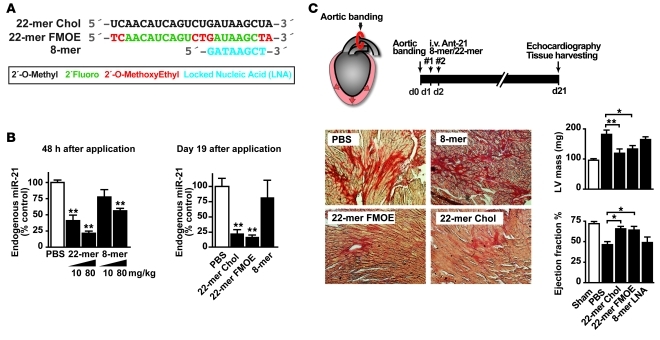
We would like to comment on a recent study that analyzed the role of microRNA-21 (miR-21) in a mouse model of cardiac disease (1). Using miR-21–deficient mice and novel, very short, 8-nucleotide anti–miR-21 oligonucleotides, the authors failed to detect any modulation of pressure overload-induced myocardial hypertrophy and fibrosis and concluded that miR-21 plays no role in cardiac disease. In contrast, we and others reported that inhibition of miR-21 with highly specific, 22- and 15-nucleotide-long anti–miR-21 oligonucleotides effectively inhibits myocardial and pulmonary fibrosis (2, 3). While genetic deletion of a target may lead to compensation during development and is often different from pharmacological inhibition of this target in the adult organism, the discrepancy between the therapeutic trials using long versus short 8-mer oligonucleotides is striking. We therefore carried out a direct head-to-head comparison of three different oligonucleotide chemistries (Figure 1A) in the same model of pressure overload-induced cardiac hypertrophy (transaortic constriction [TAC]). The two 22-mer oligonucleotides were complementary to the full-length miR-21, while the 8-mer was complementary to nucleotides 2 to 9 of miR-21, locked nucleic acid modified (LNA modified), and identical to the oligonucleotide used in the report by Patrick et al. (Figure 1A).
Figure 1. Comparison of different miR-21 oligonucleotide inhibitors.
(A) Different chemistries of miR-21 inhibitors (modifications of oligonucleotides are indicated with corresponding colors in the key). (B) miR-21 expression 48 hours and 19 days after treatment (21 days after TAC) with different miR-21 inhibitors (10–80 mg/kg). (C) Efficacy of different miR-21 inhibitors in a mouse model of left ventricular pressure overload (TAC) on left ventricular fibrosis formation, left ventricular mass, and ejection fraction. The different miR-21 oligonucleotide inhibitors have been applied twice on two consecutive days (#1 and #2). Original magnification, ×200. Ant-21, anti–miR-21; Chol, cholesterol. Data are mean ± SEM. *P < 0.05, **P < 0.01.
Therapeutic efficacy of different anti–miR-21s in a mouse model of cardiac disease.
Consistent with the results by Patrick et al., 8-mer anti–miR-21 led to repression of cardiac miR-21 on day 2 after the last dose (Figure 1B). However, treatment with 22-mer anti–miR-21s resulted in a more efficacious repression of miR-21 (by 80%). In addition, the repression was maintained only in samples treated with 22-mers but not 8-mer throughout the course of the experiment (on day 19 after the last dose, Figure 1B). It is well established that the number of phosphothioate bonds is inversely correlated to the excretion rate of oligonucleotides (4), which could partially explain the lack of efficacy of 8-mer anti–miR-21 in the cardiac fibrosis model. In addition to comparing the ability of three different anti–miR-21 oligonucleotides to repress miR-21, we also tested their ability to modify disease phenotypes of TAC. As shown in Figure 1C, interstitial fibrosis and cardiac mass were significantly increased three weeks after TAC in control mice but were strongly attenuated by treatment with both cholesterol- and F/MOE-modified long 22-mer oligonucleotides. In addition, 22-mers prevented the decline in cardiac function, as determined by echocardiography. In contrast and consistent with the findings reported by Patrick et al., application of short 8-mer oligonucleotides against miR-21 did not affect pressure overload-induced cardiac hypertrophy, fibrosis, and cardiac dysfunction. Currently, we do not know why the reported phenotype of the miR-21–deficient mice differs from that of mice that received treatment with long miR-21 inhibitors. Possible reasons are various means of genetic compensation upon constitutive deletion of the Mir21 gene as well as potential off-target effects of anti-miRs that evade current analysis.
Taken together, we confirmed that the 8-mer anti–miR-21 is ineffective in preventing cardiac disease in a mouse model of left ventricular pressure overload, a finding which is likely due to the modest and transient nature of miR-21 suppression by 8-mers. For long-term inhibition of miR-21 function in vivo, interventions based on longer anti-miRs are likely to prove superior, due to their high potency and treatment duration. In contrast, short 8-mer LNA-modified oligonucleotides against miR-21 are of less potency and without therapeutic effects in vivo.
Footnotes
Conflict of interest: Thomas Thum, Johann Bauersachs, and Stefan Engelhardt have filed a patent application for the use of miR-21 and have received royalty fees through the University of Wuerzburg. Nelson Chau, Balkrishen Bhat, and Peter S. Linsley are employees of Regulus.
Citation for this Letter: J Clin Invest. 2011;121(2):461–462. doi:10.1172/JCI45938.
References
- 1. Patrick DM, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008; 456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 3. Liu G, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levin AA, et al. Basic principles of the pharmacokinetics of antisense oligonucleotide drugs. In: Crooke ST, ed.Antisense Drug Technology: Principles, Strategies, and Applications . 2nd ed. Boca Raton, Florida, USA: CRC Press; 2007:183–215 . [Google Scholar]