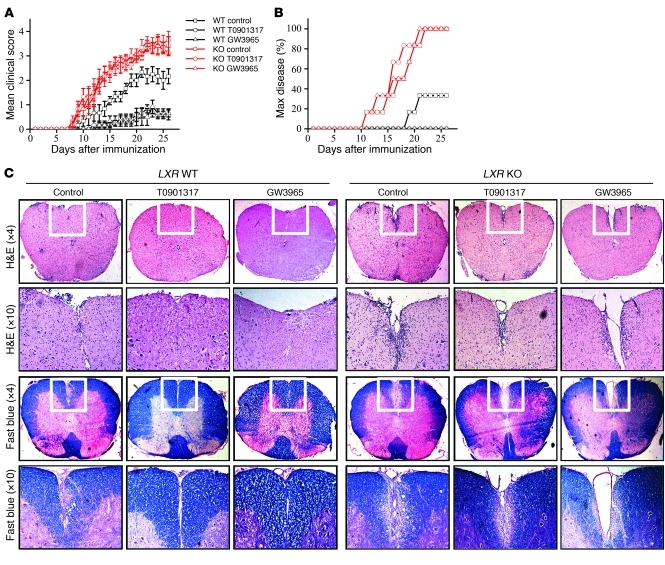
Previous investigation had suggested that T0901317 suppressed EAE, but considering the dual activator property of T0901317, it was urgent to clarify the underlying mechanism. EAE was induced in LXR KO mice and WT littermate control mice with or without the LXR agonist T0901317 or GW3965 treatment. Administration of either GW3965 or T0901317 reduced the clinical scores and EAE maximum disease incidence in WT mice, but not in LXR KO mice (Figure 1, A and B). Histological data also revealed severe demyelination and prominent inflammatory infiltration in the spinal cords of both WT and LXR KO mice. The demyelination and inflammation were dramatically ameliorated after LXR agonist treatment in WT mice but not in LXR KO mice (Figure 1C). These results suggest that T0901317 and GW3965 acted through LXR. It was interesting to note that LXR KO mice developed even more severe EAE than WT mice, which indicated that the LXR protein itself is a negative regulator of EAE development.
Figure 1. Oral administration of LXR agonists ameliorates EAE in LXR WT mice but not in LXR KO mice.
(A) EAE was induced in LXR KO mice or WT littermate control mice with or without daily oral administration of LXR agonist from day 3 after immunization to day 17 (6 or 7 mice per group). Data are expressed as mean ± SEM and represent 2 independent experiments with similar results. P = 0.009, WT control versus WT T0901317; P = 0.006, WT control group versus WT GW3965; P = 0.832, KO control versus KO T0901317; P = 0.651, KO control versus KO GW3965; P = 0.039, WT control versus KO control. (B) Maximum (Max) disease was set with a clinical score ≥3. P = 0.002, WT control versus WT T0901317; P = 0.002, WT control versus WT GW3965; P = 0.857, KO control versus KO T0901317; P = 0.807, KO control versus KO GW3965; P = 0.007, WT control versus KO control. (C) Histological staining of spinal cord sections from each group on day 15 after immunization. The white boxes in the upper panels (×4 objective) are enlarged in the lower panels (×10 objective). Total original magnification is ×40 and ×100, respectively.
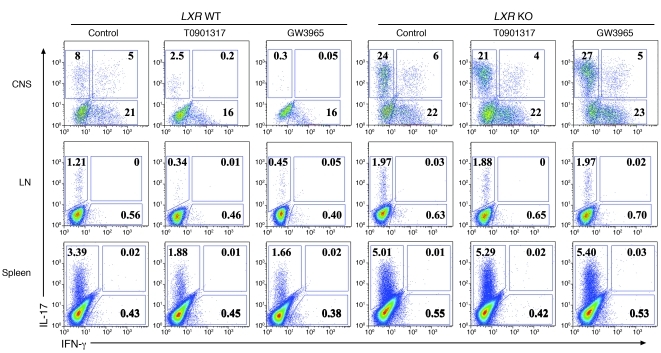
Because Th17 is dominant in the development of EAE, we also investigated the in vivo production of the inflammatory cytokines IL-17 and IFN-γ. IL-17 secretion in the CNS, spleen, and lymph node was markedly suppressed after drug administration in WT mice, but not LXR KO mice, while IFN-γ was only moderately affected (Figure 2). Next, we focused on the mechanisms underlying the Th17 regulation by LXR in an in vitro setting.
Figure 2. Oral administration of LXR agonists decreases IL-17 production in vivo in LXR WT mice but not in LXR KO mice.
Intracellular staining of IL-17 and IFN-γ on CD4+ T cells isolated from the CNS, groin lymph nodes, and spleen, as indicated, 15 days after immunization (6 mice per group). Values in the quadrants indicate the percentage of IL-17+IFN-γ– cells, IL-17+IFN-γ+ cells, and IL-17–IFN-γ+ cells.
LXR negatively regulates in vitro Th17 differentiation.
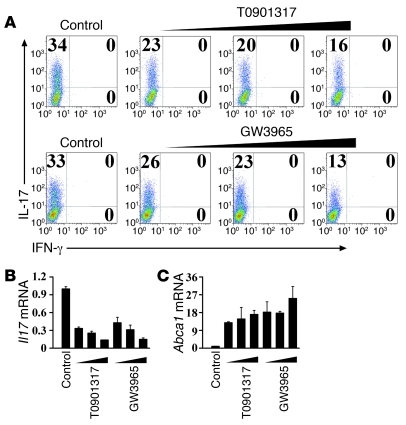
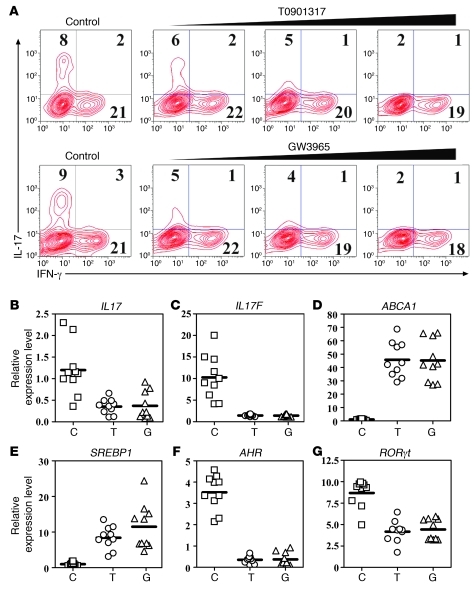
The Th17 differentiation system was established by stimulating naive CD4+ T cells with TGF-β and IL-6 in the presence of TCR activation. The inclusion of GW3965 and T0901317 led to substantial suppression of Th17 cells in a dose-dependent manner (Figure 3, A and B). Abca1, a downstream target of LXR, was induced following the addition of GW3965 and T0901317, suggesting that LXR was functionally activated (Figure 3C).
Figure 3. LXR activation suppresses in vitro Th17 cell differentiation.
(A) Mouse naive CD4+ T cells were cultured under Th17-inducing conditions for 4 days in the presence of LXR agonists T0901317 (0.5 μM, 1 μM, 2 μM) and GW3965 (2.5 μM, 5 μM, 10 μM) and subjected to intracellular staining of IL-17 and IFN-γ. This experiment was repeated more than 10 times with similar results. Values in the quadrants indicate the percentage of IL-17+IFN-γ– cells, IL-17+IFN-γ+ cells, and IL-17–IFN-γ+ cells. Cells from A were harvested for real-time PCR analysis of the Il17 (B) and Abca1 (C) mRNA levels. Data are expressed as mean ± SD in B and C.
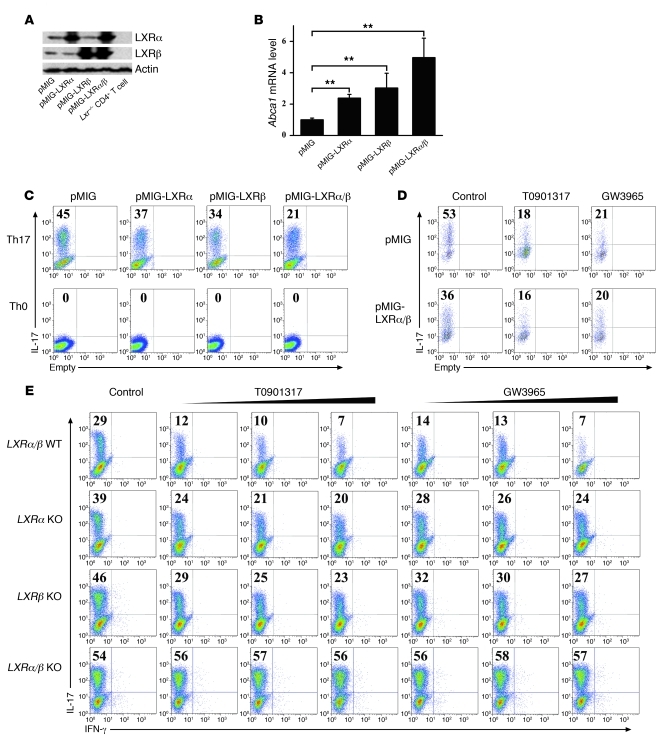
To study the individual role of LXRα and LXRβ in Th17 differentiation, these two isoforms of LXR were ectopically expressed in primary CD4+ T cells during Th17 induction. Western blot analysis with antibodies to LXRα and LXRβ (Figure 4A) and real-time PCR assay of the LXR target gene Abca1 (Figure 4B) indicated that LXRα and LXRβ were functionally expressed in CD4+ T cells. Overexpression of either LXRα or LXRβ caused a decrease in the expression of IL-17, while the combination of the two isoforms had a synergistic effect (Figure 4C), suggesting that both were involved in the regulation of Th17 differentiation. Furthermore, the LXR agonists GW3965 and T0901317 suppressed Th17 differentiation in LXR WT or LXR-overexpressing T cells (Figure 4D). Single deficiency of either gene resulted in a partial abrogation of the suppression of Th17 in response to LXR ligation, while the absence of both isoforms led to a resistance to Th17 repression upon LXR activation. This experimental evidence supported the notion that both isoforms of LXR were involved in regulation of Th17 generation (Figure 4E). Moreover, T cells from LXRα single-KO mice, LXRβ single-KO mice, or LXRα/β double-KO mice displayed increased Th17 induction compared with T cells from LXR WT littermate control mice (Figure 4E), suggesting a negative regulatory role of LXRα and LXRβ in Th17 cell differentiation.
Figure 4. LXRα/β negatively regulates in vitro Th17 differentiation.
(A) Mouse naive CD4+ T cells were cultured under Th17-inducing conditions with or without retroviral expression of LXRα and LXRβ for 4 days before protein isolation and Western blot analysis. Protein prepared from LXR KO CD4+ T cells was loaded as a control. (B) Cells from A were harvested for real-time PCR analysis of the Abca1 mRNA levels. **P < 0.01. Data in B are expressed as mean ± SD. (C) Naive CD4+ T cells were cultured under Th17- or Th0-inducing conditions with or without retroviral expression of LXRα and LXRβ for 4 days before IL-17 staining, with the gate set on GFP+CD4+ cells. This experiment was repeated at least 3 times with similar results. (D) Naive CD4+ T cells were cultured under Th17-inducing conditions with or without retroviral expression of LXRα/β and subjected to IL-17 staining with the gate set on GFP+CD4+ cells. LXR agonists T0901317 (2 μM) and GW3965 (10 μM) were added as indicated. This experiment was repeated at least 3 times with similar results. (E) Naive CD4+ T cells from LXR WT littermate control mice, LXRα KO, LXRβ KO, and LXRα/β KO mice were cultured under Th17-inducing conditions for 4 days in the presence of LXR agonists T0901317 (0.5 μM, 1 μM, 2 μM) and GW3965 (2.5 μM, 5 μM, 10 μM) before IL-17 and IFN-γ staining (3 mice per group). Values in C–E indicate the percentage of IL-17+ cells.
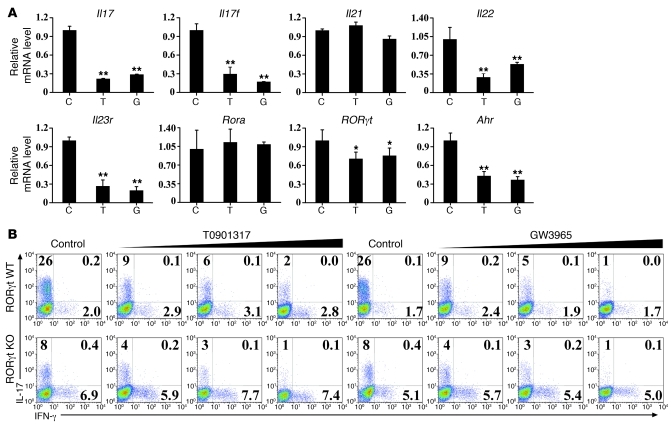
LXR agonists decrease expression of RORγt and other Th17-related genes in Th17 cells.
RORγt is a key transcription factor and plays an important role in Th17 cell differentiation. To clarify whether LXR activation reduces expression of RORγt and other Th17-related genes, we performed real-time PCR and found that T0901317 and GW3965 treatment resulted in decreased expression of RORγt mRNA levels and suppressed Th17-related genes including Il17, Il17f, Il22, Il23r, and Ahr, whereas Il21 and Rora mRNA expression levels remained unchanged (Figure 5A). This is in accordance with a recent study reporting that LXR agonist T0901317 exhibited an inverse agonistic effect on transcriptional factor RORγt (41). However, the suppressive effect of LXR agonists on Th17 cell generation was not totally abolished in RORγt-deficient cells (Figure 5B), suggesting that other pathways were also involved in Th17 differentiation suppression by LXR.
Figure 5. LXR agonists suppress expression of RORγt and other Th17-related genes.
(A) Naive CD4+ T cells were cultured under Th17-inducing conditions for 4 days in the presence of vehicle control (C), T0901317 (T), and GW3965 (G) before cells were harvested for real-time PCR analysis of the genes as indicated above. Data are expressed as mean ± SD, and this experiment was repeated at least 3 times with similar results. *P < 0.05; **P < 0.01. (B) Naive CD4+ T cells isolated from RORγt WT mice or RORγt KO mice were cultured under Th17-inducing conditions with or without LXR agonists T0901317 (0 μM, 0.5 μM, 1 μM, 2 μM) and GW3965 (0 μM, 2.5 μM, 5 μM, 10 μM) for 4 days and subjected to intracellular staining of IL-17 and IFN-γ. This experiment was repeated at least 3 times with similar results. Values in the quadrants indicate the percentage of IL-17+IFN-γ– cells, IL-17+IFN-γ+ cells, and IL-17–IFN-γ+ cells.
LXR controls Th17 cell differentiation through Srebp-1a and Srebp-1c activation.
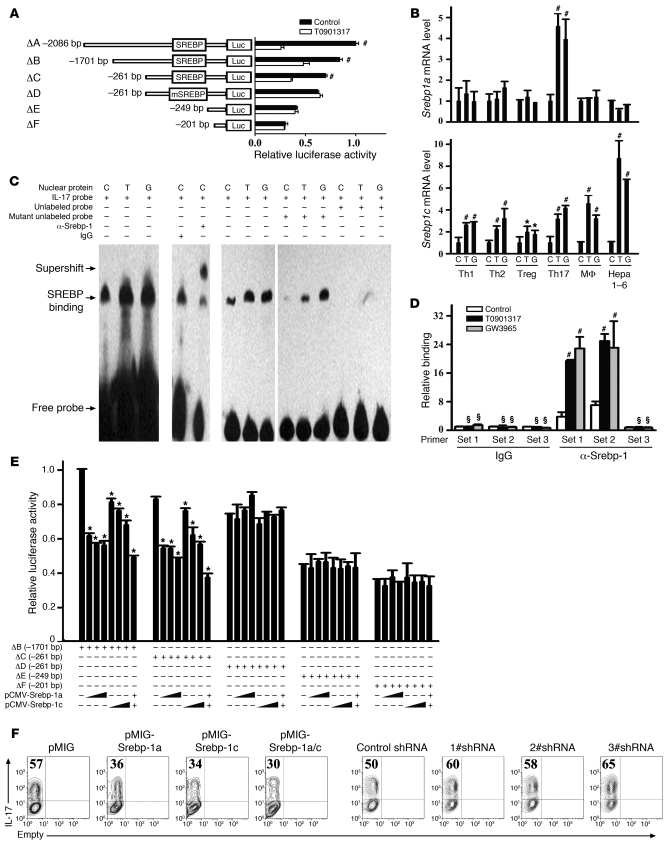
We next investigated the mechanisms underlying LXR inhibition of Th17 cell differentiation through an Il17 promoter activity assay. A series of Il17 promoter deletions linked to firefly luciferase constructs were transfected into Jurkat cells and cultured in the presence or absence of T0901317. Interestingly, T0901317 significantly decreased the promoter activity until the promoter was shortened to –249 bp upstream of the transcription initiation site (Figure 6A). Analysis of this region of the Il17 promoter (spanning –261 bp to –249 bp) using the Transcription Element Search System (TESS) program (provided by the Computational Biology and Informatics Laboratory at the University of Pennsylvania Schools of Medicine Engineering and Applied Science) revealed an E-box element, a putative Srebp-1–binding site. Deletion or mutation of this putative binding site for Srebp-1 (ΔD and ΔE) abrogated the suppression of Il17 transcriptional activity by T0901317, suggesting that Srebp-1 may be responsible for the suppression of Th17 cell differentiation through LXR activation. Therefore, we investigated whether LXR agonists regulated the expression levels of the two isoforms of Srebp-1, Srebp-1a and Srebp-1c, during Th17 differentiation. A previous study had indicated that Srebp-1c, rather than Srebp-1a, was selectively induced after LXR activation in the liver, intestine, and white fat (42). This finding was confirmed in a macrophage cell line and a hepatocyte cell line in our study using real-time PCR (Figure 6B). T cell subsets (Th1, Th2, and Treg) except for Th17 cells showed a similar selectivity of Srebp1c upon LXR activation. However, in Th17 cells, not only Srebp1c, but also Srebp1a, was induced in response to LXR agonists, implying a specific role of Srebp-1a in Th17 cell differentiation (Figure 6B). These data suggest there may be different regulatory mechanisms for Srebp-1 isoforms in various types of cells.
Figure 6. LXR activation suppresses IL-17 transcriptional activity through the promotion of Srebp-1 binding to the E-box element on the Il17 promoter.
(A) Jurkat cells were transfected with fragments of the mouse Il17 promoter linked to a firefly luciferase construct and cultured with or without T0901317 (2 μM) before luciferase reporter assays. (B) Mouse naive CD4+ T cells were cultured under Th1-, Th2-, Treg-, or Th17-inducing conditions for 4 days in the presence of the indicated drugs before real-time PCR analysis of Srebp1a and Srebp1c. Mouse macrophage cell line RAW264.7 (MF) and hepatocyte cell line Hepa 1-6 were included as controls. (C and D) Srebp-1 binding to the E-box element on the Il17 promoter in in vitro differentiated Th17 cells was assessed by EMSA (C) and ChIP (D). The primers used to detect ChIP signals are schematically represented in Supplemental Figure 1. Statistical analysis was performed between the LXR agonist–treated and untreated groups. (E) Srebp-1a and Srebp-1c expression plasmids were transfected into Jurkat cells for luciferase reporter assay. (F) Naive CD4+ T cells were cultured under Th17-inducing conditions with or without retroviral overexpression or knockdown of Srebp-1a/c before IL-17 staining. This experiment was repeated at least 3 times with similar results. Values indicate the percentage of IL-17+ cells.*P < 0.05, #P < 0.01, §P > 0.05. Data are expressed as mean ± SD in A, B, D, and E.
To determine whether Srebp-1 could physically bind to this putative Srebp-1–binding site in the Il17 promoter, we performed EMSA (Figure 6C) and ChIP assays (Figure 6D). A synthesized probe containing this –261 bp to –249 bp region of the Il17 promoter bound to Srebp-1. Inclusion of anti–Srebp-1 in the protein/probe binding mixture led to a supershift, whereas the nonspecific control IgG had no effect. Moreover, unlabeled probes were able to compete for binding to the Srebp-1 protein, whereas mutant unlabeled probes had no competitive effect (Figure 6C). Furthermore, as shown by the ChIP assay results, Srebp-1 was recruited to a region containing the E-box element, but not an adjacent region of the Il17 promoter after LXR activation (Figure 6D and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI42974DS1).
The role of Srebp-1 in Il17 transcription was investigated using the Il17 promoter activity assay (Figure 6E). Both Srebp-1a and Srebp-1c dose-dependently suppressed Il17 transcriptional activity, as long as the deletion construct contained an intact Srebp-1–binding site. To further investigate the role of Srebp-1a and Srebp-1c in Th17 cell differentiation, we performed retroviral overexpression or knockdown of Srebp-1 by shRNA. The efficiency of Srebp-1 overexpression or knockdown in CD4+ T cells was determined by real-time PCR, as there is currently no antibody available to discriminate between the two isoforms (Supplemental Figure 2). As expected, forced expression of Srebp-1a and Srebp-1c inhibited Th17 cell differentiation, while the knockdown of either isoform of Srebp-1 led to increased Th17 cell differentiation (Figure 6F). These data suggest that the LXR-induced suppression of Th17 cell differentiation is mediated through Srebp-1.
Srebp-1 physically interacts with Ahr to interfere with Th17 cell differentiation.
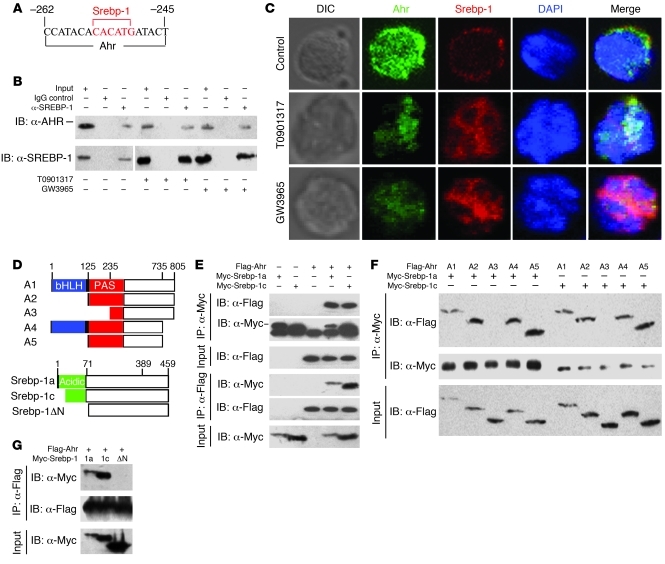
Detailed analysis of the Il17 promoter using the TESS program revealed a putative binding site for Ahr adjacent to the Srebp-1–binding site (Figure 7A). Ahr positively regulates Th17 cell differentiation, and the overlapping locations of the two binding sites suggest that Srebp-1 may negatively regulate Th17 cell differentiation by disrupting Ahr-induced Th17 promotion. ChIP assay with antibody to Ahr showed binding of Ahr to Il17 promoter, and this binding was affected by LXR activation in Th17 cells (Supplemental Figure 3), while LXR agonists treatment promoted Srebp-1 binding onto the Il17 promoter at the same region (Figure 6D). These data suggest that Ahr may be displaced by Srebp-1 following LXR activation. To determine whether Srebp-1 and Ahr physically interacted with each other, lysates from in vitro differentiated Th17 cells with or without T0901317 or GW3965 treatment were immunoprecipitated by anti–Srebp-1 antibody and immunoblotted with anti-Ahr antibody. Ahr was detected among the immunoprecipitated proteins (Figure 7B). This coimmunoprecipitation experiment was also performed in another direction (Supplemental Figure 4). Interestingly, a weaker interaction between Ahr and Srebp-1 was also observed in Th17 cells without LXR agonist treatment, implying that Srebp-1–dependent antagonism of Ahr might act as an intrinsic restraint on Th17 polarization and maintain a healthy balance in the immune system. We also examined the localization of Ahr and Srebp-1 in Th17 cells by immunostaining. Before activation, Srebp-1 was located in the cytoplasm and attached to the nuclear envelope and endoplasmic reticulum. In the presence of LXR agonists, mature Srebp-1 was generated through proteolytic cleavage of the N-terminal of the pre-mature Srebp-1 and translocated into the nucleus, as shown in Figure 7C. Ahr, as a positive regulator of the Th17 program, was highly expressed under Th17-inducing conditions and colocalized with Srebp-1 at a few sites in the nucleus (Figure 7C, top row). Confocal microscopy examination suggested that colocalization of Ahr and Srebp-1 in the nucleus of Th17 cells was dramatically enhanced following LXR activation (Figure 7C, middle and bottom rows, and Supplemental Figure 5). We also noted that LXR agonists decreased Ahr expression levels in the nucleus (Figure 7C).
Figure 7. Srebp-1 physically interacts with Ahr.
(A) Schematic representation of a fragment of the mouse Il17 promoter containing putative binding sites for Srebp-1 and Ahr. (B to C) Naive CD4+ T cells were cultured under Th17-inducing conditions with the indicated drugs before (B) coimmunoprecipitation and (C) confocal microscopy (×40 oil objective). A differential interference contrast (DIC) photo reveals the overall cellular structure, and the nucleus was stained with DAPI. The vertical line in B indicates that the lanes were run on the same gel but were noncontiguous. (D) Schematic representations of Ahr or Srebp-1 or deletion constructs (A1, A2, A3, A4, and A5 for Ahr; Srebp-1a, Srebp-1c, and Srebp-1ΔN for Srebp-1). bHLH, basic helix-loop-helix. (E–G) Flag-tagged Ahr and Myc-tagged Srebp-1 (E), Flag-tagged Ahr or deletions and Myc-tagged Srebp-1 (F), or Flag-tagged Ahr and Myc-tagged Srebp-1 or deletion mutants (G) were transiently transfected into HEK293T cells for coimmunoprecipitation.
To determine the exact domains responsible for the contact between Ahr and Srebp-1, we expressed myc-tagged Srebp-1a, Srebp-1c, or a deletion construct (Srebp-1ΔN) together with FLAG-tagged full-length Ahr (A1) or a series of deletion constructs (A2–A5) in HEK293 cells, and performed coimmunoprecipitation (Figure 7, D–G). As shown in Figure 7E, anti-Myc immunoprecipitated FLAG-tagged protein, and vice versa. Moreover, when the PAS domain of Ahr was deleted, the physical interaction was disrupted (Figure 7F), a result that was also obtained following deletion of the N-terminal acidic region of Srebp-1 (Figure 7G), suggesting a critical role of the PAS domain of Ahr and the N-terminal acidic region of Srebp-1 in the physical interaction between these two proteins.
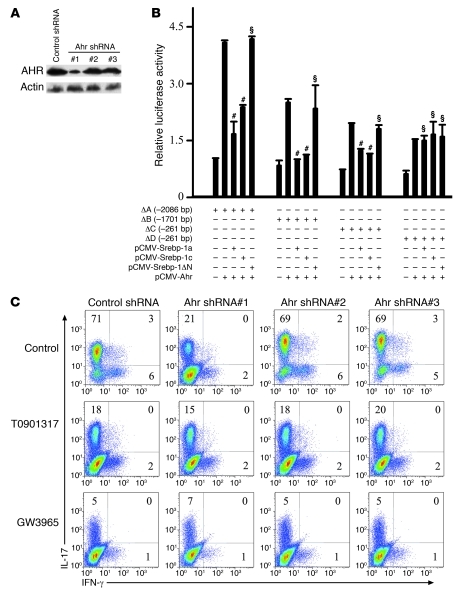
After confirmation of the physical interaction between Srebp-1 and Ahr, the effect of Srebp-1 on the transcriptional activity of Ahr was investigated. Ectopic expression of Ahr upregulated Il17 transcriptional activity, but this was disrupted by cotransfection with either Srebp-1a or Srebp-1c, but not Srebp-1ΔN (Figure 8B). Interestingly, when the E-box element on the Il17 promoter was mutated, the suppressive effect of Srebp-1 was no longer observed (Figure 8B). Furthermore, in naive CD4+ T cells, either LXR activation or Ahr knockdown by shRNA (#1) led to a dramatic decrease in IL-17 expression, while shRNA (#2 and #3) without an effective knockdown of Ahr had no influence on Th17 cells (Figure 8, A and C). Taken together, these data indicate that Srebp-1 inhibited Th17 cell differentiation by physically interacting with the PAS domain of Ahr to suppress Ahr-induced Il17 transcription.
Figure 8. Srebp-1 interferes with Ahr-promoted ILI7 transcription.
(A) Western blot was performed using cells (C) to assess the efficiency of Ahr knockdown. (B) Ahr, Srebp-1a, or Srebp-1c constructs were transfected into Jurkat cells for luciferase reporter assay. #P < 0.01, §P > 0.05 compared with the Ahr single transfection group. Data are expressed as mean ± SD. (C) Mouse naive CD4+ T cells were cultured under Th17-inducing conditions with the indicated drugs and retroviral shRNA for 4 days before intracellular staining. This experiment was repeated at least 3 times with similar results. Values in the quadrants indicate the percentage of IL-17+IFN-γ– cells, IL-17+IFN-γ+ cells, and IL-17–IFN-γ+ cells.
LXR activation decreases in vitro human Th17 differentiation.
We further investigated the role of LXR in human Th17 cell differentiation in relation to SREBP-1–dependent antagonism of AHR. The addition of GW3965 or T0901317 dose-dependently decreased Th17 polarization (Figure 9A). This was also confirmed by the finding that the IL17 and IL17F mRNA levels were inhibited by T0901317 or GW3965 (Figure 9, B and C). The induction of ABCA1 suggested that LXR was functionally activated (Figure 9D). The SREBP1 mRNA level was promoted, while expression of AHR and RORγt was decreased (Figure 9, E–G). These results suggest that LXR’s suppressive role in Th17 cell differentiation in the human was similar to that in the mouse immune system.
Figure 9. LXR activation suppresses in vitro human Th17 cell differentiation.
(A) Human naive CD4+ T cells isolated from the peripheral blood mononuclear cells of a healthy donor were cultured under Th17-inducing conditions for 4 days in the presence of LXR agonists T0901317 (0.5 μM, 1 μM, 2 μM) and GW3965 (2.5 μM, 5 μM, 10 μM) and subjected to the intracellular staining of IL-17 and IFN-γ. Fifteen individual specimens were studied, with similar results. Values in the quadrants indicate the percentage of IL-17+IFN-γ– cells, IL-17+IFN-γ+ cells, and IL-17–IFN-γ+ cells. (B–E) Cells from A were harvested for real-time PCR analysis of the mRNA levels of IL17 (B), IL17F (C), ABCA1 (D), SREBP1 (E), AHR (F), and RORγt (G). The y-axis in B–G represents the relative expression level of genes using arbitrary unit. T0901317 decreased the IL17 (P < 0.01), IL17F (P < 0.01), AHR (P < 0.01), and RORγt (P < 0.05) mRNA levels and increased ABCA1 (P < 0.01) and SREBP1 (P < 0.01). GW3965 suppressed the expression of IL17 (P < 0.01), IL17F (P < 0.01), AHR (P < 0.01), and RORγt (P < 0.05) and increased the mRNA level of ABCA1 (P < 0.01) and SREBP1 (P < 0.01).
Discussion
The role of LXR in immune regulation is still under investigation. Studies of LXR in macrophages (33, 36) and T cells (35) suggested that the role of LXR in immunity was very complicated, with either activating or suppressive functions under different conditions. In 2006, Hindinger et al. reported that the LXR agonist T0901317 ameliorated EAE by inhibiting T cell activation (38). A more recent study also showed that T0901317 inhibited IL-23 receptor expression and suppressed IL-17 production in CD90+ splenocytes from EAE mice (39). Because T0901317 is a dual agonist of both LXR and FXR (40), it is difficult to differentiate the pathway of the drug activity, and to understand the mechanism underlying the drug effect. To clarify this point, we used LXR gene KO mice and another agonist of LXR, GW3965, in addition to T0901317, to investigate the role of LXR in EAE development and Th17 differentiation from naive CD4+ T cells. T0901317 and GW3965 ameliorated EAE in WT mice, but not in LXR KO mice, suggesting both agonists suppressed autoimmunity via LXR. Furthermore, LXR activation and deficiency repressed and exacerbated EAE development, respectively. Both agonists exhibited dose-dependent inhibition of the differentiation of Th17 cells from human and mouse naive CD4+ T cells, but not in LXR-deficient cells. Overexpression of LXR in naive CD4+ T cells inhibited differentiation toward Th17 polarization, while deficiency of LXR promoted this process. These data strongly suggest that LXR plays a critical negative regulatory role during EAE development and Th17 differentiation. Further investigation with adoptively transferred naive CD4+ T cells from LXR WT or LXR KO mice to irradiated Rag1–/– mice before EAE induction demonstrated an intrinsic role of LXR in EAE development and Th17 differentiation (Supplemental Figure 6). As Rag1–/– mice lack mature T cells and B cells, and irradiation results in impairment of the growth of antigen-presenting cells, EAE development would be highly dependent on the adoptively transferred CD4+ T cells. LXR KO CD4+ T cells conferred much stronger susceptibility to EAE induction than LXR WT cells.
Kumar et al. have demonstrated recently that LXR agonist T0901317 is a novel RORα/γ inverse agonist (41). Our data also showed that LXR activation led to decreased RORγt expression, although RORα was not affected. However, further investigation revealed that LXR agonists were still effective in suppressing Th17 cell differentiation in RORγt-deficient cells. This result indicates that mechanisms in addition to RORγt inhibition are likely involved in this process. To elucidate the precise mechanism underlying the suppression of Th17 differentiation by LXR, we performed an Il17 promoter deletion assay and analyzed the Il17 promoter with the TESS program. We found that a putative Srebp-1–binding site may be involved in Th17 cell suppression by LXR. Two isoforms of Srebp-1, Srebp-1a and Srebp-1c, are traditionally considered to be modulators of cholesterol homeostasis and fatty acid synthesis (43, 44). Srebp1c has been suggested to be a target gene of LXR, because LXR activation selectively promotes Srebp1c transcription in the intestine, liver, and white fat (42). In our study, upon LXR activation, expression of both of the Srebp-1a and Srebp-1c isoforms was increased, and this event was involved in the suppression of Th17 cell differentiation. Because Srebp-1 has a transactivation domain in its N-terminus, Srebp-1 may indirectly exert an inhibitory effect on the Il17 promoter so as to reduce Il17 transcription after translocation into the nucleus following LXR activation. Detailed analysis of the Il17 promoter using the TESS program revealed a putative binding site for Ahr adjacent to the Srebp-1–binding site. Ahr positively regulates Th17 cell differentiation, and the overlapping of the two binding sites suggests that Srebp-1 may negatively regulate Th17 cell differentiation by disrupting Ahr-induced Th17 promotion.
Ahr is an important positive regulator of Th17 cell differentiation (7, 8). Kimura et al. suggested that Ahr directly interacted with the inhibitory factors STAT1 and STAT5 during Th17 differentiation, inhibiting STAT1 phosphorylation and activation and thus promoting Th17 polarization (9). Our data also showed that Ahr activated Il17 transcription, and this effect was suppressed by LXR-activated Srebp-1. These results suggest that Srebp-1–dependent antagonism of Ahr plays a very important role in LXR-mediated suppression of Th17 cell differentiation. It is noteworthy that LXR agonists were still effective in inhibiting Th17 cells even in Ahr knockdown cells, although to a much lesser extent than in cells containing wild-type Ahr. This indicates that Ahr antagonism is the major contributor to Th17 suppression after LXR activation, but that it does not act exclusively. It is interesting to note that the Ahr expression level in the nuclei of Th17 cells was moderately decreased in the presence of LXR agonists, which implies that LXR downregulated not only Ahr transcriptional activity, but also its protein level, an observation that is currently under further investigation. The physical interaction between Ahr and Srebp-1 and their colocalization were remarkably enhanced by the LXR agonists. However, in the absence of LXR activation treatment, a weak interaction between these two proteins and colocalization were still detectable, suggesting that Srebp-1–dependent antagonism of Ahr transcriptional activity may be an intrinsic mechanism during Th17 cell differentiation. The process of Th17 cell differentiation is probably controlled by the critical balance between Ahr and Srebp-1. A cytokine milieu of TGF-β and IL-6 contributes to Ahr expression and shifts the balance to Th17 cell differentiation, whereas LXR-activated Srebp-1 interferes with the Th17-promoting activity of Ahr and reduces the differentiation of this subset of inflammatory T cells, thus maintaining a healthy balance in the immune system.
Since the discovery of the subset of Th17 inflammatory T cells, RORα (NR1F1), RORγ (NR1F3), NR2F6, and a ligand-regulated PAS superfamily member, Ahr, have been reported to play important roles during their differentiation. In this study, we addressed the role of LXR, an important orphan nuclear receptor for cholesterol homeostasis, as a critical negative regulator of Th17 cell differentiation, along with its underlying mechanism. We demonstrated that LXRα (NR1H3) and LXRβ (NR1H2) negatively regulated Th17 cell differentiation through the activation of Srebp-1, which subsequently bound to the Il17 promoter and directly interacted with AHR, a positive regulator of Th17 differentiation, antagonizing its promotion of Il17 transcription.
Furthermore, LXR, which serves as a cholesterol sensor and metabolic checkpoint during inflammatory T cell differentiation, provides a link between cellular cholesterol levels and cell differentiation. Cholesterol may provide a metabolic negative signal to restrain T cell differentiation into the Th17 subset and thus impact the development of autoimmune diseases such as multiple sclerosis (MS). More than 50 years ago, two clinical studies (45, 46) suggested that MS patients had lower cholesterol levels than healthy people. Our study may provide an explanation for this observation given that a lower cholesterol level favors Th17 polarization and hence the subsequent development of Th17 inflammatory cell-dominant autoimmune diseases such as MS.
In conclusion, we report a signaling pathway in which Srebp-1–dependent antagonism of Ahr mediates the suppression of Th17 cell differentiation by LXR. This study also provides evidence for the role of LXR as a negative regulator of autoimmunity and inflammatory T cell differentiation and reveals crosstalk between different regulators of Th17 polarization.
Methods
Study subjects.
Blood samples were obtained from healthy volunteers with the help of Xuhui Central Hospital. Samples were collected after written informed consent was obtained, and the study was reviewed and approved by the Institutional Review Board of the Institute for Nutritional Sciences, Chinese Academy of Sciences in Shanghai, China.
Animals.
LXR KO mice were provided by David Mangelsdorf (University of Texas Southwestern, Dallas, Texas, USA). Exons 3–6 of LXRα were replaced with the neo resistance gene (47). Exons 5–6 of LXRβ were replaced with the neo resistance gene. Briefly, a BglII and SacII fragment of the gene that cut out the entire DBD exon and part of the next LBD exon (exons 5–6) was replaced with a PGK-neo cassette by homologous recombination. The expression of DBD and LBD was entirely eliminated in KO mice (48). WT littermate mice were used as controls, as indicated. RORγt KO mice were obtained from The Jackson Laboratory. Mice were maintained under pathogen-free conditions, and the animal study was reviewed and approved by the Institutional Review Board of the Institute for Nutritional Sciences, Chinese Academy of Sciences in Shanghai, China.
Induction and treatment of EAE.
LXR KO mice or WT littermate control mice (8–10 weeks) were immunized subcutaneously with a synthetic peptide of myelin oligodendrocyte glycoprotein peptide (MOG residues 35–55) (BioAsia Biotechnology) in complete Freund’s adjuvant (CFA) (Difco Laboratories). Pertussis toxin (List Biological Laboratories) was administered i.p. on the day of immunization and 48 hours later. Mice were orally administered vehicle, GW3965 (20 mg/kg body weight), or T0901317 (25 mg/kg body weight) daily from day 3 to day 17. Disease symptoms were scored according to the standard scale: 0, no sign; 1, limp tail; 2, paraparesis (incomplete paralysis of 1 or 2 hind limbs); 3, paraplegia (complete paralysis of 2 hind limbs); 4, paraplegia with forelimb weakness or paralysis; 5, moribund state or death.
Histology.
Mice were sacrificed, and spinal cords were removed and fixed in 4% PFA before being embedded in paraffin. Spinal cord sections (10 μm thick) were transferred to gelatin-coated slides. Sections were stained with H&E for detection of mononuclear cell infiltration or Luxol fast blue (Sigma-Aldrich) to determine demyelination and examined under a light microscope.
Preparation of mononuclear cells from mouse CNS tissue.
Mononuclear cells from CNS tissue were isolated from the brain and spinal cord using gradient centrifugation. Mice were perfused with PBS to eliminate blood before brain and spinal cord tissue were dissected. The cell suspension was centrifuged on a Percoll gradient (GE Healthcare). Cells at the interface between the two gradients were considered to be mononuclear cells.
Cell purification.
Mouse CD4+ T cells were isolated from splenocytes using a mouse CD4-negative selection kit (Dynal Biotech). For isolation of CD4+CD25– T cells, the purified CD4+ T cell populations were incubated with PE-labeled anti-CD25 antibody and anti-PE magnetic beads and isolated using a MACS separation column (Miltenyi Biotec). Naive CD4+CD25–CD62L+ T cells were prepared after a further purification using the CD62L microbeads component from a CD4+CD62L+ T cell isolation kit (Miltenyi Biotec). For human naive CD4+ T cell isolation, mononuclear cells were isolated from peripheral blood through Ficoll-Hypaque centrifugation before purification of CD4+CD25– T cells using a kit from Miltenyi Biotec.
Cell culture.
For mouse T cell differentiation, the above purified naive CD4+ T cells from 8- to 10-week-old mice were cultured in RPMI supplemented with 10% fetal bovine serum in the presence of anti-CD3/28 antibodies. 10 ng/ml IL-12 and 10 μg/ml anti–IL-4 antibody (for Th1); 10 ng/ml IL-4 and 10 μg/ml anti–IFN-γ (for Th2); 2 ng/ml TGF-β, 10 μg/ml anti–IL-4, and 10 μg/ml anti–IFN-γ (for Treg); or 10 ng/ml IL-6, 2 ng/ml TGF-β, 10 μg/ml anti–IL-4, and 10 μg/ml anti–IFN-γ (for Th17) were added to drive T cell polarization. For human Th17 differentiation, human CD4+CD25– T cells were activated with anti-CD3/28 antibodies in the presence of 50 ng/ml IL-6, 10 ng/ml IL-1β, 10 μg/ml anti–IL-4, and 10 μg/ml anti–IFN-γ. After 4 days, cells were restimulated with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of GolgiPlug (BD Biosciences — Pharmingen) for 5 hours before intracellular staining.
Flow cytometry.
For surface staining of CD4, T cells were resuspended in PBS containing 1% BSA and were incubated on ice for 30 minutes with fluorochrome-conjugated antibody (eBioscience). For intracellular staining of IL-17 and IFN-γ, cells were fixed, permeabilized, and stained with fluorochrome-conjugated antibodies (BD). The stained cells were then analyzed using a FACSCalibur or BD FACSAria instrument.
Retroviral transduction.
To overexpress genes of interest in CD4+ T cells, cDNAs of the genes were cloned into the retroviral vector pMXs-IRES-GFP (Cell Biolabs Inc.). To knock down expression of Srebp-1 or Ahr, synthesized DNA short-hairpin sequences targeting Srebp-1 (#1 shRNA, 5′-AACAGACACTGGCCGAGATGT-3′; #2 shRNA, 5′-AAGACATGCTCCAGCTCATCA-3′; #3 shRNA, 5′-AAAGCTTGGCCTCCCAGCAGC-3′) or Ahr (#1 shRNA, 5′-AACATCACCTATGCCAGCCGC-3′; #2 shRNA, 5′-AACACAGAGTTAGACCGCCTG-3′; #3 shRNA, 5′-AAAGTCATGGGATAAACTCAC-3′) were ligated into pSilencer 5.1 Retro (Ambion). These constructs were transfected into a Platinum-E cell line (Cell Biolabs Inc.) to generate retrovirus supernatant. Naive CD4+ T cells (0.5 × 106) were activated under Th17 or Th0 culture conditions for 16–24 hours before incubation with Platinum-E supernatant containing retroviruses supplemented with 8 μg/ml Polybrene (Sigma-Alrich) and centrifuged for 1 hour at 600 g at 30°C. Two to 3 days after infection, the cells were tested for IL-17 production using intracellular staining.
Real-time PCR.
RNA (0.5–1 μg) was reverse transcribed into cDNA using an oligo (dT) primer (Takara), and 1 μl of the synthesized cDNA product was then added to the PCR reaction mixture. Quantitative real-time RT-PCR was performed using an ABI Prism 7500 sequence detection system (Applied Biosystems). Gene expression was normalized to the level of β-actin in each sample. The sequences of the primers were: mouse Il17 forward: 5′-CTCCAGAAGGCCCTCAGACTAC-3′, mouse Il17 reverse: 5′-AGCTTTCCCTCCGCATTGACACAG-3′; mouse Il17f forward: 5′-GAGGATAACACTGTGAGAGTTGAC-3′, mouse Il17f reverse: 5′-GAGTTCATGGTGCTGTCTTCC-3′; mouse Il21 forward: 5′-GGCCAAACTCAAGCCATCAA-3′, mouse Il21 reverse: 5′-GCCACGAGGTCAATGATGAA-3′; mouse Il22 forward: 5′-CTTTCCTGACCAAACTCAGCAA-3′, mouse Il22 reverse: 5′-TGGTCGTCACCGCTGATG-3′; mouse Il23r forward: 5′-AGAGGACATCCTGCTTCAGGTAAT-3′, mouse Il23r reverse: 5′-GATGGCCAAGAAGACCATTCC-3′; mouse Ahr forward: 5′-CGAGATCTCCAGCCCTTTCTC-3′, mouse Ahr reverse: 5′-TTGCTTTTGGTGCGTATTGG-3′; mouse Rora forward: 5′-GCACCTGACCGAAGACGAA-3′, mouse Rora reverse: 5′-GATCCGCTGACATCAGTACGAA-3′; mouse RORγt forward: 5′-CCGCTGAGAGGGCTTCAC-3′, mouse RORγt reverse: 5′-TGCAGGAGTAGGCCACATTACA-3′; mouse Srebp1a forward: 5′-GGCCGAGATGTGCGAACT-3′, mouse Srebp1c forward: 5′-GCCATGGATTGCACATTTGA-3′, mouse Srebp1a/c reverse: 5′-GCTGGAGCATGTCTTCGATGT-3′; mouse Abca1 forward: 5′-CACCCCTTGAACCTCACTAAACA-3′, mouse Abca1 reverse: 5′-AAAGGACATCGCAAAGATGACA-3′; mouse β-actin forward: 5′-TGACAGGATGCAGAAGGAGA-3′, mouse β-actin reverse: 5′-GTACTTGCGCTCAGGAGGAG-3′; human IL17 forward: 5′-TCTGTGATCTGGGAGGCAAAG-3′, human IL17 reverse: 5′-CGTTCCCATCAGCGTTGAT-3′; human IL17F forward: 5′-GTCCGGAGGAAGCACCAA-3′, human IL17F reverse: 5′-TCACCAGCACCTTCTCCAACT-3′; human RORγt forward: 5′-GCCAAGGCTCAGTCATGAGAA-3′, human RORγt reverse: 5′-TTGTCCCCACAGATTTTGCA-3′; human SREBP1 forward: 5′-CGGAACCATCTTGGCAACA-3′, human SREBP1 reverse: 5′-GCCGGTTGATAGGCAGCTT-3′; human ABCA1 forward: 5′-TCCAGGCCAGTACGGAATTC-3′, human ABCA1 reverse: 5′-TCCTCGCCAAACCAGTAGGA-3′; human β-actin forward: 5′-GCGCGGCTACAGCTTCA-3′, human β-actin reverse: 5′-CTTAATGTCACGCACGATTTCC-3′.
Luciferase assay.
Forty-two hours after transfection of reporter plasmids, expression plasmids, and pSV40-β-gal into Jurkat cells with DMRIE-C Reagent (Invitrogen), the transfected Jurkat cells were stimulated with PMA and ionomycin for 6 hours before harvesting. The luciferase activity was measured with a luciferase assay kit (Promega) using a luminometer (Berthold Technologies) and normalized to the β-gal activity.
ChIP.
In vitro differentiated Th17 cells with or without LXR agonist treatment were crosslinked using formaldehyde before harvest. Extracts were immunoprecipitated with a control antibody or antibody against Srebp-1. DNA purified from the immunoprecipitates and input extracts were analyzed by PCR with 3 pairs of primers specific for the Il17 promoter: primer set 1 forward: 5′-TATGGAGCCCAGCTCTGCAG-3′, primer set 1 reverse: 5′-CTTTGCTGTGATTCAGTATC-3′; primer set 2 forward: 5′-GCAGCAGCTTCAGATATGTCCATA-3′, primer set 2 reverse: 5′-TGGGAGCTGAACAGAGATGCTT-3′; primer set 3 forward: 5′-CATGGAGAGGAGAGAACATGAGAGA-3′, primer set 3 reverse: 5′-TAACAGGAGGAGATGAGGGATGA-3′; β-actin (for ChIP) forward: 5′-CAGCCAACTTTACGCCTAGC-3′, β-actin (for ChIP) reverse: 5′-TTTGGACAAAGACCCAGAGG-3′.
EMSA.
EMSAs were performed using the LightShift Chemiluminescent EMSA kit (Pierce) according to the manufacturer’s instructions. Briefly, nuclear proteins were prepared from in vitro differentiated Th17 cells with or without LXR agonist treatment, incubated with biotin-labeled probes, and resolved on a 6% native polyacrylamide gel. The protein-DNA complex was transferred to a nylon membrane, crosslinked to the membrane with a UV lamp, and incubated with a streptavidin-HRP conjugate. Signals were detected by chemiluminescence. Unlabeled probes or unlabeled mutant probes were included as controls — labeled Il17 probe: biotin-5′-CATACACACATGATACTGAA-3′; unlabeled Il17 probe: 5′-CATACACACATGATACTGAA-3′; unlabeled mutant Il17 probe: 5′-CATACATACATGATACTGAA-3′.
Western blot analysis, immunoprecipitation, and immunocytochemistry.
Cells were lysed and extracted protein resolved by 10% SDS-PAGE before transfer onto PVDF membranes (Millipore). The membranes were blocked with 5% milk in Tris-buffered saline supplemented with Tween-20 (TBST) and incubated overnight at 4°C with antibodies against LXRα, LXRβ (Santa Cruz Biotechnology Inc.), actin, Myc, FLAG, Ahr (Abcam), and Srebp-1 (BD), followed by incubation with appropriate HRP-conjugated secondary antibodies for 1 hour at room temperature. Signals were detected by enhanced chemiluminescence (Pierce) according to the manufacturer’s instructions. For co-immunoprecipitation experiments, cell lysates were immunoprecipitated with antibodies against Myc, FLAG, Ahr, or Srebp-1 and the immunoprecipitates were resolved by 10% SDS-PAGE and transferred to PVDF membranes. These membranes were blotted with antibodies as indicated. For immunocytochemistry, T cells were fixed with 4% formaldehyde and permeabilized with 0.1% Triton X-100, followed by incubation with primary antibody and fluorescent-labeled secondary antibody. DAPI was used to stain nuclei, and images were captured with a LSM510 confocal microscope using a ×40 oil objective.
Statistics.
One-way ANOVA was used to compare the clinical score between 2 groups at every time point followed by a Mann-Whitney U test to determine the differences between the 2 curves in the EAE study. The Mann-Whitney U test was used to calculate differences elsewhere, and P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank David Mangelsdorf for providing the LXR KO mice. This work was supported in part by the National Natural Science Foundation (no. 31070802), the Chinese Academy of Sciences (KSCX2-YW-G-057), and the Ministry of Science and Technology of China (2007CB914801 and 2009CB919000).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(2):658–670. doi:10.1172/JCI42974.
See the related Commentary beginning on page 519.
References
- 1. Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2. Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4. Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 5. Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6. Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 8. Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 9. Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huber M, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105(52):20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du C, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 15. Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 17. Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204(12):2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Q, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29(6):899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hermann-Kleiter N, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29(2):205–216. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ichiyama K, et al. Gfi1 negatively regulates T(h)17 differentiation by inhibiting RORgammat activity. Int Immunol. 2009;21(7):881–889. doi: 10.1093/intimm/dxp054. [DOI] [PubMed] [Google Scholar]
- 21. Klotz L, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206(10):2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183(10):6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- 23. Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111(3):1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 26. Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol. 1994;14(10):7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 28. Shinar DM, Endo N, Rutledge SJ, Vogel R, Rodan GA, Schmidt A. NER, a new member of the gene family encoding the human steroid hormone nuclear receptor. Gene. 1994;147(2):273–276. doi: 10.1016/0378-1119(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 29. Song C, Kokontis JM, Hiipakka RA, Liao S. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc Natl Acad Sci U S A. 1994;91(23):10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 31. Walcher D, et al. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006;26(5):1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- 32. Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17(6):985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 33. Joseph SB, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119(2):299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 34. Korf H, et al. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009;119(6):1626–1637. doi: 10.1172/JCI35288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134(1):97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asquith DL, et al. Liver X receptor agonism promotes articular inflammation in murine collagen-induced arthritis. Arthritis Rheum. 2009;60(9):2655–2665. doi: 10.1002/art.24717. [DOI] [PubMed] [Google Scholar]
- 38. Hindinger C, et al. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J Neurosci Res. 2006;84(6):1225–1234. doi: 10.1002/jnr.21038. [DOI] [PubMed] [Google Scholar]
- 39. Xu J, Wagoner G, Douglas JC, Drew PD. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol. 2009;86(2):401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Houck KA, et al. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83(1–2):184–187. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 41. Kumar N, et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluorometh yl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol. 2010;77(2):228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98(7):1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99(5):846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker RW, Sanders H, Thompson RH, Zilkha KJ. Serum cholesterol linoleate levels in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1965;28:212–217. doi: 10.1136/jnnp.28.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baker RW, Thompson RH, Zilkha KJ. Serum fatty acids in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1964;27:408–414. doi: 10.1136/jnnp.27.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 48. Repa JJ, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.