Abstract
Activation of NF-κB and 5-lipoxygenase–mediated (5-LO–mediated) biosynthesis of the lipid mediator leukotriene B4 (LTB4) are pivotal components of host defense and inflammatory responses. However, the role of LTB4 in mediating innate immune responses elicited by specific TLR ligands and cytokines is unknown. Here we have shown that responses dependent on MyD88 (an adaptor protein that mediates signaling through all of the known TLRs, except TLR3, as well as IL-1β and IL-18) are reduced in mice lacking either 5-LO or the LTB4 receptor BTL1, and that macrophages from these mice are impaired in MyD88-dependent activation of NF-κB. This macrophage defect was associated with lower basal and inducible expression of MyD88 and reflected impaired activation of STAT1 and overexpression of the STAT1 inhibitor SOCS1. Expression of MyD88 and responsiveness to the TLR4 ligand LPS were decreased by Stat1 siRNA silencing in WT macrophages and restored by Socs1 siRNA in 5-LO–deficient macrophages. These results uncover a pivotal role in macrophages for the GPCR BLT1 in regulating activation of NF-κB through Stat1-dependent expression of MyD88.
Introduction
Inappropriate inflammatory responses contribute to many pathological conditions, including autoimmune diseases, asthma, chronic obstructive pulmonary disease, and atherosclerosis. A central feature of inflammation is leukocyte activation, which requires coordination among numerous receptors, signaling pathways, and mediators. TLRs and cytokine receptors drive inflammatory programs in macrophages (1). In addition to sensing microbial products, TLRs sense endogenous danger signals produced during tissue injury. These include extracellular matrix components, such as hyaluronic acid and biglycan, as well as intracellular proteins, including high-mobility group box–1 (1).
TLR family members and the IL-1β receptor (IL-1βR) share a conserved cytoplasmic Toll–IL-1R (TIR) domain that binds adaptor proteins, including myeloid differentiation factor 88 (MyD88). MyD88 mediates signaling through all of the known TLRs except TLR3, although its importance for individual TLRs varies (2). Although it is similarly crucial for initiating signaling responses to IL-1β and certain cytokines, such as IL-18, MyD88 does not mediate responses to other cytokines, such as TNF-α (3). MyD88 is necessary for host defense against a variety of experimental infections (4), but also promotes development of atherosclerosis in Apoe–/– mice as well as neutrophil recruitment and myocardial injury following ischemia-reperfusion (5); autoimmune responses, diabetes, and colitis (6); and familial Mediterranean fever (7). MyD88 expression can be upregulated beyond its basal level by proinflammatory mediators, including IL-6 (8) and the phorbol ester PMA (9) as well as LPS, IFN-α, CpG-DNA, and IFN-γ (9, 10).
TLR-mediated responses are characterized both by activation of the pivotal transcription factor NF-κB (11) and by synthesis of the 5-lipoxygenase–derived (5-LO–derived) lipid mediator leukotriene B4 (LTB4) (Figure 1A and ref. 12). The latter, acting via its high-affinity GPCR BLT1, is best known as a leukocyte chemoattractant but is also a potent enhancer of leukocyte functions, including cytokine secretion and microbial phagocytosis and killing (13). Like MyD88, LTB4 has also been implicated in antimicrobial defense as well as a variety of inflammatory disease states, such as atherosclerosis, ischemia-reperfusion injury, and immune responses (12). 5-LO metabolites have been suggested to participate in in vivo and in vitro LPS responses (14), but these reports were limited by the off-target effects of the pharmacologic inhibitors used (15) and by ambiguity regarding the specific 5-LO products required for TLR responses (14, 16–18). The role of specific 5-LO metabolites and receptors in various TLR- and cytokine receptor–mediated responses is therefore poorly understood. Here we demonstrate that LTB4 synthesis and signaling via BLT1 are necessary for optimal MyD88 expression and NF-κB activation in macrophages in vitro and in vivo and identify the operative molecular mechanisms by which LTB4 amplifies NF-κB activation.
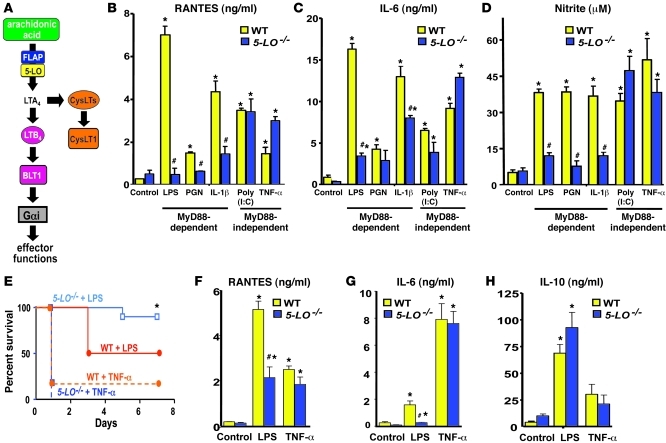
Figure 1. 5-LO metabolites are required for MyD88-dependent inflammatory responses in vitro and in vivo.
(A) Overview of LT biosynthesis and receptors. (B–D) RANTES (B), IL-6 (C), and nitrite (D) levels from WT- and 5-LO–/– elicited peritoneal macrophages stimulated for 24 hours as indicated, determined by ELISA or the Griess reaction. Cytokine and nitrite data represent mean ± SEM from 3 individual experiments, with each value determined in triplicate. *P < 0.05 versus control; #P < 0.001 versus WT. (E) WT and 5-LO–/– mice (5 mice per group in each of 2 independent experiments) were challenged with LPS or TNF-α i.p. as described in Methods, and survival was assessed over time. *P < 0.05 versus LPS-treated WT. (F–H) Levels of RANTES (F), IL-6 (G), and IL-10 (H) from WT and 5-LO–/– mice challenged with LPS or TNF-α, determined by ELISA on serum collected 6 hours after challenge. Cytokine data represent mean ± SEM from 3–5 animals per group in each of 3 individual experiments, with each value determined in triplicate. *P < 0.05 versus WT; #P < 0.05 versus WT.
Results
5-LO metabolites are required for in vitro and in vivo MyD88-dependent macrophage responses.
LPS responses are mediated via TLR4; although signaling from this receptor is dependent on both adaptor proteins MyD88 and TRIF, other TLRs and cytokine receptors use either MyD88 or TRIF to elicit cellular responses (19). To evaluate whether 5-LO metabolites are required for NF-κB activation, as well as the MyD88 dependence of any such requirement, we stimulated elicited peritoneal macrophages from WT and 5-LO–/– mice for 24 hours with the MyD88-dependent agonists LPS, peptidoglycan (PGN), and IL-1β and with the MyD88-independent agonists poly(I:C) and TNF-α (2), then measured the secretion of proinflammatory molecules known to be dependent on NF-κB activation (20). All of the TLR and cytokine receptor agonists used stimulated the generation of the NF-κB–dependent gene products RANTES, IL-6, and NO (measured as nitrite) (Figure 1, B–D). Compared with WT cells, 5-LO–/– macrophages exhibited a marked reduction in mediator generation in response to MyD88-dependent agonists, but no such reduction in response to MyD88-independent agonists. LTB4 itself was also capable of promoting the release of RANTES and IL-6, particularly in 5-LO–/– cells (Supplemental Figure 1, A and B; supplemental material available online with this article; doi: 10.1172/JCI43302DS1). A similar pattern of LTB4 dependence of LPS, but not poly(I:C), responses was observed in 2 populations of resting macrophages, namely bone marrow–derived macrophages (BMDMs) and resident peritoneal macrophages (Supplemental Figure 2, A and B, and data not shown).
Since these data indicated that 5-LO metabolites are required for optimal MyD88-induced generation of proinflammatory molecules in vitro, we investigated their in vivo importance in LPS-induced shock. Whereas 50% of WT mice died from i.p. LPS injection, only 10% of 5-LO–/– mice died from the same endotoxin dose (P < 0.05; Figure 1E). In contrast, WT and 5-LO–/– mice exhibited similar mortality, in excess of 80%, after i.p. challenge with the MyD88-independent agonist TNF-α. We next sought to determine whether the lower mortality in LPS-treated 5-LO–/– animals correlates with levels of NF-κB–dependent cytokines in the serum and peritoneal lavage fluid measured 6 hours after LPS challenge. Both serum (Figure 1, F and G) and peritoneal fluid (Supplemental Figure 1, C and D) levels of RANTES and IL-6 were significantly lower in 5-LO–/– mice than WT mice following LPS injection, whereas TNF-α injection induced similar levels of these cytokines in the serum from both genotypes. We also investigated serum levels of IL-10 — a cytokine whose expression depends on transcription factors other than NF-κB, including CREB and Sp1/Sp3 (21, 22) — in order to evaluate the specificity of leukotriene (LT) dependence for NF-κB activation. In contrast to the NF-κB–dependent cytokines, IL-10 production was no different in 5-LO–/– than in WT mice challenged with LPS (or TNF-α; Figure 1H). Taken together, these results suggest that production of NF-κB–dependent cytokines as well as systemic inflammatory responses integral to lethal shock that are driven by MyD88-dependent agonists depend on 5-LO metabolites.
5-LO metabolites are required for MyD88-dependent NF-κB activation.
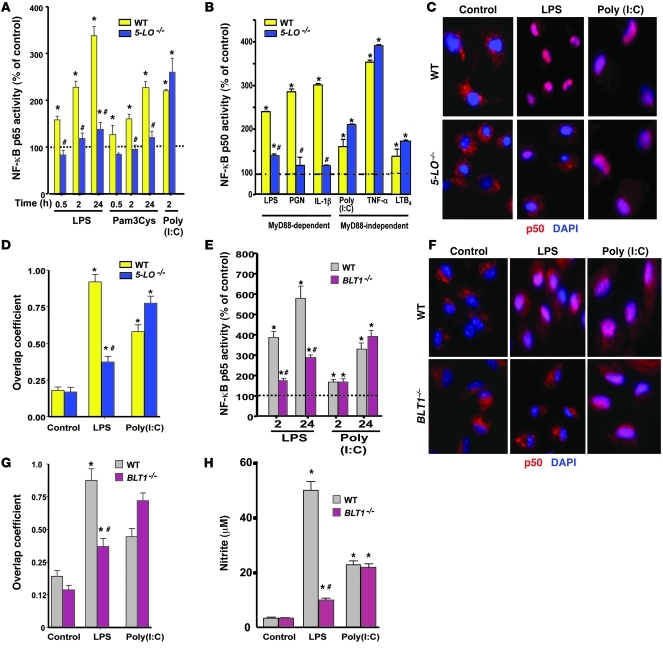
We sought to more directly determine the requirement of 5-LO for TLR-induced NF-κB activation. ELISA assays demonstrated DNA binding activity of the NF-κB p65 subunit in WT macrophages stimulated with the TLR4 agonist LPS, the TLR2 agonist Pam3Cys, and the TLR3 agonist poly(I:C); binding was detectable as early as 30 minutes and maximal at 24 hours (Figure 2A). 5-LO–/– macrophages exhibited markedly blunted p65 binding relative to WT cells at all time points in response to LPS and Pam3Cys, although p65 binding in response to poly(I:C) was intact (Figure 2A). In order to more comprehensively evaluate the MyD88 dependence of NF-κB binding to DNA, and to verify binding of transcriptionally active p65/p50 heterodimers, we used a larger panel of MyD88-dependent and -independent agonists in ELISA assays of p50 DNA binding. As observed for p65, binding activity of p50 in 5-LO–/– macrophages at 24 hours was markedly diminished in response to the MyD88-dependent agonists LPS, PGN, and IL1-β, but not to the MyD88-independent agonists TNF-α and poly(I:C) or to LTB4 itself (Figure 2B). These findings were also confirmed by assessing nuclear translocation of the NF-κB p50 subunit by immunofluorescence microscopy (Figure 2C) and by quantifying the nuclear colocalization of p50 with DAPI immunofluorescence using the Image J plug-in Jacop (Figure 2D and ref. 23). Poly(I:C) enhanced p50 nuclear translocation in elicited macrophages from both WT and 5-LO–/– mice. However, LPS-induced p50 translocation was attenuated in macrophages from 5-LO–/– animals, and the same was true for p65 translocation (Supplemental Figure 3A). Phosphorylation of the cytosolic inhibitor IκBα initiates its degradation and is required for subsequent NF-κB activation (24). We therefore assessed IκBα phosphorylation in WT and 5-LO–/– macrophages following TLR stimulation. Activation of IκBα in WT macrophages occurred as soon as 5 minutes after LPS challenge, peaked at 15 minutes, and persisted for up to 60 minutes (Supplemental Figure 3B). In 5-LO–/– macrophages, onset of activation was delayed, and the magnitude and duration of activation were also diminished. Stimulation with a panel of TLR agonists confirmed that, as observed for other response parameters, IκBα phosphorylation was attenuated in 5-LO–/– cells exclusively in response to MyD88-dependent stimuli (Supplemental Figure 3C). We confirmed the requirement of 5-LO metabolism in LPS-induced IκBα phosphorylation and NO generation by pretreating WT cells with the 5-LO inhibitor AA-861 for 48 hours (Supplemental Figure 4, C and D). Finally, in order to determine whether 5-LO control of MyD88-dependent NF-κB activation is limited to inflammatory macrophages, we examined LPS- and poly(I:C)-induced p65 activation in resting BMDMs from both genotypes. As with thioglycollate macrophages, p65 activation in BMDMs in response to LPS, but not poly(I:C), was blunted in 5-LO–/– cells (Supplemental Figure 4C).
Figure 2. LTB4/BLT1 signaling is required for MyD88-dependent NF-κB activation.
(A) WT and 5-LO–/– macrophages were stimulated for different time points as indicated, and nuclear p65 activity was determined as described in Methods. (B) WT and 5-LO–/– macrophages were stimulated with the indicated MyD88-dependent and -independent agonists for 24 hours, and nuclear p50 activity was determined as described in Methods. (C) Immunofluorescence microscopic assessment of p50 nuclear translocation in WT and 5-LO–/– macrophages stimulated as indicated for 24 hours. (D) Quantification of p50/DAPI colocalization was determined as described in Methods. (E) WT and BLT1–/– macrophages were stimulated as indicated, and nuclear p65 activity was determined as in A. (F) Immunofluorescence microscopic assessment of p50 nuclear translocation in WT and BLT1–/– macrophages stimulated as indicated for 24 hours. (G) Quantification of p50/DAPI colocalization was determined as described in Methods. (H) Nitrite levels from WT- and BLT1–/– elicited peritoneal macrophages stimulated for 24 hours as indicated, determined by the Griess reaction. In C and F, p50 is stained red and nuclei blue (DAPI); each field is representative of 100 examined (original magnification, ×400) per each of 3 independent experiments. In A, B, D, E, G, and H, data are from 3 individual experiments, each performed in triplicate. *P < 0.05 versus control; #P < 0.05 versus WT.
LTB4/BLT1 signaling is required for MyD88-dependent NF-κB activation.
Macrophage 5-LO metabolism yields a variety of bioactive metabolites, most notably LTB4 and the cysteinyl LTs (CysLTs) C4, D4, and E4 (Figure 1A and ref. 12). However, 5-LO oxygenation of arachidonic acid also leads to the generation of reactive oxygen species (25), and NF-κB activation is known to be sensitive to oxidant tone (15). Thus, we sought to verify that LPS-induced IκBα phosphorylation in WT macrophages depends on lipid metabolites of 5-LO and to determine the relative roles of LTB4 versus CysLTs in this response. As an initial approach to addressing these questions, we pretreated WT cells for 48 hours with antagonists to their respective high-affinity GPCRs, BLT1 (CP105,696) and CysLT1 (MK571). Only the BLT1 antagonist prevented LPS-induced IκBα phosphorylation and NO secretion (Supplemental Figure 4, C and D). Exogenous LTB4 also synergized with LPS to dose-dependently enhance iNOS expression and NO production (data not shown). These data strongly suggest that LTB4 is the main 5-LO product required for NF-κB activation triggered by MyD88-dependent agonists, but it is not necessary for the TLR3-dependent response.
To more definitively confirm the specific role of BLT1 signaling in MyD88-dependent NF-κB activation, we used elicited peritoneal macrophages from BLT1–/– mice. DNA binding activity of p65 in response to LPS treatment was impaired in BLT1–/– relative to WT macrophages at both the 2- and 24-hour time points, whereas both genotypes were equally responsive to poly(I:C) (Figure 2E). These findings were confirmed by assessing p50 NF-κB nuclear translocation in BLT1–/– or WT elicited peritoneal macrophages upon LPS or poly(I:C) challenge. Compared with WT, BLT1 deficiency impaired p50 nuclear translocation in response to LPS, but not poly(I:C) (Figure 2, F and G). A similar selective impairment of BLT1–/– macrophages in NO production in response to LPS, but not poly(I:C) (Figure 2H), reinforced the conclusion that BLT1 signaling is required for MyD88-dependent NF-κB activation.
We next sought to assess the role of LTB4 in NF-κB activation in an in vivo setting and in response to a genuine microbial stimulus rather than a purified or synthetic TLR ligand. We thus infected WT and 5-LO–/– mice intranasally with the important respiratory pathogen Streptococcus pneumoniae, which is recognized mainly by TLR2 (26); administered aerosolized LTB4 or vehicle control 24 hours later; and assessed p50 localization by immunofluorescence microscopic examination of lung cells obtained by lavage 24 hours thereafter (Supplemental Figure 5). Compared with that observed in uninfected animals, p50 nuclear translocation was increased in alveolar macrophages from infected and vehicle-treated WT, but not 5-LO–/–, mice. Administration of LTB4 by aerosol augmented nuclear p50 staining in macrophages from both genotypes, restoring the p50 translocation response in 5-LO–/– mice to the level observed in infected WT animals. Consistent with these results, we recently reported that administration of LTB4 using this protocol also enhances lung bacterial clearance in 5-LO–/– animals (27). Interestingly, we did not observe p50 nuclear translocation in neutrophils from either genotype in the absence or presence of aerosolized LTB4 (data not shown). Thus, both pharmacologic and genetic approaches demonstrated that LTB4 generation and BLT1 signaling are essential for optimal MyD88-dependent activation of NF-κB in vivo and in vitro.
LTB4 is required for MyD88 expression in phagocytes.
Because downstream signaling pathways linking various adaptors to NF-κB activation are largely redundant, yet findings in Figures 1 and 2 indicated an impairment in 5-LO–/– macrophage responses that was limited to particular agonists, mechanistic exploration was focused on proximal pathway components. 5-LO–/– macrophages manifested no reduction in the expression levels of TLR4 or CD14 (data not shown), consistent with the findings above that impaired responses in these cells extended to MyD88-dependent agonists besides LPS. We next determined whether elicited macrophages from 5-LO–/– mice exhibit altered expression of MyD88 or of the other TLR adaptors TIRAP, TRIF, and TIRP compared with WT macrophages. The LT-deficient cells manifested decreased basal MyD88 protein and mRNA expression. Pretreatment of WT macrophages with the 5-LO inhibitor AA-861 for 48 hours also decreased basal MyD88 expression (Supplemental Figure 4A), and preliminary studies verified a similar effect in human monocytes as well (data not shown). Pretreatment for 48 hours with a BLT1 antagonist, but not a CysLT1 antagonist, also decreased basal MyD88 expression in WT elicited macrophages (Supplemental Figure 4A). The reduction in MyD88 expression in 5-LO–/– macrophages was restored to levels above WT by incubation with exogenous LTB4 in a time-dependent manner (Figure 3, A–C, and Supplemental Figure 6). Furthermore, pretreatment of 5-LO–/– macrophages with LTB4 for 24 hours fully restored both LPS and IL-1β responsiveness in these cells, as reflected by NO generation (Figure 3G). Only a modest enhancement of MyD88 expression was effected by provision of exogenous LTD4 (Supplemental Figure 4B). Similar reductions in MyD88 protein and mRNA levels were also observed in BLT1–/– macrophages, but, as would be expected in the absence of its cognate receptor, reductions in the adaptor could not be overcome by addition of LTB4 (Figure 3, A–C). No decrease in expression of any of the other adaptors was noted in 5-LO–/– versus WT macrophages; however, TRIF expression was consistently higher in 5-LO–/– than WT macrophages (Figure 3A). While this suggests a possible compensatory mechanism in 5-LO–/– macrophages, its functional significance is uncertain, since levels of mediators generated in response to poly(I:C) by these cells were not consistently higher than in WT macrophages.
Figure 3. LTB4 is required for MyD88 expression in phagocytes.
(A) MyD88, TIRAP, TRIF, and TIRP protein expression in WT macrophages and in 5-LO–/– and BLT1–/– macrophages treated for 24 hours with or without LTB4. (B) Densitometry of MyD88 protein in 5-LO–/– and BLT1–/– macrophages. (C) Myd88 mRNA expression in 5-LO–/– and BLT1–/– macrophages treated as in A. (B and C) Data are representative of 3 experiments, performed in triplicate; values are relative to control WT macrophages (dashed line). *P < 0.05 versus WT; #P < 0.001 versus untreated 5-LO–/–. (D) MyD88 protein expression in resident peritoneal (PM), alveolar (AM), and splenic (SM) macrophages as well as splenic DCs, T and B lymphocytes, and lung fibroblasts from WT and 5-LO–/– mice. Data are representative of 3 experiments. (E) Myd88 mRNA decay in WT and 5-LO–/– macrophages harvested after treatment with actinomycin D (2.5 mg/ml). Data are from 3 experiments in triplicate; values are relative to untreated macrophages from both genotypes. (F) MyD88 protein in WT and 5-LO–/– macrophages incubated for 24 hours as indicated. Immunoblot results are from 2–3 independent experiments (relative MyD88 density shown by numbers beneath). Lanes were run on the same gel but were noncontiguous (white line). (G) Nitrite production in WT and 5-LO–/– macrophages pretreated for 24 hours with or without LTB4, followed by LPS for another 24 hours. Data are representative of 3 experiments. *P < 0.05 versus control; #P < 0.05 versus non–LTB4-treated stimulated WT; &P < 0.01 versus LPS or IL-1β alone.
MyD88 deficiency in 5-LO–/– animals was restricted to tissue phagocytes, including resident peritoneal, alveolar, and spleen macrophages as well as spleen DCs and BMDMs, but was not observed in T or B lymphocytes or lung fibroblasts (Figure 3D). This cell-specific pattern of 5-LO dependence for MyD88 expression correlates nicely with the well-known robust capacity for LTB4 synthesis of phagocytes (13). Since levels of Myd88 mRNA are dictated by both transcription (28) and degradation (29), we compared its decay in WT and 5-LO–/– macrophages. At various time points following addition of actinomycin D to block the formation of new transcripts, cells were processed for real-time RT-PCR analysis. No difference in mRNA stability was observed between 5-LO–/– and WT macrophages (Figure 3E), which suggests that reduced Myd88 mRNA in 5-LO–/– cells reflects a transcriptional defect.
MyD88 expression can be potentiated above basal levels by treatment with inflammatory mediators (2). We therefore compared the effects of proinflammatory molecules known to increase MyD88 expression on levels of this adaptor in 5-LO–/– and WT macrophages (Figure 3F). Both MyD88-dependent and -independent agonists augmented MyD88 protein expression in WT cells, but this response was markedly blunted in 5-LO–/– macrophages. Of the mediators tested, only exogenous LTB4 was able to increase MyD88 expression to a considerable degree in cells from LT-deficient mice, attesting to the specific importance of LTB4 in increasing MyD88 expression. Taken together, our findings show that LTB4, but not CysLTs, is required for upregulating transcriptional programs required for both basal and induced MyD88 expression.
Enhanced JAK/STAT1 activation mediates LTB4 control of MyD88 expression.
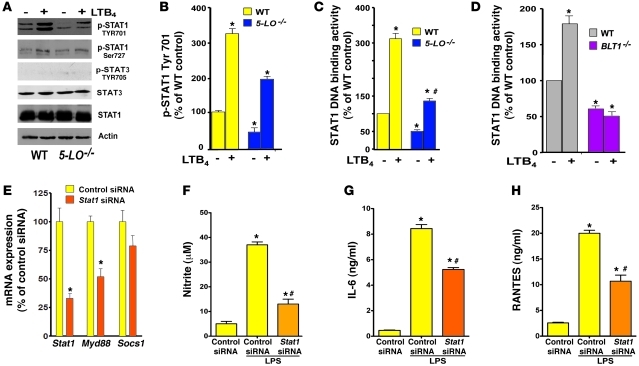
STAT1 is a key transcription factor governing MyD88 expression (28). STAT1 is well known to be activated by IFNs (30), but little is known about its activation by macrophage GPCRs. Because of the important regulatory influence of LTB4 on MyD88 gene expression, we sought to determine whether LTB4/BLT1 signaling is required for STAT1 activation. Compared with WT macrophages, 5-LO–/– cells displayed lower basal phosphorylation of STAT1 at Tyr701, a residue important for its nuclear translocation (31), and the degree of phosphorylation was enhanced in cells of both genotypes by LTB4 treatment (Figure 4, A and B). No such difference between the genotypes was noted in STAT1 phosphorylation at Ser727 (Figure 4A and data not shown), a residue important for DNA binding activity (31). STAT1 phosphorylation at Tyr701 was also evaluated in WT macrophages pretreated with a 5-LO inhibitor as well as CysLT1 or BLT1 antagonists. Basal phosphorylation was diminished in response to the 5-LO inhibitor and the BLT1 antagonist, but not the CysLT1 antagonist (Supplemental Figure 7). These data suggest that the impairment in basal STAT1 phosphorylation at Tyr701 is likely a critical determinant of MyD88 deficiency in cells incapable of LTB4 synthesis and signaling, but exclude a role for CysLTs in this process. As a complementary approach, we determined nuclear STAT1 activity in WT and 5-LO–/– as well as BLT1–/– macrophages. Both 5-LO–/– and BLT1–/– macrophages exhibited lower STAT1 activity than their corresponding WT macrophages. Exogenous LTB4 enhanced STAT1 activation in WT and 5-LO–/– macrophages, but not in BLT1–/– cells (Figure 4, C and D). To address the importance of STAT1 in mediating MyD88 expression, we used siRNA to knock down Stat1 and achieved a 70% decrease in Stat1 mRNA compared with control siRNA (Figure 4E). Stat1 knockdown decreased Myd88 mRNA expression by 45%. This decrease in MyD88 expression following STAT1 knockdown correlated with a lower capability of WT macrophages to produce NO, RANTES, and IL-6 upon LPS stimulation (Figure 4, F–H), thus recapitulating the phenotype observed in 5-LO–/– and BLT1–/– cells, which themselves expressed decreased MyD88.
Figure 4. LTB4/BLT1-induced STAT1 activation is required for MyD88 expression and TLR4 responses.
(A) WT and 5-LO–/– macrophages were treated for 30 minutes with or without LTB4, and pSTAT1 (Tyr701), pSTAT1 (Ser727), total STAT1, pSTAT3 (Tyr705), total STAT3, and actin were determined by immunoblotting. (B) Densitometric analysis of pSTAT1 (Tyr701) protein levels in 5-LO–/– macrophages, normalized for total STAT1 and expressed as percent of WT control cells. (C and D) WT and 5-LO–/– macrophages (C) and WT and BLT1–/– cells (D) were incubated for 30 minutes with or without LTB4, and nuclear STAT1 activity was determined as described in Methods. (E) WT macrophages were treated for 48 hours in the presence of scrambled siRNA (control siRNA) and Stat1 siRNA, and the expression of Stat1, Myd88, and Socs1 mRNA was determined by real-time RT-PCR and expressed relative to control siRNA values. (F) NO, (G) IL-6, and (H) RANTES secretion in Stat1-silenced WT macrophages incubated with or without LPS for 24 hours. Data represent mean ± SEM from 3 individual experiments, each performed in triplicate. (B–D) *P < 0.05 versus unstimulated WT; #P < 0.05 versus stimulated WT. (E–H) *P < 0.05 versus control siRNA; #P < 0.05 versus LPS-challenged control siRNA.
Tyrosine phosphorylation and activation of STAT1 are mediated by the tyrosine kinases JAK1/2 and Tyk2 (32). 5-LO–/– and BLT1–/– macrophages exhibited less basal activation of JAK2, but not JAK1 or Tyk2, than did their respective WT cells, and exogenous LTB4 enhanced its phosphorylation in 5-LO–/–, but not BLT1–/–, macrophages (Figure 5A). To verify that LTB4-induced activation of JAK2 is required for STAT1 phosphorylation, we used pharmacological inhibitors of JAK2 activation, AG490 (33) and JAK2 inhibitor I (2-[1,1-Dimethylethyl]-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one, P6, Pyridone 6; ref. 34), in WT macrophages. JAK2 inhibition abolished LTB4-induced STAT1 phosphorylation at Tyr701 (Figure 5B).
Figure 5. JAK2 activation is required for LTB4-induced STAT1 phosphorylation.
(A) WT, 5-LO–/–, and BLT1–/– macrophages were treated for 30 minutes with or without LTB4, and pJAK2, pJAK1, pTyk2, total JAK2, and actin were determined by immunoblotting. Densitometric analysis of pJAK2 protein levels in 5-LO–/– and BLT1–/– macrophages is also shown, expressed relative to their respective strain-matched WT cells. (B) WT macrophages were pretreated for 30 minutes with JAK2 inhibitors AG490 (1 μM) and JAK2 inhibitor I (JAK2 i; 10 nM), followed by 30 minutes of treatment with or without LTB4; pJAK2 (Tyr1007/1008) and pSTAT1 (Tyr701) were subsequently determined by immunoblotting. Densitometric analysis of pJAK2 and pSTAT1 protein levels in WT macrophages treated with LTB4 in the absence or presence of JAK2 inhibitors is also shown. Immunoblots are representative of at least 3 independent experiments; densitometry values are mean ± SEM from 3 independent experiments. *P < 0.05 versus untreated WT; #P < 0.05 versus LTB4 alone.
We have previously shown that the effects of BLT1 on resident alveolar macrophage antimicrobial functions are mediated primarily by Gαi signaling (35). However, it is not known whether Gαi signaling is required for BLT1 effects, including JAK2/STAT1 activation, in elicited peritoneal macrophages. The role of Gαi proteins was determined by pretreating WT elicited macrophages with the Gαi inhibitor pertussis toxin (PTX; ref. 35). PTX prevented LTB4-induced phosphorylation of both JAK2 and STAT1, implicating Gαi in these events (Figure 6A). We also studied the role of Gαi signaling in determining basal as well as LTB4-enhanced Myd88 expression. PTX decreased basal MyD88 expression and prevented LTB4/BLT1-mediated upregulation of MyD88 expression in WT macrophages. Conversely, 5-LO–/– macrophages showed no further decrease in MyD88 after PTX pretreatment (Figure 6B), which suggests that BLT1 is the main Gαi-coupled receptor regulating MyD88 expression. Together, these data show that the LTB4/BLT1/Gαi axis is required for constitutive as well as stimulated JAK2/STAT1 activation, and hence MyD88 expression.
Figure 6. Gαi signaling is required for LTB4/BLT1-induced JAK2/STAT1 phosphorylation and MyD88 expression.
(A) WT macrophages were pretreated for 24 hours with or without the Gαi inhibitor PTX (600 ng/ml), followed by 30 minutes of treatment with or without LTB4. pSTAT1 (Tyr701), pJAK2 (Tyr1007/1008), total JAK2, and actin were determined by immunoblotting. Densitometric analysis of pSTAT1 and pJAK2 protein levels in WT macrophages is also shown, expressed relative to untreated WT cells; data are mean ± SEM from 3 individual experiments. *P < 0.05 versus untreated WT; #P < 0.001 versus LTB4 alone. (B) WT and 5-LO–/– macrophages were pretreated with PTX (600 ng/ml) for 24 hours and incubated with or without LTB4 for another 24 hours, after which RNA was isolated for Myd88 mRNA determination by real-time RT-PCR. Data are mean ± SEM from 3 individual experiments, each performed in triplicate. *P < 0.05 versus untreated WT; #P < 0.05 versus LTB4 alone.
LTB4 decreases SOCS1 expression in macrophages.
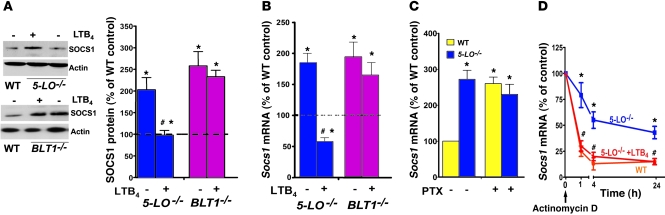
JAK2 activity is restrained by SOCS1 (36). We therefore considered the possibility that enhancement of JAK2/STAT1 activation by endogenous and exogenous LTB4 reflects downregulation of SOCS1 expression. Indeed, both 5-LO–/– and BLT1–/– cells expressed significantly higher basal levels of SOCS1 protein and mRNA than did WT macrophages, and LTB4 decreased SOCS1 expression in 5-LO–/– macrophages (Figure 7, A and B). No difference in mRNA levels of other SOCS family members (Socs2–Socs5) was observed in 5-LO–/– macrophages versus WT cells (data not shown). In addition, pharmacologic 5-LO inhibition and BLT1 antagonism increased SOCS1 levels, but CysLT1 antagonism had no such effect (Supplemental Figure 7). In parallel with its ability to reduce Myd88 expression (Figure 6B), Gαi inactivation by PTX enhanced basal Socs1 mRNA expression in WT macrophages, but did not further enhance Socs1 mRNA expression in 5-LO–/– cells (Figure 7C). The increased basal Socs1 mRNA expression in 5-LO–/– macrophages could reflect an increase in mRNA transcription or stability. Socs1 mRNA levels were therefore determined by real-time RT-PCR at various time points following the addition of actinomycin D. In 5-LO–/– macrophages, Socs1 mRNA levels declined by approximately 20% at 1 hour and by approximately 50% over the interval between 4 and 24 hours (Figure 7D). In contrast, Socs1 mRNA in WT macrophages exhibited faster turnover, decreasing by approximately 70% at 1 hour and by approximately 85% at 4 hours. Addition of exogenous LTB4 increased the rate of mRNA degradation in 5-LO–/– cells to a rate indistinguishable from that of WT cells. These data suggest that enhanced mRNA degradation is an important mechanism by which endogenous and exogenous LTB4 reduces SOCS1 expression.
Figure 7. LTB4/BLT1 signaling is required to restrain SOCS1 expression.
(A) WT and 5-LO–/– or BLT1–/– macrophages were treated for 24 hours with or without LTB4 and SOCS1 expression was determined by immunoblotting. Immunoblots are representative of 3 independent experiments. Mean ± SEM densitometric values for SOCS1 protein in 5-LO–/– and BLT1–/– macrophages from 3 independent experiments were normalized for actin and expressed relative to untreated WT control (dashed line). (B) Socs1 mRNA expression by real-time RT-PCR in 5-LO–/– and BLT1–/– macrophages treated for 24 hours with or without LTB4, expressed relative to untreated WT control (dashed line). Data are mean ± SEM from 3 individual experiments, each performed in triplicate. (C) WT and 5-LO–/– macrophages were pretreated with PTX (600 ng/ml) for 48 hours, after which RNA was isolated for Socs1 mRNA determination by real-time RT-PCR. Data are mean ± SEM from 3 individual experiments, each performed in triplicate. (D) Socs1 mRNA decay in WT and 5-LO–/– macrophages. Cells were pretreated with or without LTB4 for 5 minutes, followed by addition of actinomycin D (2.5 mg/ml); cells were harvested at the indicated times and Socs1 mRNA was measured by real-time RT-PCR. Data are mean ± SEM from 3 individual experiments, each performed in triplicate; values are relative to untreated macrophages of the appropriate genotype. *P < 0.05 versus WT control; #P < 0.05 versus untreated 5-LO–/–.
Pivotal role of increased SOCS1 in the attenuation of MyD88 expression and NF-κB activation in LT-deficient macrophages.
To establish that the increase of SOCS1 expression in cells from 5-LO–/– mice is in fact responsible for their decreased MyD88 expression and NF-κB–dependent responses, we tested the effects of Socs1 knockdown using siRNA. Socs1 siRNA decreased Socs1 mRNA expression in WT and 5-LO–/– macrophages by approximately 70% (Figure 8A), but had no effect on Socs3 expression (data not shown). A control siRNA had no significant effect on Socs1 expression. Importantly, silencing of Socs1, but not Socs3, enhanced MyD88 protein and mRNA expression in WT and especially 5-LO–/– macrophages (Figure 8, B and C) and completely restored the generation of NO, RANTES, and IL-6 by LPS-stimulated 5-LO–/– macrophages to a level comparable to that of WT cells treated with control siRNA (Figure 8, E–G). Collectively, our findings showed that LTB4 increases Socs1 mRNA degradation, which allows STAT1-mediated MyD88 expression and hence optimal TLR-induced secretion of proinflammatory mediators.
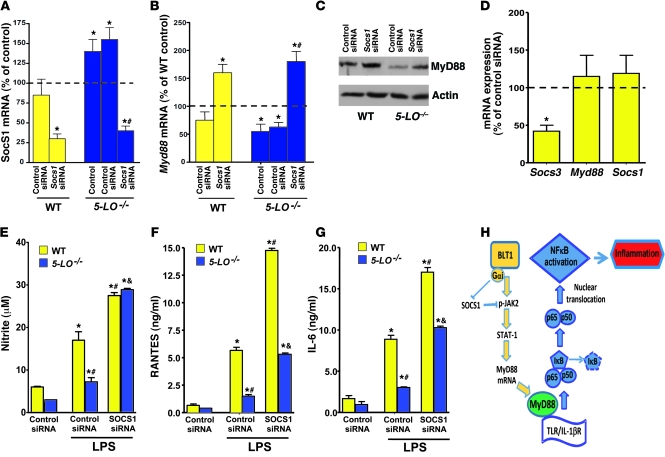
Figure 8. SOCS1 silencing restores MyD88 expression and responsiveness in 5-LO–/– macrophages.
(A) WT and 5-LO–/– macrophages were treated for 48 hours in the absence (control) or presence of Socs1 siRNA or scrambled siRNA (control siRNA), and the expression of Socs1 mRNA was determined by real-time RT-PCR and expressed relative to values in untreated WT cells (dashed line). (B) Myd88 mRNA and (C) MyD88 protein expression in WT and 5-LO–/– macrophages treated with Socs1 and control siRNA, as determined by real-time RT-PCR and immunoblotting, respectively; mRNA levels are expressed relative to those in untreated WT cells (dashed line). Immunoblot is representative of 3 independent experiments. (D) WT macrophages were treated for 48 hours in the presence of scrambled control and Socs3 siRNAs, and the expression of Socs3, Myd88, and Socs1 mRNA was determined by real-time RT-PCR and expressed relative to values in control siRNA cells. (E) NO, (F) RANTES, and (G) IL-6 secretion in Socs1-silenced WT and 5-LO–/– macrophages incubated with or without LPS. (H) Proposed model of LTB4/BLT1 regulation of SOCS1 and MyD88 expression and enhancement of MyD88-dependent NF-κB activation. Data are mean ± SEM from 3 individual experiments, each performed in triplicate. *P < 0.05 versus control; #P < 0.05 versus WT or 5-LO–/– control siRNA; &P < 0.05 versus LPS-challenged control siRNA.
Discussion
Here we demonstrate a nonredundant regulatory role of the lipid mediator LTB4 in amplifying macrophage MyD88 expression, which thus permits enhanced TLR/IL-1βR–dependent NF-κB activation (Figure 8H). To our knowledge, this is the first demonstration of an essential role for a GPCR in MyD88-dependent responses. Our results specifically showed that (a) LTB4/BLT1 signaling is required for MyD88-dependent NF-κB activation and production of proinflammatory molecules in vivo and in macrophages in vitro; (b) LTB4/BLT1 is required for basal and inducible Myd88 gene expression; (c) the MyD88 transcription factor STAT1 is activated by LTB4 in a manner dependent on Gαi activation and JAK2 phosphorylation; (d) Stat1 silencing prevents MyD88 expression and LPS responsiveness; (e) LTB4/BLT1 effects are mediated by enhanced degradation of mRNA encoding SOCS1, an important brake on the activation of JAK2 and STAT1; and (f) Socs1 knockdown restores MyD88 expression and LPS responsiveness in 5-LO–/– macrophages. The fact that this regulatory influence of LTB4/BLT1 was manifested in 2 different background mouse strains (Sv/129 for 5-LO–/– and C57BL/6 for BLT1–/– animals) underscores its generalizability. Moreover, 2 different models demonstrated the in vivo significance of this form of regulation. In the first, 5-LO–/– mice were protected from endotoxin shock and concomitant generation of inflammatory mediators. In the second, alveolar macrophage NF-κB activation during pneumococcal pneumonia was impaired in 5-LO–/– mice, but restored by intrapulmonary administration of LTB4.
Since both BLT1 and TLRs are pivotal drivers of inflammatory responses, cross-talk between these 2 classes of receptors is not unexpected. Indeed, TLR ligands have been shown to promote LT biosynthesis (37, 38). Furthermore, both LTB4 (39) and CysLTs (40) have themselves been reported to activate NF-κB in specific cells and contexts. However, whether 5-LO metabolites influence TLR responses, whether they are in fact necessary for TLR responses, and which specific 5-LO metabolites exert such actions and by what molecular mechanisms, are not well understood. Here, using both genetic and pharmacological approaches, we showed that MyD88-dependent NF-κB activation in macrophages depends on LTB4 biosynthesis and BLT1 signaling in both in vitro and in vivo settings. We have shown that the other major class of 5-LO metabolite, the CysLTs, did not exert effects on SOCS1, STAT1, and MyD88, emphasizing the specific and nonredundant role of LTB4 in regulating MyD88-dependent responses in macrophages. MyD88 expression in macrophages can be augmented above basal levels upon stimulation with proinflammatory molecules such as LPS (9), IFNs (9, 10), IL-6 (8), TNF-α (9), and IL-12 (10). Notably, LTB4 was required not only for constitutive, but also for inducible, expression of MyD88. Also striking was the fact that the upregulatory influence on MyD88 expression and TLR responsiveness was observed not only following the addition of exogenous LTB4, but also under basal conditions, in which levels of constitutive endogenous LTB4 generation would be expected to be quite low. Basal release of LTB4 by elicited peritoneal macrophages from WT animals over 90 minutes was approximately 100 pg/ml, which is equivalent to 0.5 nM. This concentration is indeed sufficient to activate BLT1, since we have shown that LTB4 amplifies AM antimicrobial functions at concentrations as low as 0.01 nM (35).
Control of MyD88-dependent responses by LTB4/BLT1 appears to be limited to cells that express abundant 5-LO and BLT1, including tissue phagocytes, and is not operative in lymphocytes and fibroblasts, which do not. It therefore dictates inflammatory responses in cells that are the pivotal first responders in innate immune processes. Studies with PTX revealed that Gαi signaling downstream from BLT1 was essential for LTB4-mediated MyD88 expression.
Little is known about the role of JAK/STAT in the physiological or pathophysiological functions of GPCRs. Gαi signaling triggered by angiotensin II has been reported to enhance JAK2 activation in a manner dependent on the tyrosine kinase Src (41). As we have previously demonstrated that LTB4 enhances Src activation in macrophages (42), we speculate that Src could be the upstream kinase responsible for tyrosine phosphorylation of JAK2 and initiation of STAT1-dependent MyD88 transcription. The lower basal phosphorylation of tyrosine residues on both JAK2 and STAT1 in 5-LO–/– macrophages led us to consider the possibility that LT deficiency increases the expression of SOCS proteins, the negative regulators of JAK/STAT signaling (36). Indeed, 5-LO–/– and BLT1–/– cells manifested significantly higher mRNA and protein expression of SOCS1 (but not of other SOCS family members), which was reduced back to WT levels by addition of LTB4 in BLT1+ cells. The finding that PTX increased Socs1 expression in WT macrophages (Figure 7C) while concomitantly reducing Myd88 expression (Figure 6B) suggests that SOCS1 expression in these cells may be enhanced by cyclic AMP. Although to our knowledge, regulation of SOCS1 by cyclic AMP has not previously been established, this possibility is consistent with a report that prostaglandin E2, via Gαs-coupled receptor–derived cyclic AMP, increased SOCS3 expression (43, 44). In contrast to this paradigm, increased expression of SOCS1 and SOCS3 has previously been reported in response to ligation of chemoattractant receptors other than BLT1, including CXCR1/2 by CXCL8/IL-8 and FPR-1 by fMLP (45). The conflicting nature of these data suggests that cyclic AMP regulation of SOCS proteins is context dependent. We found that endogenous and exogenous LTB4 reduced Socs1 mRNA primarily by increasing its rate of degradation; to our knowledge, this is the first demonstration of a GPCR regulating Socs1 mRNA stability. Among the complex determinants of mRNA stability are the nucleotide sequence itself (46), destabilizing zinc-finger proteins (47), and microRNAs (46). Further studies will be necessary to elucidate the mechanisms by which LTB4/BLT1 signaling regulates Socs1 mRNA stability.
Our findings have direct translational importance, as pharmacologic 5-LO inhibitors or BLT1 antagonists that are currently available or under development would be expected to reduce macrophage MyD88 expression and attenuate the excessive NF-κB activation characteristic of a variety of inflammatory diseases. These include activation of TLR4 by danger signals released during tissue injury (48) or of unchecked IL-1βR activation in autoinflammatory conditions (7). At the same time, however, acquired states of LTB4 deficiency, such as HIV infection and malnutrition (13), would be expected to limit NF-κB activation and thereby compromise innate immune defense against microbial pathogens. Indeed, the recent finding that alveolar macrophages from HIV+ individuals exhibit a signaling defect limited to MyD88-dependent TLRs (49) could potentially be explained by the findings reported herein, together with the LTB4 deficiency previously described for these cells (13). States of immunosuppression such as these might be overcome by administration of exogenous LTB4.
Methods
Animals.
8-week-old female 5-LO–/– (129-Alox5tm1Fun; ref. 50) and strain-matched WT sv/129 mice, and BLT1–/– (B6.129S4-Ltb4r1tm1Adl/J; ref. 51) and strain-matched WT C57BL/6 mice (The Jackson Laboratory), were treated according to NIH guidelines for the use of experimental animals, with the approval of the University of Michigan Committee for the Use and Care of Animals.
Cell harvest.
Macrophages were harvested from the peritoneal cavities of mice by lavage with PBS 4 days after the injection of 2 ml of 3% thioglycollate, as described previously (52).
Purification of leukocyte populations.
Purification of spleen macrophages, DCs, T cells, and B cells was accomplished using magnetic beads conjugated to CD11b, CD11c, TCRα/β, and CD19, respectively, as described by the manufacturer (Miltenyi). Alveolar macrophages (35) and lung fibroblasts (53) were isolated as described previously.
Macrophage stimulation.
Cells were stimulated with LPS (from E. coli serotype 0111:B4; 100 ng/ml), PGN (1 μg/ml), Pam3Cys (2 μg/ml), or poly(I:C) (50 μg/ml; all from Invivogen); IL-1β (2 ng/ml; Novus Biologicals); TNF-α (2 ng/ml; Biosource); and LTB4 (10 nM) or LTD4 (100 nM; both from Biomol) for the time periods indicated in the figure legends.
Immunoblotting.
Western blots were performed as previously described (52). Protein samples were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with commercially available primary antibodies against MyD88, TRIF, TIRP, SOCS1 (all 1:500, Abcam); p65, TIRAP, phosphorylated IκBα, p50, p65 (all 1:500, Santa Cruz); Tyr701- and Ser727-phosphorylated as well as total STAT1, STAT3, and JAK2 and phosphorylated JAK2 (Tyr1007/1008), JAK1 (Tyr1022/1023), Tyk2 (Tyr1052/1053), and STAT3 (Tyr705) (all 1:1,000; Cell Signaling); and β-actin (1:10,000; Sigma-Aldrich). Densitometric analysis was as described previously (35).
Immunofluorescence microscopy and image analysis.
Macrophages adhered on coverslips were stimulated with LPS or poly(I:C) for 24 hours (42). In other experiments, leukocytes were obtained from the lung lavage fluid of mice infected with S. pneumoniae and subsequently treated with aerosolized vehicle or LTB4 24 hours after infection. The NF-κB p50 or p65 subunit was detected by incubation with rabbit anti-mouse antibody (1:200 dilution) for 60 minutes. Mounts were washed 3 times with 1% BSA-PBS, and rhodamine-conjugated goat anti-rabbit secondary (1:200; Invitrogen) was added for 1 hour at 37°C. Fluorescence was visualized with a Nikon Labophot 2 microscope equipped for epifluorescence at ×400 objective magnification. The extent of colocalization between NF-κB p50 and nuclear DAPI was quantitated using the Jacop plug-in for Image J (23). The background of the collected images was corrected by the Image J rolling ball algorithm plug-in. The specific algorithm used was based on the Manders overlap coefficient (23), which ranges from 0 to 1, the former corresponding to nonoverlapping images and the latter reflecting 100% colocalization between both images. For each experiment, at least 100 randomly selected cells were scored.
Measurement of nitrite, cytokine, and chemokine levels.
Levels of RANTES, IL-6, and IL-10 were determined by ELISA (R&D Duoset; R&D Systems) by the University of Michigan Cancer Center Cellular Immunology Core. Nitrite, the stable oxidized derivative of NO, was determined using the Griess reaction (52).
RNA isolation and semiquantitative real-time RT-PCR.
RNA from cultured cells was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions, and real-time RT-PCR was performed as previously described (54). To determine the decay of Myd88 and Socs1 mRNA, WT and 5-LO–/– macrophages were treated with or without 2.5 μg/ml actinomycin D (Sigma-Aldrich) in the presence or absence of exogenous LTB4, and the amount of mRNA was determined after harvesting at different time points. Socs1 or Myd88 mRNA were normalized to β-actin, and the respective WT control was set to 100%. Percentages were plotted against time, and decay curves were calculated.
RNA interference.
RNA interference was performed according to a protocol provided by Dharmacon. WT and 5-LO–/– macrophages were transfected using DharmaFECT 1 reagent with 30 nM of nonspecific control or specific ON-TARGET SMARTpool Socs1, Stat1, and Socs3 siRNAs. After 48 hours of transfection, macrophages were harvested for mRNA or protein analysis or treated with LPS for 24 hours to analyze NO, RANTES, and IL-6 secretion.
NF-κB p50, NF-κB p65, and STAT1 activity.
Macrophages were stimulated for the specified times with TLR agonists IL-1β, TNF-α, or LTB4, and DNA binding activity in nuclear extracts (10 μg protein) was assayed using transcription factor ELISAs for NF-κB p50 and p65 subunits (Panomics) as well as STAT1 (Panomics), according to the manufacturer’s instructions.
S. pneumoniae infection.
S. pneumoniae serotype 3, 6,303 (ATCC) was grown, and mice were infected, as described previously (27).
In vivo injection with LPS and TNF-α.
LPS (10 μg/kg) from E. coli serotype 0111:B4 (Sigma-Aldrich) or recombinant TNF-α (25 μg/ml; Biolegend) was reconstituted in PBS with 1% BSA and administered to mice via i.p. injection. Mice were monitored over a 7-day period for survival analysis; results were pooled from n = 5 per group in each of 2 independent experiments. Cytokine levels were measured in serum and peritoneal lavage fluid harvested 6 hours after challenge with LPS or TNF-α.
Statistics.
Data are presented as mean ± SEM. Comparisons among groups were assessed with ANOVA followed by Bonferroni analysis. Differences were considered significant for P values less than 0.05.
Supplementary Material
Acknowledgments
We thank members of the Peters-Golden laboratory and Ana Paula Moreira for their thoughtful input. This work was supported by NIH grant HL058897 (to M. Peters-Golden); by an American Lung Association Senior Postdoctoral Research Fellowship, an American Heart Association Career Development Award, and NIH grant K99HL103777 (to C.H. Serezani); and by FAPESP (to S. Jancar).
Footnotes
Conflict of interest: M. Peters-Golden has received research support from Nycomed.
Citation for this article: J Clin Invest. 2011;121(2):671–682. doi:10.1172/JCI43302.
References
- 1. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 3. Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009;10(4):333–339. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 4. Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27(9):474–482. doi: 10.1016/S0968-0004(02)02145-X. [DOI] [PubMed] [Google Scholar]
- 5. Michelsen KS, Arditi M. Toll-like receptor signaling and atherosclerosis. Curr Opin Hematol. 2006;13(3):163–168. doi: 10.1097/01.moh.0000219662.88409.7c. [DOI] [PubMed] [Google Scholar]
- 6. O’Neill LA. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3(4):396–403. doi: 10.1016/S1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 7. Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360(23):2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5(7):1095–1097. [PubMed] [Google Scholar]
- 9. Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168(2):554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 10. Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165(4):1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 11. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 12. Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357(18):1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 13. Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;174(2):589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 14. Won JS, Im YB, Khan M, Singh AK, Singh I. Involvement of phospholipase A2 and lipoxygenase in lipopolysaccharide-induced inducible nitric oxide synthase expression in glial cells. Glia. 2005;51(1):13–21. doi: 10.1002/glia.20178. [DOI] [PubMed] [Google Scholar]
- 15. Piette J, et al. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem. 1997;378(11):1237–1245. [PubMed] [Google Scholar]
- 16. Paul L, Fraifeld V, Kaplanski J. Evidence supporting involvement of leukotrienes in LPS-induced hypothermia in mice. Am J Physiol. 1999;276(1 pt 2):R52–R58. doi: 10.1152/ajpregu.1999.276.1.R52. [DOI] [PubMed] [Google Scholar]
- 17. Shiratori Y, Tanaka M, Umihara J, Kawase T, Shiina S, Sugimoto T. Leukotriene inhibitors modulate hepatic injury induced by lipopolysaccharide-activated macrophages. J Hepatol. 1990;10(1):51–61. doi: 10.1016/0168-8278(90)90073-Z. [DOI] [PubMed] [Google Scholar]
- 18. Collin M, et al. Reduction of the multiple organ injury and dysfunction caused by endotoxemia in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. J Leukoc Biol. 2004;76(5):961–970. doi: 10.1189/jlb.0604338. [DOI] [PubMed] [Google Scholar]
- 19. O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 20. Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9(10):692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez Y, Municio C, Alonso S, Sanchez Crespo M, Fernandez N. The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J Immunol. 2009;183(2):1471–1479. doi: 10.4049/jimmunol.0900312. [DOI] [PubMed] [Google Scholar]
- 22. Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165(1):286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 23. Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. . J Microsc. 2006;224(pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 24. Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 25. Bonizzi G, et al. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19(3):1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163(1):1–5. [PubMed] [Google Scholar]
- 27. Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun. 2010;78(5):2264–2271. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harroch S, Gothelf Y, Revel M, Chebath J. 5' upstream sequences of MyD88, an IL-6 primary response gene in M1 cells: detection of functional IRF-1 and Stat factors binding sites. Nucleic Acids Res. 1995;23(17):3539–3546. doi: 10.1093/nar/23.17.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem. 2005;280(7):5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- 30. Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 31. Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 32. Finbloom DS, Larner AC. Regulation of the Jak/STAT signalling pathway. Cell Signal. 1995;7(8):739–745. doi: 10.1016/0898-6568(95)02004-7. [DOI] [PubMed] [Google Scholar]
- 33. Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 34. Lucet IS, et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107(1):176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 35. Peres CM, Aronoff DM, Serezani CH, Flamand N, Faccioli LH, Peters-Golden M. Specific leukotriene receptors couple to distinct G proteins to effect stimulation of alveolar macrophage host defense functions. J Immunol. 2007;179(8):5454–5461. doi: 10.4049/jimmunol.179.8.5454. [DOI] [PubMed] [Google Scholar]
- 36. Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 37. McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170(4):1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 38. Lindner SC, Kohl U, Maier TJ, Steinhilber D, Sorg BL. TLR2 ligands augment cPLA2alpha activity and lead to enhanced leukotriene release in human monocytes. J Leukoc Biol. 2009;86(2):389–399. doi: 10.1189/jlb.1008591. [DOI] [PubMed] [Google Scholar]
- 39. Sanchez-Galan E, et al. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81(1):216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
- 40. Hashimoto K, Ichiyama T, Hasegawa M, Hasegawa S, Matsubara T, Furukawa S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-kappaB pathways. Int Arch Allergy Immunol. 2009;149(3):275–282. doi: 10.1159/000199724. [DOI] [PubMed] [Google Scholar]
- 41. Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20(5):953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 42. Serezani CH, Aronoff DM, Sitrin RG, Peters-Golden M. FcgammaRI ligation leads to a complex with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions. Blood. 2009;114(15):3316–3324. doi: 10.1182/blood-2009-01-199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheon H, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177(2):1092–1100. doi: 10.4049/jimmunol.177.2.1092. [DOI] [PubMed] [Google Scholar]
- 44. Gasperini S, et al. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur Cytokine Netw. 2002;13(1):47–53. [PubMed] [Google Scholar]
- 45. Stevenson NJ, et al. The chemoattractants, IL-8 and formyl-methionyl-leucyl-phenylalanine, regulate granulocyte colony-stimulating factor signaling by inducing suppressor of cytokine signaling-1 expression. J Immunol. 2004;173(5):3243–3249. doi: 10.4049/jimmunol.173.5.3243. [DOI] [PubMed] [Google Scholar]
- 46. Liu J. Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008;20(2):214–221. doi: 10.1016/j.ceb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 47. Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36(pt 3):491–496. doi: 10.1042/BST0360491. [DOI] [PubMed] [Google Scholar]
- 48. Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 49. Tachado SD, et al. MyD88-dependent TLR4 signaling is selectively impaired in alveolar macrophages from asymptomatic HIV+ persons. Blood. 2010;115(17):3606–3615. doi: 10.1182/blood-2009-10-250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372(6502):179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 51. Tager AM, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4(10):982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 52. Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. . J Immunol. 2006;177(5):3201–3208. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 53. Moore BB, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174(9):5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 54. Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37(5):562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.