Abstract
Transplantation of allogeneic stem cells into the early gestational fetus, a treatment termed in utero hematopoietic cell transplantation (IUHCTx), could potentially overcome the limitations of bone marrow transplants, including graft rejection and the chronic immunosuppression required to prevent rejection. However, clinical use of IUHCTx has been hampered by poor engraftment, possibly due to a host immune response against the graft. Since the fetal immune system is relatively immature, we hypothesized that maternal cells trafficking into the fetus may pose the true barrier to effective IUHCTx. Here, we have demonstrated that there is macrochimerism of maternal leukocytes in the blood of unmanipulated mouse fetuses, with substantial increases in T cell trafficking after IUHCTx. To determine the contribution of these maternal lymphocytes to rejection after IUHCTx, we bred T and/or B cell–deficient mothers to wild-type fathers and performed allogeneic IUHCTx into the immunocompetent fetuses. There was a marked improvement in engraftment if the mother lacked T cells but not B cells, indicating that maternal T cells are the main barrier to engraftment. Furthermore, when the graft was matched to the mother, there was no difference in engraftment between syngeneic and allogeneic fetal recipients. Our study suggests that the clinical success of IUHCTx may be improved by transplanting cells matched to the mother.
Introduction
Stem cell transplantation is a promising treatment strategy for many genetic disorders such as hemoglobinopathies, immunodeficiencies, or inborn errors of metabolism, but clinical applications are limited by graft rejection and toxic immunosuppression. Transplantation of allogeneic stem cells into the early gestational fetus offers an avenue to overcome this limitation. Theoretically, introduction of allogeneic cells prior to the maturation of the developing immune system may result in donor-specific tolerance. Since the seminal experiments of Billingham, Brent, and Medawar (1), animal models of in utero hematopoietic cell transplantation (IUHCTx) have shown that the fetal environment offers considerable advantages for the success of stem cell transplantation (reviewed in ref. 2). In the mouse model, fetal mice can be tolerized to fully allogeneic stem cells without any immunosuppression (3, 4). The treatment of hemoglobinopathies has also been achieved in mice using this approach (5). These results have been confirmed in large animal models: fetal lambs can accept xenogeneic human HSCs with long-term hematopoietic chimerism (6), and we have observed long-term engraftment and tissue-specific differentiation of human mesenchymal stem cells in sheep using this approach (7).
In spite of the results from animal models, the clinical success of IUHCTx for congenital disorders has been hampered by poor donor cell engraftment except in severe combined immunodeficiency (8, 9), in which there is a clear survival advantage for transplanted cells without a host adaptive immune response. While there are many potential barriers to engraftment, such as cell dose, technique of transplantation, and lack of competitive advantage of transplanted cells (10), the host immune system, even in the fetus, may be an important barrier to consider. Even in the mouse model, it has been described that not all transplanted fetuses ultimately engraft with allogeneic cells, possibly secondary to a host immune response (11). This observation has created a conundrum in the field, since the fetus can also be tolerized to foreign antigens such as non-inherited maternal antigens (NIMAs) during gestation (reviewed in refs. 12, 13). One potential explanation, then, is that it is the maternal, not fetal, immune response which is the real barrier. In fact, it was recently reported that maternal alloantibodies, transmitted postnatally via breast milk, impede engraftment after transplantation of adult BM cells in fetal mice (14).
In addition to antibodies, there is considerable evidence that maternal leukocytes cross into the fetus during gestation. Maternal-fetal cellular trafficking (MFCT) is the bidirectional passage of cells across the placenta, resulting in long-lived fetal cells in mothers (15) and maternal cells in children (16). Although the functional significance of this phenomenon is unclear, there is speculation that microchimerism resulting from this trafficking may be involved in the pathogenesis of autoimmune disease or in the tissue response to injury (17, 18). In humans, the presence of microchimeric maternal cells in fetuses was recently shown to induce fetal Tregs against NIMAs (19), suggesting that the fetus can develop dominant tolerance to foreign antigens encountered during development. However, the role of trafficking maternal leukocytes in fetal transplantation has not, to our knowledge, been studied. It is possible that they could instead induce an immune response that limits the engraftment of cells transplanted into the fetus.
In this study, we examined the trafficking of maternal leukocytes into the fetus and the role of the maternal immune system in limiting the engraftment of in utero transplanted cells in mice. We report that maternal T cells pose a significant barrier to engraftment after IUHCTx and suggest that the clinical success of fetal transplantation may be improved by transplanting stem cells harvested from the mother or by HLA-matching the transplanted cells to the mother.
Results
The adaptive immune system limits engraftment after IUHCTx.
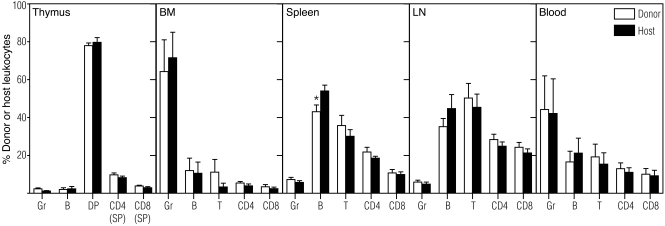
We chose to use a mouse model of in utero fetal liver (FL) transplantation, based on previous publications showing increased engraftment with FL compared with adult BM in the fetal environment (20). To validate our model of IUHCTx, we first performed FL transplants in congenic fetuses by using C57BL/6.CD45.1 (designated CD45.1) mice as donors and C57BL/6.CD45.2 (designated B6) mice as recipients. Donor-derived cells were detected in the blood of transplant recipients by flow cytometry (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI44907DS1). The frequency of engraftment (chimerism level, >1%) at 4 weeks after transplantation in congenic recipients was 82% (n = 17), similar to what has been previously reported (11). Chimerism levels were stable for 28 weeks, the last time point tested. Analysis of donor and host T cell, B cell, and granulocyte composition in lymphoid organs confirmed multilineage engraftment of donor cells (Figure 1), indicating that using this stem cell source, injection technique, and dose, engraftment is not limited by the availability of hematopoietic niches in the recipient. Given that this method of IUHCTx generates stable, mixed chimeras, we then used our model to focus on the role of the immune system on engraftment.
Figure 1. Lineage analysis of donor- and host-derived leukocytes in congenic recipients.
Multilineage engraftment of FL-derived hematopoietic cells was seen in primary (thymus and BM) and secondary (spleen and lymph node [LN]) lymphoid organs and peripheral blood at 43–57 weeks after in utero transplantation (n ≥ 5 mice per group). Donor- and host-derived double-positive (DP: CD4+CD8+) and single-positive (SP: CD4+CD8– or CD4–CD8+) thymocytes, T lymphocytes (T, CD4, and CD8), B lymphocytes (B), and granulocytes (Gr) are shown as a percentage of their respective CD45+ leukocyte gate (donor, CD45.1; host, B6; CD45.2). No significant differences were observed between the percentages of donor and host leukocyte subpopulations, with the exception of a decreased percentage of donor-derived B cells in spleen. *P < 0.05 by t test.
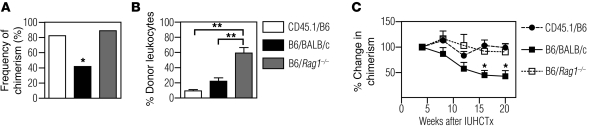
Previous studies have suggested that the adaptive immune response plays a role in limiting the engraftment of adult BM-derived hematopoietic cells after in utero transplantation (11). To determine whether the adaptive immune system also limits engraftment of FL-derived hematopoietic cells, we compared the frequencies of engraftment in congenic (CD45.1 into B6) and allogeneic recipients (B6 into BALB/c or BALB/c into B6). Donor-derived cells were detected in the blood of transplanted hosts using flow cytometry (Supplemental Figure 1B). The frequency of engraftment after transplantation of B6 FL into BALB/c fetuses was 42% (n = 43), significantly lower than the 82% achieved in the congenic setting (Figure 2A, c2 test, P < 0.005 compared with congenic). Similarly, the rate of chimerism after transplantation of BALB/c FL into B6 fetuses was 53% (n = 40, c2 test < 0.05 compared with congenic). These data indicate that the alloimmune response is a barrier to early engraftment after IUHCTx with FL. To determine whether the barrier is due to an innate immune response, such as NK cell activation, or an adaptive immune response, we performed allogeneic in utero FL transplants into BALB/c.Rag1–/– (Rag1–/–) recipients which lack T and B cells but have an intact innate immune system (21). In this instance, the frequency of engraftment of B6 FL in Rag1–/– recipients was 89% (Figure 2A, n = 9, c2 test, P = 0.83 compared with congenic controls), indicating that the barrier to IUHCTx in our model was likely due to an adaptive alloimmune response. While NK cells have been shown to be important in engraftment after IUHCTx (22, 23), their effect can be overcome using high-cell doses, as we are doing in this model (24). As expected, the overall levels of chimerism in these animals were much higher (59.2% ± 7.2% compared with 22.2% ± 4.5% for allogeneic wild-type recipients, P = 0.0001), likely secondary to the competitive advantage of transplanted cells in the Rag1–/– hosts (ref. 25 and Figure 2B). Lineage analysis of chimeric cells in these animals indicated that the increase in chimerism levels in the Rag1–/– hosts was limited to the lymphoid component, while granulocyte chimerism levels were low and similar to those obtained in wild-type hosts (Supplemental Figure 2). Among wild-type recipients of IUHCTx, the levels of chimerism were not different in any of the experiments in this report and are detailed in Supplemental Figure 3.
Figure 2. The adaptive immune response limits engraftment after allogeneic IUHCTx.
(A) Frequency of chimerism (number of chimeric animals/number of surviving animals) after in utero transplantation of B6 FL cells into congenic (CD45.1 into B6 [CD45.1/B6], n = 17), allogeneic (B6 into BALB/c [B6/BALB/c], n = 43), or immunodeficient (B6 into BABL/c.Rag1–/– [B6/Rag1–/–], n = 9) recipients. *P < 0.005, c2 test for allogeneic versus congenic. (B) Levels of chimerism in individual engrafted animals at 4 weeks after in utero transplantation (CD45.1/B6, n = 18; B6/BALB/c, n = 16; B6/Rag1–/–, n = 10). **P < 0.05, ANOVA with Tukey’s multiple comparison test. (C) Change in levels of chimerism over time when normalized to the initial level of chimerism at 4 weeks after transplantation (CD45.1/B6, n = 18; B6/BALB/c, n = 10; B6/Rag1–/–, n = 10). *P < 0.05 comparing CD45.1/B6 and B6/BALB/c, and CD45.1/B6 and B6/Rag1–/– using ANOVA with Tukey’s multiple comparison test.
When we analyzed our chimeric animals over time, we observed a gradual loss of engraftment in allogeneic, but not congenic or immunodeficient, chimeras, suggesting that the adaptive immune system also contributes to a late-phase graft loss. There was a significant difference (P < 0.05) between the levels of chimerism in congenic and allogeneic recipients that remained chimeric 16 and 20 weeks after transplantation (Figure 2C).
Engrafted mice are tolerant to donor antigen.
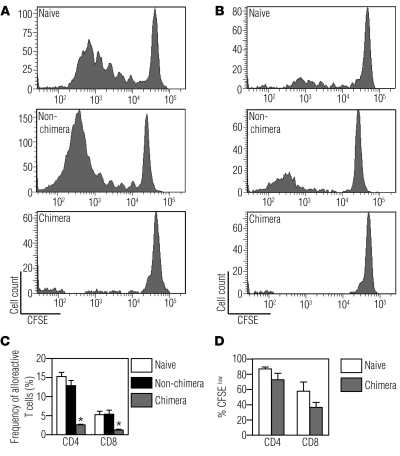
To confirm that chimeric animals exhibited allospecific tolerance, we stimulated lymphocytes from chimeric mice with the donor alloantigen using an in vivo mixed lymphocyte reaction (MLR) (26). We injected CFSE-labeled lymphocytes from naive (uninjected), chimeric (adult animals that were stably chimeric as a result of their in utero injection), or non-chimeric animals (adult animals that were never engrafted after their in utero injection) into B6 × BALB/c F1 recipients and examined the proliferation of injected CD4+ (Figure 3A) and CD8+ (Figure 3B) T cells 3 days later. Using this assay, the frequencies of donor-reactive T cells can be calculated based on their proliferative histories recorded in the CFSE fluorescence intensities. In naive animals, the frequency of alloreactive CD4+ T cells was 15.3% ± 1.1% and that of CD8+ T cells was 5.3% ± 0.9%, which was in concordance with previously described results (26). All chimeric mice exhibited marked reduction in response to donor alloantigens compared with both naive animals and injected non-chimeras (Figure 3C) while retaining the ability to respond to a third-party antigen (Figure 3D). We observed decreased frequencies of both alloreactive CD4+ (2.6% ± 0.2%, P < 0.0001) and CD8+ T cells (1.2% ± 0.2%, P = 0.0005) in chimeras when compared with naive mice. These results indicate that chimeric mice exhibit donor-specific tolerance, although this assay does not distinguish between deletion and anergy.
Figure 3. Chimeras are tolerant to donor alloantigen.
Lymphocytes harvested from spleens and lymph nodes of naive mice, injected non-chimeras, and chimeras were labeled with CFSE and injected into (A–C) B6 × BALB/c or (D) C3H × DBA/2 (third-party control) F1 recipients. Representative flow cytometric histograms showing CFSE profiles after gating on donor-derived (A) CD4+ or (B) CD8+ T lymphocytes are shown. (C) Percentage of alloreactive T cells in naive mice, non-chimeras, and chimeras. The data shown are representative of at least 4 independent experiments (naive, n = 10; non-chimera, n = 7; chimera, n = 10). *P < 0.05 comparing chimeras with naive mice and non-chimeras using ANOVA with Tukey’s multiple comparison test. (D) Percentage of donor-derived proliferating cells (%CFSElow) from naive and chimeric mice in response to a third-party antigen. Data are representative of at least 2 independent experiments (naive, n = 3; chimera, n = 5).
Maternal leukocytes are present in the circulation of normal fetuses and increase after in utero transplantation.
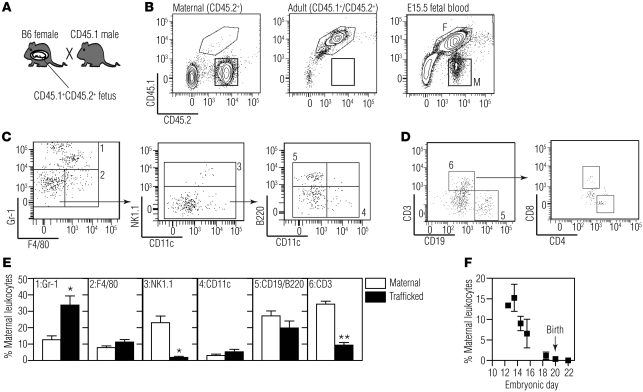
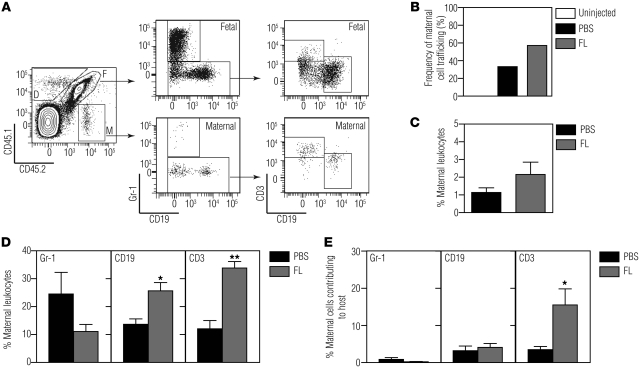
The immune system of fetal mice at E14.5 is still immature, and antigenic encounter at this age should lead to tolerance, which is at odds with our observation that the adaptive immune response limits engraftment in half of the recipients. We hypothesized that maternal leukocytes may instead be responsible for limiting engraftment in our model. We first asked whether maternal leukocytes are present in the fetus at the time of in utero transplantation using a flow cytometry–based method to detect maternal cells in fetuses. For these experiments, we bred B6 mothers with CD45.1 fathers, such that the fetuses were heterozygous (CD45.1+CD45.2+) and maternal cells present in the fetuses could be identified by their lack of CD45.1 (Figure 4, A and B). We analyzed blood and tissues from the fetuses of these matings by flow cytometry after staining for CD45.1, CD45.2, and leukocyte lineage markers. Using this strategy, we found that there is a sizeable population of maternal cells in the blood of mid-gestational fetuses (Figure 4B). At E13.5–E14.5, the age at which we performed in utero transplantations, the CD45+ population in the fetal blood contained 8.9% ± 1.4% maternal cells (n = 12).
Figure 4. Maternal macrochimerism in mid-gestation fetal blood.
(A) The breeding scheme used to identify maternal leukocytes in fetal blood. (B) Representative flow cytometric plots depicting the profile of CD45.2+ (maternal, left panel), CD45.1+/CD45.2+ (adult control, middle panel), and E15.5 fetal blood (right panel; F, fetal; M, maternal). Lineage analysis of maternal leukocytes found in fetal blood (gate M in B) was performed using cell surface markers for (C) innate and (D) adaptive immune cells. (C) The gating strategy for identifying innate immune cells involved first detecting Gr-1+ or F4/80+ leukocytes. NK cells were identified among the Gr-1–F4/80– cells. Gr1–F4/80–NK1.1– cells were further divided into CD11c+ and B220+ leukocytes. (D) Adaptive immune cells were characterized by identifying CD3+ and CD19+ maternal leukocytes. The CD3+ subpopulation was further characterized based on CD4 and CD8 expression. (E) Percentages of various leukocyte subsets found in the mother (maternal) and in the fetus (trafficked) at E12.5–E15.5 (n ≥ 3; *P < 0.01, **P < 1 × 10–8 by t test). (F) Percentage of maternal leukocytes (number of CD45.2+ cells/total CD45+ cells) in fetal circulation at various embryonic days of gestation (E12.5, n = 1; E13.5, n = 4; E14.5, n = 8; E15.5, n = 5; E18.5, n = 14; E20, n = 12; E22, n = 3). There was a significant negative correlation between maternal macrochimerism and gestational age (Pearson r = –0.94, P = 0.002).
We further analyzed the lineage composition of the trafficking maternal cells using markers for T cells (CD3, CD4, CD8), B cells (CD19, B220), NK cells (NK1.1), macrophages (F4/80), granulocytes (Gr-1), and dendritic cells (CD11c). At E12.5–E15.5, we detected populations of maternal Gr-1+, F4/80+, NK 1.1+, CD11c+, CD19+, B220+, and CD3+ leukocytes (Figure 4, C and D). While CD3+ T cells were not observed in all fetuses at baseline, when they were present, both CD4+ and CD8+ populations were detected (Figure 4D). When we compared the lineage composition of maternal cells in the fetus with that in the mother, we found significant differences in the lineage distribution of maternal cells in fetal blood compared with those in maternal blood (Figure 4E). The maternal leukocytes in fetal circulation contained a significantly higher proportion of Gr-1+ cells and a significantly lower proportion of T cells and NK cells when compared with the cells in maternal circulation (Figure 4E). These results confirm that the observed maternal cells are not secondary to contamination of maternal blood during harvesting and further suggest that trafficking of maternal leukocytes to the fetus is selective.
Maternal cells were not detectable by flow cytometry in the liver, spleen, or thymus at any time point tested, likely secondary to dilution of small numbers of maternal cells by the higher concentration of fetal CD45+ cells in these lymphoid organs. When we analyzed fetuses at later gestational ages, we found a strong negative correlation between peripheral blood maternal macrochimerism and gestational age (Figure 4F, Pearson r = –0.94, P = 0.002), such that maternal cells were rarely detected late in gestation and not detectable after birth.
We next examined whether fetal intervention leads to changes in maternal-fetal cellular trafficking. We compared the number of maternal cells in fetuses from the B6 female and CD45.1 male matings after in utero transplantation with allogeneic NOD.CD45.1 FL at E14.5. Control fetuses received PBS injection or no injection, and all animals were harvested on E18.5–E19.5. This experimental scheme allowed us to detect the presence of the transplanted NOD cells (CD45.1+CD45.2–) and trafficking maternal leukocytes (CD45.1–CD45.2+) among fetal cells (CD45.1+CD45.2+) (Figure 5A). As expected, NOD donor cells were found in 95% (n = 21) of fetal recipients at this early time point, supporting our interpretation that graft loss occurs gradually and is not due to technical variations in the injection method.
Figure 5. Maternal-fetal cellular trafficking after fetal intervention.
B6 mothers were mated with CD45.1 fathers, and the CD45.1+/CD45.2+ fetuses were injected with allogeneic NOD.CD45.1 FL cells or PBS on E14.5. Injected (and uninjected control) fetuses were sacrificed on E18.5–E19.5, and the number of maternal leukocytes (CD45.2+) in fetal blood was quantified. (A) Flow cytometric analysis of donor (gate D), maternal (gate M), and fetal (gate F) leukocytes. (B) Frequency of fetuses with circulating maternal leukocytes (number of fetuses with circulating maternal leukocytes/total number of fetuses) after PBS injection (n = 11/33, 33%) and allogeneic FL injection (n = 12/21, 57%) and in age-matched uninjected controls (n = 0/21, 0%). (C) Percentage of maternal leukocytes (CD45.2+ maternal leukocytes/total CD45.2+ cells) in fetal circulation after PBS (n = 11) and allogeneic FL injection (n = 12). (D) Lineage analysis of trafficking maternal cells shown as percentage of maternal leukocytes (e.g., trafficked maternal Gr-1+ cells/total trafficked maternal leukocytes). *P < 0.005, **P < 0.0001 by t test. (E) Lineage analysis of trafficking maternal cells shown as the percentage of maternal cells contributing to each of the leukocyte subsets in fetal circulation (e.g., trafficked maternal Gr-1+ cells/total number of fetal and trafficked maternal Gr-1+ cells). *P < 0.05 by t test.
Analysis of maternal cells in these animals showed no maternal cell trafficking in unmanipulated fetuses (n = 21), while 33% (n = 33) of PBS-injected and 57% (n = 21) of FL-injected pups had greater than 0.5% maternal cells (Figure 5B, c2 test, P = 0.15 between FL and PBS), indicating that nonspecific tissue injury after fetal intrahepatic injection alters trafficking, with further increases in maternal cells after FL transplantation. Analysis of the percentage of maternal leukocytes in fetal blood in each animal revealed a trend toward increased maternal cell chimerism with FL transplantation compared with PBS (Figure 5C, 1.1% ± 0.3% PBS vs. 2.2% ± 0.7% FL, P = 0.21). These results indicate either increased maternal cell trafficking into the fetus or increased survival of trafficking maternal cells after fetal intervention.
Although the overall percentage of maternal cells found in the fetus was not significantly different between the two groups, we detected significant increases in both maternal T and B cells found in the fetus after allogeneic FL transplantation compared with PBS injection (Figure 5D, T cell: 12.1% ± 2.9% PBS vs. 33.8% ± 2.3% FL, P < 0.0001; B cell: 13.7 ± 1.9 PBS vs. 25.7% ± 3.0% FL, P < 0.005). Since the fetus at this gestational age has very few circulating T cells, we then determined whether the trafficked maternal cells contributed to a significant population of the T cell pool. We found that maternal T cells represented 15.5% ± 4.3% of the total circulating T cell pool in the host after FL transplantation, whereas they represented 3.5% ± 0.8% of the total T cell population after PBS injection (Figure 5E, P < 0.05). Thus, IUHCTx leads to a significant increase in the levels of maternal T cells in the fetal circulation, suggesting that these cells may play a functional role in the engraftment of allogeneic cells.
Maternal B cells and antibodies are not the critical component in limiting engraftment after in utero transplantation.
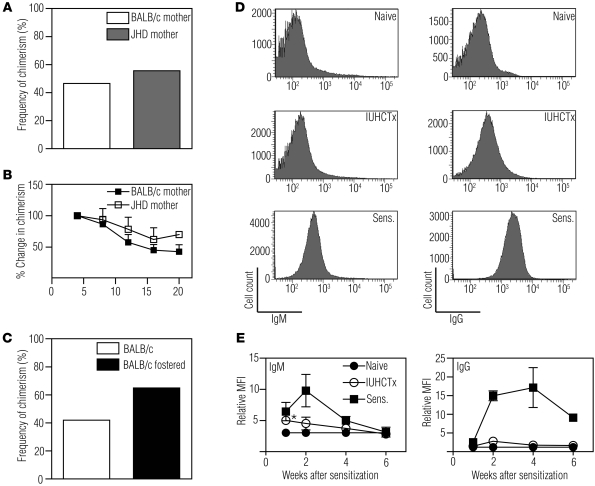
To determine whether trafficking maternal B cells contribute to an immune response against the cells transplanted into the fetus, we bred B cell–deficient JHD mothers (27) with wild-type BALB/c fathers (such that the fetuses were heterozygous and immunocompetent) and transplanted allogeneic B6 FL into the fetuses. The frequency of chimerism in this setting was 56% (n = 9, Figure 6A), which is not different from what is seen when the mother is immunocompetent (c2 test, P = 0.11). The chimerism levels over time were similar compared with animals born to wild-type mothers (Figure 6B).
Figure 6. The rejection of in utero transplanted allogeneic hematopoietic cells occurs independent of maternal B cells and maternal alloantibodies.
(A) Frequency of chimerism after IUHCTx of B6 FL cells into fetuses born to a wild-type BALB/c father and either a wild-type BALB/c mother (n = 43) or a B cell–deficient (JHD) mother (n = 9). (B) Change in levels of chimerism over time in engrafted animals when normalized to the initial level of chimerism at 4 weeks after transplantation (BALB/c mother, n = 10; JHD mother, n = 4). (C) Frequency of chimerism in pups fostered by naive mothers (BALB/c fostered, n = 20) and in non-fostered pups (BALB/c, n = 43) after IUHCTx with B6 FL. (D) Serum from BALB/c mothers whose fetuses received allogeneic IUHCTx (n ≥ 10) was analyzed by flow cytometry to quantify total serum IgM (left panels) and IgG (right panels) alloantibody. Comparison groups include naive (n = 7) and sensitized (Sens., n ≥ 3) mice. (E) Total IgM (left panel) and IgG (right panel) alloantibody production at 1, 2, 4, and 6 weeks after sensitization is shown as the MFI relative to a no-serum sample (relative MFI). *P < 0.05 comparing IUHCTx with naive by ANOVA with Tukey’s multiple comparison test.
In the course of our investigation, it was reported that maternal alloantibodies passing through breast milk limit engraftment after IUHCTx in mice (14). In this model, the authors transplanted 20 × 106 mature BM cells using a vitelline vein injection method and determined that this approach led to maternal alloantibody formation and engraftment in only 30% of the recipients; fostering of animals born after IUHCTx with naive mothers led to 100% engraftment. We compared these results with those obtained using our method of intrahepatic injection of FL cells. When we fostered BALB/c pups born after allogeneic IUHCTx with naive mothers, the frequency of engraftment was 65% (n = 20, Figure 6C), which was slightly increased but not significantly different (c2 test, P = 0.11) from that of non-fostered animals. We also analyzed mothers for the presence of donor-specific antibodies (IgG and IgM) after allogeneic IUHCTx (Figure 6, D and E). We determined a modest and transient increase in donor-specific IgM only at 1 week after IUHCTx, while the overall levels of IgM and IgG were much lower than those seen among sensitized positive controls. Taken together with the results of the experiments using JHD mothers, these data indicate that in our model, maternal B cells are not the critical component in limiting engraftment.
Maternal T cells limit engraftment after in utero transplantation.
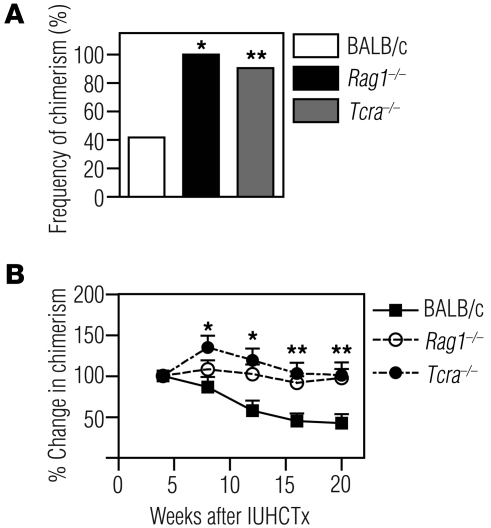
We next determined whether the maternal cellular immune system plays a role in the engraftment of in utero transplanted allogeneic hematopoietic cells. We bred Rag1–/– mothers to wild-type BALB/c fathers, such that the fetuses were immunocompetent, and performed allogeneic IUHCTx with B6 FL. We compared the rate of chimerism in these animals to those born to wild-type BALB/c mothers after IUHCTx with B6 FL. All surviving pups demonstrated engraftment of donor hematopoietic cells (Figure 7A, n = 10, c2 test, P = 0.004), indicating that maternal lymphocytes limit donor cell engraftment after IUHCTx.
Figure 7. Maternal T cells limit engraftment and contribute to ongoing losses in chimerism after allogeneic IUHCTx.
Engraftment after transplantation of B6 FL cells into fetuses born to wild-type BALB/c fathers and either wild-type BALB/c or immunodeficient (Rag1–/– or Tcra–/–) mothers. (A) Frequency of chimerism (BALB/c, n = 43; Rag1–/–, n = 10; Tcra–/–, n = 21). *P < 0.005 comparing Rag1–/– with BALB/c, c2 test; **P < 0.001 comparing Tcra–/– with BALB/c, c2 test. (B) Change in levels of chimerism over time when normalized to the initial level of chimerism at 4 weeks after transplantation (BALB/c, n = 10; Rag1–/–, n = 12; Tcra–/–, n = 13). *P < 0.05 comparing BALB/c and Tcra–/–; **P < 0.05 comparing both BALB/c and Tcra–/–, and BALB/c and Rag1–/–. Comparisons were performed using ANOVA with Tukey’s multiple comparison test.
We next analyzed the effect of selectively removing T cells from mothers by breeding Tcra–/– mothers (28) to wild-type BALB/c fathers and transplanting the fetuses (immunologically replete) with B6 FL cells. In these experiments, 91% of the recipients became chimeric (Figure 7A, n = 21, c2 test, P < 0.001 compared with animals born to wild-type mothers after IUHCTx with B6 FL), indicating that maternal T cells are the key limiting factor in engraftment. Interestingly, when we analyzed the chimerism levels over time in pups born to Rag1–/– or Tcra–/– mothers, none of the engrafted animals demonstrated a loss of chimerism compared with the ongoing losses seen in pups born to wild-type BALB/c mothers, suggesting that maternal T cells also contribute to late-phase graft losses in the recipients (Figure 7B). This result, combined with our observation of increased maternal T cell trafficking after fetal intervention, indicates that maternal T cells play a critical role in the rejection of allogeneic hematopoietic cells after in utero transplantation.
MHC matching of the graft to the mother improves engraftment.
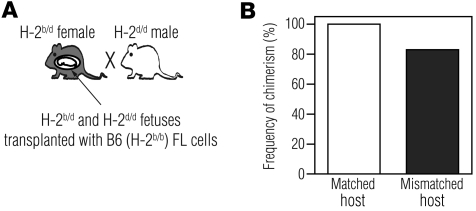
The obvious clinical implication of the above observations is that MHC matching the graft to the mother, instead of the fetus, should result in improved engraftment. To determine this experimentally, we used the F1 backcross model described by Zhang and Miller (29) to generate crosses in which the transplanted B6 cells would be matched to the mother and allogeneic to the fetuses. We crossed B6 × BALB/c F1 (H-2b/d) mothers to BALB/c (H-2d/d) fathers, such that half of the fetuses in each litter were H-2b/d and the other half were H-2d/d. We transplanted all of the fetuses with B6 (H-2b/b) FL, such that the grafts were non-immunogenic to the mother and the H-2b/d fetuses and were allogeneic to the H-2d/d fetuses, creating an internal control for assessing the fetal host immune response in each litter (Figure 8A). The frequency of engraftment in allogeneic H-2d/d fetuses was 83% (Figure 8B, n = 12), comparable to that found in H-2b/d fetuses (100%, n = 11, c2 test, P = 0.564). These results indicate that if the graft is matched to the mother, the fetal host immune response does not limit engraftment.
Figure 8. MHC matching between mother and graft improves engraftment in MHC-mismatched fetuses.
(A) Breeding scheme used to MHC match the mother with donor cells. B6 × BALB/c F1 (H-2b/d) female mice were mated to BALB/c (H-2d/d) males, and fetuses received B6 H-2b/b FL cells. (B) Frequency of chimerism among fetuses that were MHC matched (H-2b/d, n = 11) or mismatched (H-2d/d, n = 12) to the donor graft.
Discussion
Transplantation of stem cells into the preimmune fetal environment can be an innovative strategy to treat congenital stem cell disorders and establish donor-specific tolerance. However, the lack of success of clinical IUHCTx for all diseases except severe combined immunodeficiency has decreased enthusiasm for this field. We investigated the cellular mechanisms of graft loss after allogeneic IUHCTx using a mouse model. We report that the fetal circulation harbors a previously unrecognized level of maternal leukocytes and that there is a particular increase in maternal T cells found in the fetus after fetal hematopoietic cell transplantation. Furthermore, by selectively removing T or B cells from the mother using knockout mice, we show that the maternal immune system, particularly T cells, plays an important role in limiting engraftment following IUHCTx. Finally, we demonstrate that MHC matching of the graft to the mother results in comparable engraftment in allogeneic and syngeneic fetal recipients, supporting a clinical application for these observations.
It is important to note that even in immunocompetent mothers, half of fetal recipients of hematopoietic cell transplants demonstrate engraftment without any conditioning. These results are consistent with numerous previous reports that hematopoietic chimerism observed naturally in twin gestations (30) and following in utero transplantation in multiple animal models (3, 5, 14, 31, 32) and in humans (33) can lead to donor-specific tolerance. Thus, fetal tolerance induction for stem cell transplantation remains an important and approachable clinical goal. Since immune reactions have been observed after postnatal embryonic stem cell transplantation (34), the fetal environment may even offer an important potential avenue to overcome the existing barriers to some clinical applications of developing stem cell technologies. Our finding of near-complete absence of donor reactivity measured by in vivo MLR in chimeric mice suggests that deletional mechanisms may be important, as has been reported previously both in a fetal model (35) and for postnatal transplantation (36). However, our results do not rule out contribution of anergy and Tregs in tolerance induction, as has been reported after fetal BM transplantation (14).
A recent study by Merianos et al. has also demonstrated the importance of the maternal immune response to engraftment after IUHCTx (14). The authors showed that only one-third of pups engraft after allogeneic IUHCTx, but the frequency of engraftment increases to 100% if they are fostered by naive dams, indicating that maternal alloantibodies transmitted through breast milk limit engraftment. Our experiments indicate that maternal T cells are the primary barrier, although it is possible that the improved engraftment in the Tcra–/– mice in our experiments may be secondary to diminished B cell responses as a result of deficient T cell help. However, in our model, the mothers did not develop high levels of alloantibodies following IUHCTx, and pups born to B cell–deficient JHD mothers had similar rates of engraftment when compared with pups born to wild-type mothers, suggesting that rejection is independent of maternal B cells. One potential explanation for our discrepant findings is that Merianos et al. transplanted a high dose of adult BM cells containing mature APCs, while we transplanted a 10-fold-lower dose of FL cells, which do not contain mature APCs. The presence of mature donor APCs can make the graft more immunogenic in several ways. First, mature APCs express higher levels of MHC molecules than stem cells and are therefore more potent stimulators of alloreactive T cells. Second, mature APCs express MHC class II molecules that can directly stimulate alloreactive CD4+ T cells, which can, in turn, help B cells to produce alloantibodies. Third, the breakdown product of MHC proteins, which are highly expressed on mature donor APCs, can be presented by host APCs to stimulate T cells that recognize alloantigens through the indirect pathway of allorecognition. Therefore, it is highly likely that the HSC grafts used by Merianos et al. are more immunogenic, leading to the sensitization of the mother. Additional experiments to directly compare these models may distinguish between these possibilities. Nonetheless, our independent conclusion that the maternal immune response is an important variable should lead to changes in the way clinical transplants are conducted, if these findings are confirmed in large animal models.
We have demonstrated a two-phase immune response to allogeneic grafts in this model. The first phase of graft rejection occurs early, leading to loss of engraftment in half of transplanted mice within 4 weeks. It is important to note that when the mice are sacrificed in the first week after transplantation, donor cells are found in 95% of recipients (similar to what was reported by Peranteau et al; ref. 11), indicating that the loss of chimerism seen in transplanted animals at 4 weeks is secondary to rejection and not to technical errors in transplantation. In the second phase, there are ongoing losses in chimerism even in engrafted animals, with a plateau at later time points. The surprising finding that chimerism levels decline in animals born to wild-type mothers whereas they are steady in those born to Rag1–/– or Tcra–/– mothers suggests that the maternal immune response contributes to this second phase of graft loss as well. There are two potential mechanisms by which maternal cells may exert such an influence: maternal cells may themselves persist in chimeras and cause an ongoing immune response; or they may prime the host immune system (for example, by inducing earlier maturation of APCs) to reject the transplanted cells. A previous study reported detection of maternal T cells in 6-week-old animals by flow cytometry (29). However, we have not been able to consistently detect maternal T cells in offspring after their birth.
In this article, we demonstrate high levels of maternal leukocytes in fetal blood in mid-gestation. Maternal cells have been detected in fetal mice using PCR, immunohistochemistry, and flow cytometry (29, 37–41), although we have not found a previous analysis of maternal leukocytes in the blood of mouse fetuses. Our particular breeding scheme allowed us to focus on CD45+ leukocytes and to fully characterize these maternal cells by flow cytometry. The fact that the composition of cells found in the fetal blood is distinct from that found in maternal circulation argues against contamination of fetal samples with maternal blood and suggests that maternal trafficking across the placenta is active and selective, rather than a result of general “leakiness” of the maternal-fetal interface. The functional significance of these maternal leukocytes during normal gestation is presently unclear. In humans, maternal-fetal cellular trafficking may contribute to fetal immune development and maternal-fetal tolerance, inducing the fetus to develop Tregs against maternal antigens (19). Changes in the levels of maternal-fetal cellular trafficking have been reported to correspond with maternal-fetal histocompatibility in the mouse model, suggesting that cellular trafficking has implications for maternal-fetal tolerance (37, 40).
In addition to baseline trafficking, we have determined that there are key changes in the composition of maternal cells in fetal blood after fetal intervention, with particular increases in the levels of maternal T cells following in utero transplantation. Several mechanisms may result in such a finding: there may be selective recruitment of T cells across the placenta, increased proliferation or decreased turnover of T cells that have already crossed, or decreased homing of maternal T cells to fetal tissues with resultant increases in the circulation. The fact that trafficking was variable among the fetuses in each litter is intriguing and may explain why some fetuses in a litter engraft while littermates do not: the percentage of fetuses with detectable maternal-fetal cellular trafficking is similar to the percentage that ultimately fails to engraft after allogeneic FL transplantation.
The finding that fetal PBS injection alone leads to maternal cell trafficking may have clinical implications in the field of fetal intervention. The amount of fetal trauma from the intrahepatic injection in our model is likely more than would be expected for human IUHCTx, which uses minimally invasive methods. Therefore, it is not known whether human fetal interventions will lead to similar alterations in trafficking of maternal cells into the fetus, although changes in the amount of fetal DNA in the mother have been described (42). If cellular trafficking is related to maternal-fetal tolerance, alterations in trafficking may correlate with the onset of preterm labor, an idea that has been defined in spontaneous preterm labor (43, 44) but not in fetal intervention. Given the growing interest in clinical fetal surgery for various anatomic anomalies, our findings may have important implications for the pathogenesis of preterm labor following fetal intervention.
If the maternal immune response limits engraftment, a potential clinical solution is to transplant cells that are harvested from (or HLA-matched to) the mother, as we have demonstrated in our F1 experiment (Figure 8). Such a strategy is also supported by observations that tolerance to NIMAs may improve transplant outcomes in some settings (45, 46). In our experiment, it is possible that there is some improvement in the engraftment of H-2b FL cells in H-2d/d fetuses because these fetuses are exposed to the NIMA, H-2b. Interestingly, it has been reported that there are strain-dependent variations in tolerance versus sensitization to NIMAs, and in utero exposure to H-2b may instead be sensitizing (47). Thus, it is especially striking that this possible sensitization was overcome with IUHCTx.
In summary, we have demonstrated a critical role for maternal T cells in limiting fetal engraftment after allogeneic IUHCTx. Our finding of increased T cell trafficking with fetal intervention indicates a mechanism by which maternal cells respond to the transplanted cells. The observation that almost all fetuses engraft once maternal T cells are removed or once the graft is matched to the mother validates the promise of using the fetal environment for inducing donor-specific tolerance.
Methods
Reagents and antibodies.
The following reagents were used: ACK Lysing Buffer (Lonza), Invitrogen Vybrant CFDA SE Cell Tracer Kit (CFSE, Invitrogen), Histopaque 1077 Ficoll (Sigma-Aldrich), Ficoll-Paque Plus (GE Healthcare). The following antibodies for flow cytometry were purchased from BD: CD3 (145-2C11), CD8 (clone 53-6.7), CD11c (HL-3), CD19 (1D3), CD45 (30-F11), CD45.1 (A20), CD45R/B220 (RA3-6B2), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), H-2Kk (clone 36-7-5), NK1.1 (PK136); eBioscience: CD4 (RM4-5), CD8 (clone 53-6.7), CD45.1 (A20), CD45.2 (104), F4/80 (BM8), anti-IgG, anti-IgM (II/41); from SouthernBiotech: CD8 (clone 53-6.7); UCSF Hybridoma Core: Gr-1 (RB6-8C5), Fc receptor (2.4G2).
Mice.
The inbred strains, BALB/c, B6, and CD45.1, and F1 hybrid strains B6 × BALB/c and C3H × DBA/2 were obtained from either NCI or The Jackson Laboratory. BALB/c.Tcra–/– (Tcra–/–), and BALB/c.JHD (JHD), mice were obtained from A. Abbas (UCSF). NOD.CD45.1.uGFP (NOD.CD45.1) mice were generated by backcrossing B6.uGFP transgenic mice (strain 004353, The Jackson Laboratory) at least 6 times to NOD (The Jackson Laboratory) mice. BALB/c.Rag1–/– mice were obtained from The Jackson Laboratory. All mice were bred and maintained in a specific pathogen–free facility at UCSF. All mouse experiments were performed according to a UCSF Institutional Animal Care and Use Committee–approved protocol.
In utero FL transplantation.
B6 or BALB/c fetal livers were harvested from E13.5–E14.5 donor fetuses in PBS. Single-cell suspensions were made by gently pipetting the fetal livers and filtering through a 70-μm Nitex filter. FL mononuclear cells (FLMCs) were isolated by density gradient separation using Histopaque 1077 or Ficoll-Paque Plus. The FLMCs were washed twice and adjusted to a concentration of 2.5 × 106 cells/5 μl. To prepare the recipient fetuses, we anesthetized pregnant dams at E13.5–E14.5 using isoflurane. After a midline laparotomy was made, the uterus was exteriorized, and 5 μl of the FL cell suspension was injected into the fetal livers of recipients using pulled glass micropipettes fabricated in our laboratory. The uterus was returned to the abdominal cavity, and the wound was closed in layers. Surviving pups were counted on the day of birth and at the time of weaning.
Determination of chimerism levels.
Blood was collected from the maxillary vein into heparinized tubes and washed, and red blood cells were lysed using ACK Lysing Buffer. The cells were then stained with antibodies to CD45, H-2Kd, and H-2Kb for chimeras of B6 and BALB/c strain combinations (Supplemental Figure 1B). Chimerism in the congenic control group (CD45.1 into B6) and in the B6 and NOD strain combination was determined by staining with antibodies to CD45.1 and CD45.2 (Supplemental Figure 1A). Samples were analyzed on a FACSCalibur cytometer (BD), and the data were analyzed using FACSDiva software (BD). Levels of chimerism in each animal were calculated by dividing the number of donor leukocytes by the total number of CD45+ leukocytes. A chimerism threshold of 1% was used, similar to that reported in ref. 14, although analysis of the data with a chimerism threshold of 0.1% did not alter any of the conclusions. Animals were first analyzed at 4 weeks after transplantation to determine the frequency of engraftment (number of chimeric animals/surviving animals). Levels of chimerism were also determined every 4 weeks and normalized to the value obtained at the initial analysis. The results for each strain combination reported represent at least 3 independent litters.
In vivo MLR.
Lymphocytes were collected from spleens and inguinal, cervical, axillary, brachial, and mesenteric lymph nodes from chimeric (>1% chimerism), injected non-chimeric (<0.1% chimerism), or naive animals and labeled with CFSE. Cells (25 × 106 to 50 × 106) were injected into the retroorbital plexus of B6 × BALB/c F1 or C3H × DBA/2 F1 (third-party controls) hybrid animals. Recipient F1 mice were sacrificed between 60 and 72 hours after injection. Lymphocytes from spleens were stained with antibodies to H-2Kb, H-2Kd, H-2Kk, CD4, and CD8, and the proliferation of donor-derived CD4+ and CD8+ cells was analyzed on an LSRII flow cytometer (BD). To estimate the frequency of alloreactive T cells, the number of precursor cells that proliferated was divided by the total (proliferated and non-proliferated) number of precursor cells. The number of precursor cells that proliferated was calculated as described in ref. 26, by quantifying the numbers of cellular events within a given CFSE peak and dividing the number of events by 2n, where n is the division cycle number. The number of non-proliferated precursors was determined by quantifying the number of events in the peak with the highest CFSE intensity (26).
Detection of maternal cells in fetal mice.
B6 mothers were bred to CD45.1 fathers, and the resulting pups were harvested at indicated time points. Pups were washed twice in PBS prior to decapitation in heparinized HBSS to minimize contamination with maternal blood. At the earlier gestational ages (E12.5–E15.5), blood from 2–3 pups was pooled to obtain enough sample for flow cytometry. After blood collection, pups were dissected under a dissecting microscope, individual organs were collected and dissociated using collagenase and DNase, and single-cell suspensions were prepared. Red blood cells in blood and spleen samples were lysed using ACK Lysing Buffer. The cells were stained for CD45.1, CD45.2, CD3, B220 (or CD19), and Gr-1 to detect maternal cells and analyze their lineage composition. The presence of maternal leukocytes was quantified by calculating the percentage of CD45.2+CD45.1– cells over the total CD45+ pool. A clear population of maternal leukocytes that was greater than 0.5% and more than 50 events was considered significant. In some experiments, a more detailed lineage analysis was performed using two panels of antibodies to detect lymphocytes of the adaptive (CD3, CD4, CD8, CD19) and innate (CD11c, Gr-1, NK1.1, F4/80) immune systems. To determine the effect of IUHCTx on maternal cell trafficking, we sacrificed fetuses 4–5 days after in utero transplantation of NOD.CD45.1 FL and analyzed them for the presence of donor and maternal lymphocytes by flow cytometry (LSRII, BD). Dead cells were gated out using DAPI. The results for uninjected, PBS-injected, and FL-injected groups were compiled from at least 3 independent litters per group.
Detection of donor-specific antibody responses.
To detect circulating alloantibodies that might be present in mothers that underwent IUHCTx, serum was collected weekly for 6 weeks after in utero transplantation and analyzed as reported in Merianos et al (14). Serum from naive BALB/c mice was used as a negative control, and serum from sensitized BALB/c mice was used as a positive control. Sensitized mice were generated by injection of BALB/c animals with 20 × 106 B6 splenocytes intraperitoneally, followed by a repeat injection 7 days later. To quantify the amount of alloantibodies in the serum, lymphocytes harvested from the spleen and lymph nodes of naive B6 mice were incubated with antibodies to Fc receptor for 15 minutes and then incubated with serum samples for 45 minutes. Cells were then washed twice in FACS buffer and incubated with anti-FcR for 5 minutes, followed by the addition of anti-IgM, anti-IgG, and CD19 antibodies. The stained cells were analyzed using flow cytometry to determine the MFI of IgM and IgG staining on CD19-negative cells. The relative MFI was normalized to that of B6 lymphocytes that were not exposed to serum.
Statistics.
Frequencies of engraftment were compared using c2 test. Comparisons involving 2 groups were evaluated using Student’s t test, and those involving multiple groups were evaluated using ANOVA with Tukey’s multiple comparison test. A P value of less than 0.05 was considered to be significant. Data represent mean ± SEM.
Supplementary Material
Acknowledgments
We acknowledge Abul Abbas, Mike McCune, Susan Fisher, Jeff Bluestone, and Sang-Mo Kang for numerous helpful discussions. We would like to thank the members of the Tang and Abbas laboratories for their advice and expertise and Greg Emmanuel and Catherine Tsai for technical assistance. Funding support includes the Irene Perstein Award (to T.C. MacKenzie), UCSF Sandler Funds (to T.C. MacKenzie and Q. Tang), an American Pediatric Surgical Association (APSA) Foundation Scholarship (to T.C. MacKenzie), NIH/NIAID grant K08 AI085042 (to T.C. MacKenzie), a California Institute for Regenerative Medicine Postdoctoral Training Grant (to A. Nijagal), a National Science Foundation Training Grant (to M. Wegorzewska), and the Joslin Diabetes and Endocrinology Research Center (DERC) flow cytometry core.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(2):582–592. doi:10.1172/JCI44907.
References
- 1. Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2. Merianos D, Heaton T, Flake AW. In utero hematopoietic stem cell transplantation: progress toward clinical application. Biol Blood Marrow Transplant. 2008;14(7):729–740. doi: 10.1016/j.bbmt.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 3. Kim HB, Shaaban AF, Yang EY, Liechty KW, Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77(1):1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 4. Hayashi S, Peranteau WH, Shaaban AF, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of in utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100(3):804–812. doi: 10.1182/blood-2002-01-0016. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi S, et al. Mixed chimerism following in utero hematopoietic stem cell transplantation in murine models of hemoglobinopathy. Exp Hematol. 2003;31(2):176–184. doi: 10.1016/S0301-472X(02)01024-X. [DOI] [PubMed] [Google Scholar]
- 6. Zanjani ED, Flake AW, Rice H, Hedrick M, Tavassoli M. Long-term repopulating ability of xenogeneic transplanted human fetal liver hematopoietic stem cells in sheep. J Clin Invest. 1994;93(3):1051–1055. doi: 10.1172/JCI117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liechty KW, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 8. Flake AW, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335(24):1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 9. Wengler GS, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDXI). Lancet. 1996;348(9040):1484–1487. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]
- 10. Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation: ontogenic opportunities and biologic barriers. Blood. 1999;94(7):2179–2191. [PubMed] [Google Scholar]
- 11. Peranteau WH, Endo M, Adibe OO, Flake AW. Evidence for an immune barrier after in utero hematopoietic-cell transplantation. Blood. 2007;109(3):1331–1333. doi: 10.1182/blood-2006-04-018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molitor ML, Burlingham WJ. Immunobiology of exposure to non-inherited maternal antigens. Front Biosci. 2007;12:3302–3311. doi: 10.2741/2313. [DOI] [PubMed] [Google Scholar]
- 13. van Rood JJ, Roelen DL, Claas FH. The effect of noninherited maternal antigens in allogeneic transplantation. Semin Hematol. 2005;42(2):104–111. doi: 10.1053/j.seminhematol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14. Merianos DJ, et al. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119(9):2590–2600. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maloney S, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104(1):41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291(9):1127–1131. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 18. Khosrotehrani K, Bianchi DW. Fetal cell microchimerism: helpful or harmful to the parous woman? Curr Opin Obstet Gynecol. 2003;15(2):195–199. doi: 10.1097/00001703-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 19. Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor PA, McElmurry RT, Lees CJ, Harrison DE, Blazar BR. Allogenic fetal liver cells have a distinct competitive engraftment advantage over adult bone marrow cells when infused into fetal as compared with adult severe combined immunodeficient recipients. Blood. 2002;99(5):1870–1872. doi: 10.1182/blood.V99.5.1870. [DOI] [PubMed] [Google Scholar]
- 21. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68(5):869–877. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 22. Durkin ET, Jones KA, Elnaggar D, Shaaban AF. Donor major histocompatibility complex class I expression determines the outcome of prenatal transplantation. J Pediatr Surg. 2008;43(6):1142–1147. doi: 10.1016/j.jpedsurg.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durkin ET, Jones KA, Rajesh D, Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112(13):5245–5253. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee LA, Sachs DH, Sykes M. Effect of natural killer cell depletion on long-term multilineage allogeneic bone marrow engraftment. Transplant Proc. 1993;25(1 pt 2):1246–1247. [PubMed] [Google Scholar]
- 25. Blazar BR, et al. Engraftment of severe combined immune deficient mice receiving allogeneic bone marrow via In utero or postnatal transfer. Blood. 1998;92(10):3949–3959. [PubMed] [Google Scholar]
- 26. Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 27. Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5(6):647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 28. Mombaerts P, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Miller RG. The correlation of prolonged survival of maternal skin grafts with the presence of naturally transferred maternal T cells. Transplantation. 1993;56(4):918–921. doi: 10.1097/00007890-199310000-00027. [DOI] [PubMed] [Google Scholar]
- 30. Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 31. Almeida-Porada G, Porada C, Gupta N, Torabi A, Thain D, Zanjani ED. The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts’ potential. Exp Hematol. 2007;35(10):1594–1600. doi: 10.1016/j.exphem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee PW, et al. In utero bone marrow transplantation induces kidney allograft tolerance across a full major histocompatibility complex barrier in Swine. Transplantation. 2005;79(9):1084–1090. doi: 10.1097/01.TP.0000161247.61727.67. [DOI] [PubMed] [Google Scholar]
- 33. Touraine JL, Raudrant D, Laplace S, Roncarolo MG. Immunological tolerance following stem cell transplantation in human fetuses in utero. Transplant Proc. 1997;29(5):2477. doi: 10.1016/S0041-1345(97)00455-7. [DOI] [PubMed] [Google Scholar]
- 34. Wu DC, Boyd AS, Wood KJ. Embryonic stem cells and their differentiated derivatives have a fragile immune privilege but still represent novel targets of immune attack. Stem Cells. 2008;26(8):1939–1950. doi: 10.1634/stemcells.2008-0078. [DOI] [PubMed] [Google Scholar]
- 35. Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor–specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surg. 1999;34(5):726–729. doi: 10.1016/S0022-3468(99)90364-0. [DOI] [PubMed] [Google Scholar]
- 36. Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62(3):380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan J, Land S. Influence of maternal-fetal histocompatibility and MHC zygosity on maternal microchimerism. J Immunol. 2005;174(11):7123–7128. doi: 10.4049/jimmunol.174.11.7123. [DOI] [PubMed] [Google Scholar]
- 38. Marleau AM, Greenwood JD, Wei Q, Singh B, Croy BA. Chimerism of murine fetal bone marrow by maternal cells occurs in late gestation and persists into adulthood. Lab Invest. 2003;83(5):673–681. doi: 10.1097/01.lab.0000067500.85003.32. [DOI] [PubMed] [Google Scholar]
- 39. Piotrowski P, Croy BA. Maternal cells are widely distributed in murine fetuses in utero. Biol Reprod. 1996;54(5):1103–1110. doi: 10.1095/biolreprod54.5.1103. [DOI] [PubMed] [Google Scholar]
- 40. Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta. 2007;28(7):639–649. doi: 10.1016/j.placenta.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41. Zhou L, et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101(4):570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wataganara T, et al. Persistent elevation of cell-free fetal DNA levels in maternal plasma after selective laser coagulation of chorionic plate anastomoses in severe midgestational twin-twin transfusion syndrome. Am J Obstet Gynecol. 2005;192(2):604–609. doi: 10.1016/j.ajog.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 43. Farina A, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005;193(2):421–425. doi: 10.1016/j.ajog.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 44. Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352(9144):1904–1905. doi: 10.1016/S0140-6736(05)60395-9. [DOI] [PubMed] [Google Scholar]
- 45. Burlingham WJ, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. . N Engl J Med. 1998;339(23):1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 46. van Rood JJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99(5):1572–1577. doi: 10.1182/blood.V99.5.1572. [DOI] [PubMed] [Google Scholar]
- 47. Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ. Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA). Am J Transplant. 2008;8(11):2307–2315. doi: 10.1111/j.1600-6143.2008.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.