Abstract
Lack of awareness (anosognosia) for one's own language impairments has rarely been investigated, despite hampering language rehabilitation. Assessment of anosognosia by means of self-report is particularly complex, as a patient's language difficulties may seriously prevent or bias the assessment. Other methods, such as measures of self-correction and error detection, have provided valuable information, although they are an indirect form of assessment of anosognosia and are not exempt from methodological criticisms. In this study we report on a new tool, the VATA-L (Visual-Analogue Test for Anosognosia for Language impairment), geared at assessing explicit anosognosia for aphasia. The VATA-L compares the patient's self-evaluation with caregivers’ evaluations of the patient's verbal communication abilities in a series of common situations. By means of non-verbal support and a system of check questions, this test minimizes some of the methodological limitations of existing diagnostic tools (e.g., structured interviews), enhancing reliability, and enabling assessment of patients with aphasia. Finally, normative data provided in the study allow a clearer interpretation of the patient's performance and facilitate assessment of anosognosia.
Keywords: Anosognosia, Unawareness, Aphasia, Brain damage, Assessment
INTRODUCTION
Patients with language impairments may be unaware of their difficulties in verbal communication (Adair, Schwartz, & Barrett, 2003; Kertesz, 2010; Rubens & Garrett, 1991; Vuilleumier, 2000). They suffer from anosognosia for their aphasia. As a result, they may deny language difficulties, fail to correct their speech errors, or adopt compensatory strategies, so that their communication is largely incomprehensible (e.g., Alajouanine, 1956; Kinsbourne & Warrington, 1963; Levelt, 1983; Maher, Rothi, & Heilman, 1994; Marshall, Rappaport, & Garcia-Bunuel, 1985; Weinstein, Lyerly, Cole, & Ozer, 1966; Wernicke, 1874). A series of studies identified anosognosia for cognitive and motor impairment as the best predictor of poor functional recovery (Appelros, Karlsson, & Hennerdal, 2007; Fillingham, Sage, & Lambon Ralph, 2006; Gialanella & Mattioli, 1992; Rüsch & Corrigan, 2002) and the most likely cause of caregivers’ distress (Prigatano, Borgaro, Baker, & Wethe, 2005). Therefore it is important to be able to quantify reliably and interpret correctly evidence of lack of awareness in order to allow comparison among patients and follow-ups.
The assessment of anosognosia for language deficits is a particularly complex process, as it is marred by the difficulty in interpreting aphasic patients’ responses to questions related to their deficits (Lebrun, 1987). Therefore information about a patient's explicit self-evaluation is rarely considered (e.g., Breier et al., 1995) or it is reported anecdotally (e.g., Kinsbourne & Warrington, 1963; see also Cocchini & Della Sala, 2010, for a critical appraisal of assessment). For this reason, to diagnose anosognosia for aphasia, researchers have relied on forms of assessment such as self-correction and error detection methods. The basic assumption of the self-correction method is that if aphasic patients are not aware of their language errors they will not attempt to correct them (e.g., Rubens & Garrett, 1991; see Adair et al., 2003, for a review). However, aphasic patients may be fully aware of their impairments and yet fail to correct themselves for other reasons. For example, patients may decide, more or less intentionally, to withdraw from any further effort when convinced that they will never find the correct word (Maher et al., 1994). Using the error detection method, patients are typically asked to detect their errors during the description of a picture or when performing a naming task (e.g., Marshall, Robson, Pring, & Chiat, 1998). However, as for self-corrections, this method consists of an ad hoc test tailored around the patient's actual performance on verbal tasks. Different patients may commit errors to different stimuli in different tests, making difficult to standardize the approach.
Therefore the diagnosis of anosognosia for language disorders is still unclear and often tailored to a particular patient, limiting systematic assessment and group studies.
An alternative method of assessing of anosognosia is by questionnaires, by means of which patients are asked to rate their own difficulties in performing specific tasks, and then their evaluation is compared with those of a caregiver or against actual performance (e.g., Marcel, Tegnér, & Nimmo-Smith, 2004). Questionnaires rely heavily on verbal communication, hence it can be hard both to interpret aphasic patients’ responses and to be certain about their comprehension of the questions. As suggested by McGlynn and Schacter (1989), this method is open to subjective interpretation and “development of methods for investigating unawareness in different aphasic groups is clearly necessary” (p. 180). To respond to this need some authors have developed clinical scales to assess lack of awareness in patients with language difficulties (e.g., Kertesz, Nadkarni, Davidson, & Thomas, 2000). However, these are mainly based on indirect measures, in which caregivers evaluate the patient's general awareness and do not provide a patient's score to be interpreted against norms.
The aim of this study is to develop a new method of assessing explicit anosognosia for language disorders with a tool, which (i) facilitates reliable participation by aphasic patients by means of non-verbal communication (i.e., drawings and visual-analogue scale; cf. Stern et al., 1997) and simplified verbal text (e.g., reading aloud core actions; cf. Worral et al., 2005); (ii) permits comparison of a patient's evaluation with norms to allow the identification of anosognosia; (iii) permits the selective assessment of anosognosia for comprehension and expressive language disorders; (iv) is reliable even in cases of poor verbal comprehension; and (v) is easy to administer.
METHOD AND MATERIALS
Participants
A total of 65 (23 female and 42 male) patients were recruited in Italian and British rehabilitation wards on a voluntary basis according to the following inclusion criteria: (i) CT or MRI demonstrated left hemisphere brain damage; (ii) age between 18 and 85; (iii) evidence of language disorder resulting from brain damage. Patients were referred to us by their clinicians, and we cannot exclude the possibility that some relevant potential participants were not contacted by clinicians or did not accept to take part in the study. For this reason this cannot be considered an epidemiological study.
Patients’ average age was 57.0 years (SD = 15.2; range 19-84), they had on average 9.9 years of formal education (SD = 4.2; range 4-19), and average onset from brain damage was 300.1 days (SD = 589.7; range 6-3963). Two patients had a bilateral lesion. Site of brain lesions encompassed (but was not limited to) frontal or parietal lobes in 45 cases, whereas 11 cases showed lesions limited to subcortical structures. Nine patients showed brain lesions in posterior sites (i.e., temporal and occipital lobes) of the cortex and subcortical structures. Nature of lesion was vascular (23 hemorrhagic, 33 ischemic) in 56 cases and traumatic in 9 cases. All patients showed some degree of language impairment on routine clinical assessment. Most of 65 patients who entered the study attended weekly rehabilitation sessions for their language deficits.
A total of 72 healthy volunteers, recruited from among the patients’ professional caregivers (e.g., therapists, medical doctors, nurses, psychologists) and non-professional caregivers (friends or relatives), also entered the study to provide data for the Visual-Analogue Test for Anosognosia for Language impairment (VATA-L). The caregivers were people who met with the patients on a regular basis (daily or weekly). For each patient, at least one caregiver was recruited. On average, professional caregivers (1 male and 10 females) were 25.5 years old (SD = 2.7; range 20-30) with 16.6 years (SD = 1.0; range 14-18) of formal education. On average non-professional caregivers (24 males and 37 females) were 51.2 years old (SD = 13.1; range 18-77), with 11.2 years of formal education (SD = 4.2; range 5-21).
All patients and caregivers gave informed consent prior to participating in the study. For those patients showing more severe aphasia, consent from family members was also obtained.
Language assessment
Language skills were examined using the Italian or the English version of the Aachen Aphasia Test (AAT) (Huber, Poeck, & Willmes, 1984; Luzzatti et al., 1987; Miller, Willmes, & de Bleser, 2000). The AAT is a highly reliable (Miller et al., 2000) battery of subtests that assess a range of language functions in different domains (i.e., spontaneous speech, repetition, naming, auditory comprehension, reading, and written language) at different levels of description. A spontaneous speech sample is elicited and is rated in terms of communicative behavior, articulation and prosody, formulaic language, semantics, phonology, and syntax. Repetition of speech sounds, words of increasing length, and sentences is assessed. Participants name object pictures, colors, and depicted events (to elicit sentences). Comprehension of spoken language is assessed via a version of the Token Test (Orgass, 1986), single-word, and sentence-to-picture matching tasks. Comprehension of written language is assessed by single-word and sentence-to-picture matching, and there is a test of reading aloud. Writing is assessed via writing to dictation of words and sentences, and assembling heard words and sentences from printed letters or words respectively. In order to obtain an indication of the patient's overall language ability, the patient's raw score on each of the AAT subtests was converted into a percentage (a higher percentage indicated better performance). The patient's overall AAT score was then calculated as the average percentage across the above tests. Recent studies (e.g., Basso, 2005) conducted on populations of aphasic individuals show that the major division of language impairment lies between subtests that require some form of spoken expression (such as the AAT naming subtest) and those requiring comprehension (such as the three AAT comprehension subtests). Hence, for each patient, performance on the naming and comprehension subtests of the AAT was compared with related norms (Luzzatti et al., 1987; Miller et al., 2000).
Vata-L
A set of illustrations depicting tasks involving language was developed. In order to determine whether the pictures were indeed providing non-verbal information about the tasks, they were used in a pilot study with healthy volunteers. The volunteers were asked to identify one drawing out of four possible drawings that best illustrated a specific language task. If the expected response was not selected by at least four out of five volunteers, that particular picture was either dropped or further modified and re-piloted. Drawings that finally obtained the expected response were considered for the final version of the VATA-L.
The VATA-L comprises one practice item, used as a run-in to make sure that the participant understands the task, followed by 18 target questions. Of these, 14 enquire about the participant's ability to perform common tasks requiring language production (8 questions), language comprehension (4 questions), or both production and comprehension (2 questions). For example: “Do you have a problem telling someone when you don't understand?” (see question 13 in the Appendix). Each question is illustrated by a simple drawing to facilitate comprehension for people with aphasia. Evidence for the benefits to people with aphasia of providing pictures, as well as formatting and simplifying text, has been recently provided by Worral et al. (2005) (see also Brumfitt & Sheeran, 1999; Della Sala, Cocchini, Beschin, & Cameron, 2009; Stern et al., 1997). Patients rated their current ability to carry out each task using a 4-point visual-analogue scale (0 = “no problem in carrying out the task”, 3 = “major problem in carrying out the task”). Scores (0-3) were displayed along a continuum, with written labels “no problem” and “problem” at either extremity, along with a smiling or non-smiling face to aid comprehension (see Visual-analogue scale in the Appendix). The patients rated their current ability in each task by verbally stating a number on the scale, by uttering the corresponding word, or by pointing to a position along the scale (Brumfitt & Sheeran, 1999; Della Sala et al., 2009; Stern et al., 1997). The caregivers rated the ability of the patient they cared for on the same questions. Participants’ responses that were made halfway between two numbers (i.e., 0.5, 1.5, 2.5) were accepted. In addition, four “check questions” were included which elicited answers only appropriate when made at either end of the response scale. For two check questions (e.g., “Would you have a problem hearing a fire engine with its sirens on?” see Question 12 in the Appendix) the expected response was “no problem” (0 or 1 rating score); for the remaining two check questions (e.g., “Would you have a problem speaking Russian?” see Question 18 in the Appendix) the expected response was “problem” (2 or 3 rating score). Moreover, two of the four check questions, one for each type of the expected responses, referred to non-language tasks (e.g., “Do you have any difficulty jumping over a lorry?” see Question 3 in the Appendix). The ratings from all the check questions are not included in the calculation of the VATA-L total score; however patients who failed to provide the expected response to any of the check questions were excluded from the final analyses.
The questions were presented in a fixed pseudo-random order (we made sure that questions related to each language domain and check questions were well distributed throughout the questionnaire). Each picture was displayed on an A4 sheet of paper, in portrait orientation and, in order to minimize the effect of possible unilateral visuo-spatial disorders, it was placed on the tabletop to the ipsilesional side of the patient's midline. The examiners read aloud the core action (e.g., “Reading the newspaper?”), repeating the entire question if necessary. In addition they pointed to draw attention to the illustration and gestured key actions (such as reading) where appropriate. The examiner emphasized that the questions were about the patient's current abilities. The caregiver and patient were tested independently. Caregiver and patient versions of the VATA-L were identical, except that the questions in the caregiver's version were altered to refer to a third person (the patient). The final version of the VATA-L is reported in the Appendix, and it is also available for downloading on the following web page: http://homepages.gold.ac.uk/gcocchini
Patients and caregivers were asked to fill out the VATA-L again after 24-72 hours to assess the reliability of the test. This specific time window was chosen to reduce bias due to possible recovery, or the patients remembering their responses.
VATA-L total score. The total score of the VATA-L was calculated by adding the scores from the 14 questions. For each question a score of 0 (no problem) to 3 (problem) was given, hence the total VATA-L score ranged from 0 to 42. Wherever possible, two caregivers (a professional and a non-professional) rated the same patient's ability. In these cases the mean caregiver rating was calculated. The patient's total score was then subtracted from the caregiver's total score to provide a caregiver-patient discrepancy value, which ranged from 42 to + 42. A discrepancy value of 0 indicates overall agreement between the caregivers and the patient. A positive discrepancy value indicates that, compared with the caregivers’ evaluation, the patient has overestimated his/her own language abilities (i.e., possible lack of awareness); while a negative discrepancy value would indicate that the patient has underestimated his/her own language abilities.
VATA-L language subscales. Two subscales were composed to assess awareness for language comprehension and language production separately. The VATA-L subscale for oral and written comprehension (VATA-L Comprehension) was based on four questions (see Appendix, questions 2, 5, 9, and 16). The score ranged from 0 to 12 and the discrepancy value ranged from 12 to + 12. The VATA-L subscale for speaking and writing (VATA-L Expressive) was based on eight questions (see Appendix, questions 1, 4, 6, 7, 10, 11, 14, and 17). The score ranged from 0 to 24 and the discrepancy value ranged from 24 to + 24. Two further questions referred to language abilities requiring both verbal comprehension and expression (see Appendix, questions 13 and 15) and were not included in either of the two subscales.
The two subscales provide positive and negative caregiver-patient discrepancy values indicating, as described above, whether the patient overestimated or underestimated the assessed ability. A discrepancy value equal to zero indicated a perfect agreement with caregivers.
RESULTS
Language impairment
One patient refused to complete all sections of the AAT. The average total AAT score was 51.5 (SD = 28.2), which generally corresponds to a moderate impairment. Based on separate comprehension and production scores, 56 patients showed a pathological performance in the AAT naming subtest, and 54 in the AAT comprehension subtests. Of these patients, 14 showed selective impairment in the naming (n = 8) or comprehension (n = 6) subtests.
VATA-L scale
Check questions. Nine patients (14% of the initial group of 65) responded incorrectly to at least one check question, so their data were not included in the final analyses. These exclusions should not necessarily be considered the result of impaired comprehension, as other factors may be involved such as poor attention span, perseveration, or lack of compliance. Three further patients were excluded as their caregivers (all non-professional) misinterpreted one of the four check questions. That these healthy volunteers did so also suggests that factors other than poor comprehension may affect reliability of data. Following these exclusions, the data of 53 patients were entered in the final analyses. The average total AAT score of these patients was 54.9% (SD = 28.5; range 6.3-92).
Unawareness cut-off scores. For 40 of these 53 patients a rating was obtained from two caregivers: one with a personal relationship and one with a professional relationship. The rating difference between personal (mean = 21.1; SD = 9.16) and professional caregiver (mean = 22.7; SO = 9.71) was far from significant (p = .423). The total AAT score obtained by these 53 patients (please note that the higher the score the better the performance) was also compared with their caregivers’ ratings (when two caregivers rated the same patient, the caregivers’ average was used). A Pearson correlation showed a significant negative correlation (r = .310, p = .05). These data are in line with previous studies showing that caregivers can provide reasonable evaluations of patients’ impairments (e.g., Smith, Della Sala, & Logie, 2000). This will be mediated via the subjective impact of the language deficit rather than relating directly to an objective score.
In order to interpret the patients’ evaluations of their own language skills and the possible discrepancies from their caregiver/s, we first estimated the absolute discrepancy between the each pair of caregivers rating the same patient (“caregiver/caregiver discrepancy”). This score ranged from 0 (i.e., perfect agreement) to 42 (i.e., complete disagreement). The mean difference between the scores given by the two caregivers was 4.88 (SD = 3.95). Next, in order to define whether the discrepancy values between patients and caregivers are meaningful, the inner and outer tolerance limits were calculated, as follows. Frequency of caregiver/caregiver discrepancy was calculated and ordered. The value below which 95% of the caregiver pairs scored represented the inner limit (this is therefore the highest discrepancy value still considered non-pathological). The discrepancy value above which only 5% of the population scored represented the outer limit (this is therefore the value above which scores are considered pathological). Only patients whose discrepancy value was above the outer limit were considered as unaware of their language deficits. Those with a discrepancy value falling between the two limits were considered as borderline (i.e., an unequivocal classification of aware or unaware was not possible). Following these criteria we reduced the risk of false alarms and false positive cases (see Brazzelli, Capitani, Della Sala, Spinnler, & Zuffi, 1994).
VATA-L total score. According to these analyses, the inner tolerance limit considering the total VATA-L score was 12 and the outer tolerance limit was 13. Therefore a patient/caregiver discrepancy value equal to or lower than 11.9 should be considered as an indicator of preserved awareness, whereas a patient/caregiver discrepancy value equal to or higher than 13.1 should be considered as an indicator of unawareness. A patient/caregiver discrepancy value between 12.0 and 13.0 should be considered as “borderline”.
VATA-L subscales. Similar analyses were run to identify the inner and outer limits for the two VATA-L subscales. For the VATA-L Comprehension, the inner and outer tolerance limits for discrepancy values were 5.0 and 6.0 respectively. Therefore a discrepancy value equal to or less that 4.9 should be considered as an indicator of awareness, whereas a discrepancy value equal to or higher than 6.1 should be considered as an indicator of unawareness. A discrepancy value between 5.0 and 6.0 should be considered as “borderline”. For the VATA-L Expression subscale, the inner and outer tolerance limits for discrepancy values were 7.0 and 9.0 respectively. Therefore a discrepancy value equal to or less that 6.9 should be considered an indicator of awareness, whereas a discrepancy value equal to or higher than 9.1 should be considered an indicator of unawareness. A discrepancy value between 7.0 and 9.0 should be considered as “borderline”.
Test-retest reliability
Test and retest data (collected 24-72 hours later) of 89 caregivers’ ratings1 (i.e., 36 pairs of caregivers rating the same patient, plus 12 professional only and 5 personal only caregivers) were entered into two separate Spearman's correlation analyses, one for professional and one for personal caregivers. Both groups showed a highly significant positive correlation, with r = .97 and r = .92, respectively (both with p<.001).
We collected retest data for 41 out of 53 patients. The correlation between test and retest of these patients was also highly significant, with r = .84 (p<.001).
Language impairment and awareness
According to the scoring as detailed above, 10 out of 53 patients (i.e., 18.9%) showed lack of awareness for their language difficulties. Three further patients (5.7%) were classified as borderline.
We also ran an analysis to investigate whether severity of language impairment (as measured by the AAT) correlated with subjective measures (patients’ VATA-L ratings) and level of awareness (i.e., VATA-L discrepancy scores). Two Pearson's correlation analyses showed negative (r = .465 and r = .310, respectively) significant correlation (p < .001 and p < .05, respectively), suggesting that the better the patient's language competence the more accurate is the subjective evaluation and awareness of language deficits. However, it should be noticed that AAT scores could only predict less than a quarter of the subjective measure and awareness, probably because of the scores achieved by anosognosic patients.
Next, the AAT scores of the 40 patients showing awareness of their language deficits, by our measure, were compared with those of the 10 patients showing clear evidence of anosognosia for aphasia. On average, anosognosic patients performed worse (mean = 34.08; SD = 30.18) than aware patients (mean = 57.89; SD = 26.47) and a t-test analysis showed that this difference was reliable (t-crit = 2.48; p < .05). Figure 1 details the performance of the two subgroups of patients on each AAT subtest. Unaware patients performed worse than aware patients in all subtests, but this difference was more evident in the subtests assessing verbal production (i.e., spontaneous speech, repetition, naming, and written language). Data of overall AAT comprehension subtests and overall AAT production subtests were entered in a 2 (Unaware vs Aware) × 2 (Production vs Comprehension) ANOVA. The difference between production and comprehension was significant, F(1, 48) = 17.1; p < .001; in our sample patients scored lower on production than comprehension language subtests. We also found a significant interaction between factors, F(l, 48) = 6.76; p<.01. Post-hoc analysis (t-tests) with Bonferroni correction showed a significant difference between overall comprehension and overall production of anosognosic patients (p = .007), indicating that unaware patients performed worse on the production than on the comprehension sections of the AAT.
Figure 1.
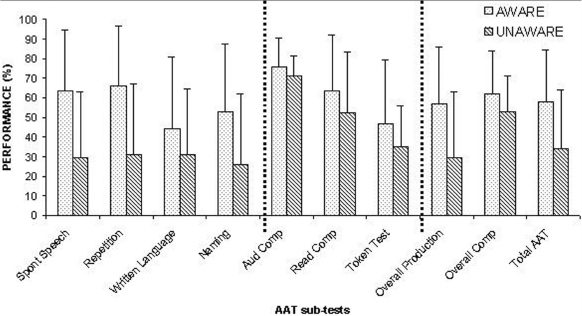
Aachen Aphasia Test (AAT) performance (means and standard deviations) of patients aware and unaware of their language disorders. Spont speech = Spontaneous speech; Aud comp = Auditory comprehension; Read comp = Reading comprehension; Overall comp = Overall comprehension.
DISCUSSION
The VATA-L
Anosognosia for aphasia has rarely been investigated by direct questioning of patients, as their language impairments may affect comprehension of the questions and can make it difficult to interpret responses (McGlynn & Schacter, 1989). Moreover, when this has been done, the assessment has been limited by relying on a single general question (e.g., Breier et al., 1995) or by resulting in the exclusion of patients with severe language disorders (e.g., Schlenck, Huber, & Willmes, 1987; see also Cocchini, Beschin, Cameron, Fotopoulou, & Della Sala, 2009). The main aim of this study was to devise a test to facilitate this type of assessment, providing a reliable tool that would allow investigation of different degrees of awareness for different aspects of language skills and clear interpretation of patients’ performance by means of normative data.
The VATA-L appears to satisfy these requirements. It consists of a series of 14 questions related to common situations involving different aspects of verbal communication. Despite the relative complexity of the questionnaire, the non-verbal support (the illustrations and the visuo-analogue scale) allowed us to reduce the exclusion rate of aphasics (to 14%), even when severe cases of language impairment were considered. Indeed the average AAT score of the 53 patients who reliably completed the VATA-L was 54.9% with the worst performance as low as 6.3%. In the meantime the system of check questions allowed us to obtain information about the reliability of the patients’ responses, and to exclude a posteriori unreliable data. About 85% of our sample of aphasic individuals were able to express reliably their conscious evaluation of their language deficits.
Each patient's evaluation was then compared to that of their caregivers. Patients are called to provide a subjective measure of their deficit: for this reason it is important to compare their evaluation with that of caregivers rather than with mere test scores. It has been pointed out that caregivers may have little information about the patient's actual condition, especially in the acute phase following brain damage, and that other factors such as stress and anxiety may affect their judgment (Orfei, Caltagirone, & Spalletta, 2010; Prigatano et al., 2005). These are certainly variables to be carefully considered. However, our findings are encouraging in the use of caregivers as raters. In our study the evaluation of patients’ language difficulties by personal and professional caregivers significantly correlated with patients’ actual performance on a psychometric language test (the AAT). They also proved to be highly reliable on retesting. (Similar findings have been observed when caregivers were asked to evaluate the patients’ motor difficulties; Della Sala et al., 2009.) Thus caregivers can provide realistic and reliable evaluations of patients’ conditions. Further, personal and professional carers provided very similar ratings, suggesting that the ability to judge cognitive competence is not seriously affected by more motivational factors, at least in sub-acute and chronic phases of disease. The comparison with normative data is another crucial issue for every study attempting to diagnose anosognosia, as the risk of false alarms is a serious concern (Baier & Karnath, 2005). The VATA-L is the first assessment that provides a scoring system and cut-off points to minimize this risk for anosognosia of language deficits. The risk of false alarms is higher when patients present with mild deficits. The milder the aphasia, the smaller is the possible caregiver-patient discrepancy and, consequently, the lower the level of possible unawareness. For example, a patient who is unaware of her very mild aphasia (e.g., caregiver rating = 0.5 and patient's rating = 0 on each of the 14 VATA-L questions) would not be diagnosed as anosognosic, as the caregiver-patient discrepancy score (7 in this example) will not exceed the cut-off for anosognosia (i.e., 11.9). VATA-L is therefore a conservative measure of identifying anosognosia.
Anosognosia for aphasia
Frequency of occurrence of anosognosia for aphasia is still unclear; data from previous studies ranged from 78% (Weinstein et al., 1966) to less than 7% (Gainotti, 1972). In our study we observed that about 20% (10 out of 53) of aphasic patients with brain lesions limited to the left hemisphere showed clear evidence of anosognosia. The different frequencies reported in different studies certainly reflect the fact that different methods of assessment have been used. The selection of patients, and sample sizes, varied considerably between studies. However, a key aspect of our investigation is that our clinical sample consisted mostly of patients with brain damage limited to the left hemisphere. This is an important issue as some authors have suggested that anosognosia is observed only following large bilateral brain lesions (e.g., Weinstein et al., 1966), implying a unique role for the right hemisphere in awareness. Even though ours is not an epidemiological study, our results suggest that, in line with other studies in the literature (Gainotti, 1972; Kertesz & Benson, 1970; Maher et al., 1994; Marshall et al., 1998), anosognosia for aphasia may occur with lesions circumscribed to the left hemisphere. Moreover, since our sample was mainly comprised of patients in the sub-acute phase (on average 10 months post-lesion), anosognosia seems to be a relatively frequent occurrence even some time following brain injury. Similar findings have been reported in recent studies on sub-acute anosognosia for motor impairment (e.g., Cocchini et al., 2009; Marcel et al., 2004; for a review see Table 1 in Cocchini, Beschin, & Della Sala, 2002) suggesting that anosognosia for motor, as for language, disorders is far from being solely a transient phenomenon. We have recently suggested that anosognosia for hemiplegia in less acute phases might have been underestimated because patients could have been repeatedly exposed to some of the questions used to assess anosognosia. Thus patients may provide the “correct” response based on what they have “learned” rather than on their actual awareness of their deficit (Cocchini et al., 2009). Similarly, aphasic patients often attend daily speech therapy sessions and relatives or clinicians might often comment on their language difficulties. For this reason it is important that patients evaluate their abilities in relation to several tasks, rather than the assessment being based on a single question (as in Breier et al., 1995).
A further interesting finding emerges from the data. Patients showing more severe language impairment were more likely to be unaware of their deficit. This has important implications. Some authors suggested that anosognosia might be due to a difficulty in patients discovering their deficit (Levine, 1990). However, while with hemiplegia the severity of the deficit makes the disability more apparent, which would facilitate its discovery, in the case of aphasia the opposite could be true. Indeed anosognosia for aphasia could be due to impaired comprehension that would in turn prevent an accurate monitoring process and the “discovery” of the deficit (e.g., Boller, Vrtunski, Kim, & Mack, 1978; Heilman, 1991; Wernicke, 1874). In other words, according to these authors, the more compromised the patient's understanding, the harder it should be for them to realize/evaluate their language impairment. In line with this interpretation, anosognosia for aphasia has often been observed in association with sub-groups such as Wernicke's, jargon, and transcortical sensory aphasia with severe comprehension deficits (for a review see Kertesz, 2010). However, studies that have analyzed self-corrections produced by aphasics in detail provide a consistent body of evidence against comprehension failure as the main factor leading to unawareness (e.g., Schlenck et al., 1987). Poeck (1972), and later Schlenck et al. (1987), did not find a clear association between anosognosia and comprehension deficits. Marshall and Tompkins (1982) investigated awareness in 42 aphasic patients, and found no differences between fluent and non-fluent aphasic patients, differing in comprehension deficits. Similarly, when we consider the type of verbal component mainly impaired in anosognosic patients we find that they performed significantly worse in verbal production than verbal comprehension subtests. Taking into account both findings in the literature and our current results, we do not exclude that comprehension deficits per se might lead to some forms of anosognosia. However, it seems likely that other causes might also be responsible of the lack of awareness for aphasia. In line with this hypothesis, some authors have observed aphasie patients able to acknowledge their language disorders only when their own mistakes were attributed to another person (Alajouanine, 1956; Kinsbourne & Warrington, 1963; Maher et al., 1994, case AS) or when they listened to a recorded performance of their own utterances (Lebrun, 1987; Maher et al., 1994, case CM; Marshall et al., 1998; Shuren, Hammond, Maher, Rothi, & Heilman, 1995). This suggests that other factors such as motivational denial or attention might also play a role in anosognosia for aphasia.
Our study has some limitations: (i) Time post-onset varied across the patients included; awareness of deficits is likely to be different in people immediately after injury as compared to people in the post-acute period. Future studies should investigate awareness for language in selected and homogeneous samples to validate the instrument in cases of persistent anaosognosia. (ii) The relative severity of anosognosia for language compared to other forms of anosognosia, e.g., for motor deficits, remains an open question. (iii) The new test presented in this study calls for further validation and comparison with other methodologies.
ACKNOWLEDGMENTS
This research was supported by the Wellcome Trust (grant n.078580) to GC and SDS. We would like to thank Dr. Lodi, Dr. Pasini, Dr. Galli, and Dr. Zaro for their support in recruiting the patients in Italian wards (Rehabilitation Ward - Gallarate Hospital) and Dr. Papps and Dr. Rado for their support in British wards (Homerton Hospital in London; Blackheath Brain Injury Rehabilitation Centre and Neurodisability Service in London). We would like to thank Ms. Katherine Broomfield and Ms. Annette Cameron for their suggestions on a preliminary version of this test. NB's work is currently supported by a grant from the Compagnia di San Paolo - Programma Neuroscienze.
Appendix
Expected ratings for check questions 3 and 18 are 2 or 3; expected ratings for check questions 8 and 12 are 0 or 1.
Example:
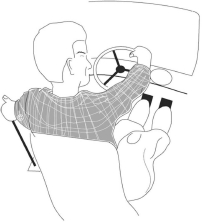
Do/would you have difficulty driving?
1.

Do you have a problem finding the right word?
2.

Do you have a problem understanding what people are talking about?
3.
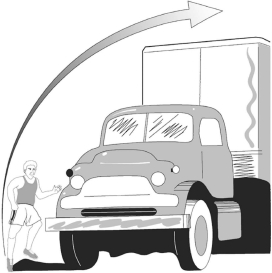
Check question: Do you have any difficulty in jumping over a lorry?
4.
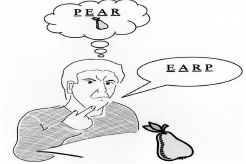
Do you have the problem of putting the sounds of words in the wrong order?
5.

Do you have a problem reading notices?
6.

Do you have a problem getting the sounds of a word?
7.
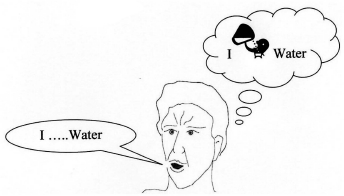
Do you have the problem of missing out words in sentences?
8.
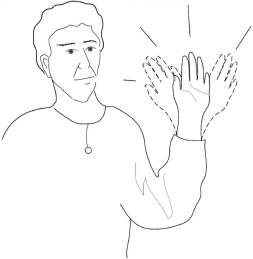
Check question. Do you have any difficulty in waving?
9.

Do you have a problem reading the newspaper?
10.
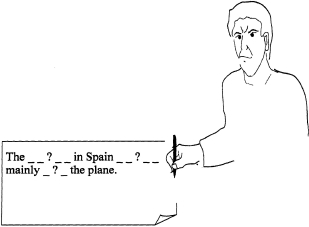
Do you have a problem writing sentences?
11.

Do you have the problem of using made-up words that don't make sense?
12.
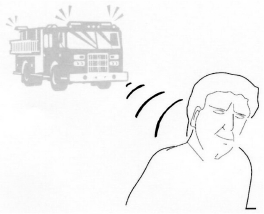
Check question: Would you have a problem hearing afire engine with its sirens on?
13.
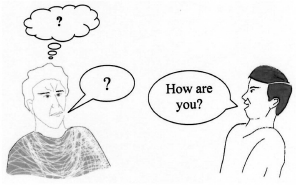
Do you have a problem telling someone when you don't understand?
14.
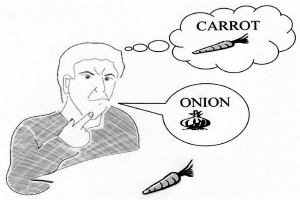
Do you have the problem of using the wrong word?
15.

Do you have a problem speaking on the telephone?
16.

Do you have a problem picking up mistakes in your speech?
17.
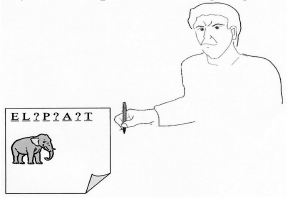
Do you have a problem writing single words?
18.

Check question: Would you have a problem speaking Russian?
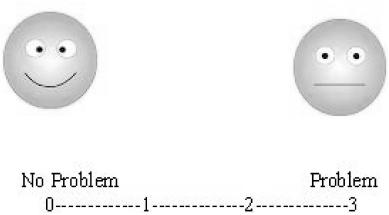
Visual-analogue scale
Footnotes
1 Note that in several instances professional caregivers rated more than one patient.
References
- Adair J. C., Schwartz R. L., Barrett A. M. Anosognosia. In: Heilman K. M., Valenstein E., editors. Clinical neuropsychology. Oxford, UK: Oxford University Press; 2003. pp. 185–214. [Google Scholar]
- Alajouanine T. Verbalization in aphasia. Brain. 1956;79:1–28. doi: 10.1093/brain/79.1.1. [DOI] [PubMed] [Google Scholar]
- Appelros P., Karlsson G. M., Hennerdal S. Anosognosia versus unilateral neglect. Coexistence and their relations to age, stroke severity, lesion site and cognition. European Journal of Neurology. 2007;14:54–59. doi: 10.1111/j.1468-1331.2006.01544.x. [DOI] [PubMed] [Google Scholar]
- Baier B., Karnath H. O. Incidence and diagnosis of anosognosia for hemiparesis revised. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:358–361. doi: 10.1136/jnnp.2004.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A. Conoscere e rieducare l'afasia. Roma: Il Pensiero scientifico Editore; 2005. [Google Scholar]
- Boller F., Vrtunski P. B., Kim Y., Mack J. L. Delayed auditory feedback and aphasia. Cortex. 1978;14:212–226. doi: 10.1016/s0010-9452(78)80047-1. [DOI] [PubMed] [Google Scholar]
- Brazzelli M., Capitani E., Della Sala S., Spinnler H., Zuffi M. A neuropsychological instrument adding to the description of patients with suspected. Neurosurgery, and Psychiatry. 1994;57:1510–1517. doi: 10.1136/jnnp.57.12.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier J. L., Adair J. C., Gold M., Fennell E. B., Gilmore R. L., Heilman K. M. Dissociation of anosognosia for hemiplegia and aphasia during left-hemisphere anesthesia. Neurology. 1995;45:65–67. doi: 10.1212/wnl.45.1.65. [DOI] [PubMed] [Google Scholar]
- Brumfitt S. M., Sheeran P. The development and validation of the Visual Analogue Self Esteem Scale (VASES) British Journal of Clinical Psychology. 1999;38:387–400. doi: 10.1348/014466599162980. [DOI] [PubMed] [Google Scholar]
- Cocchini G., Beschin N., Della Sala S. Chronic anosognosia: A case report and theoretical account. Neuropsychologia. 2002;40:2030–2038. doi: 10.1016/s0028-3932(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Cocchini G., Beschin N., Cameron A., Fotopoulou A., Della Sala S. Anosognosia for motor impairment following left-brain damage. Neuropsychology. 2009;23:223–230. doi: 10.1037/a0014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchini G., Della Sala S. Assessing anosognosia for motor and language impairments. In: Prigatano G., editor. The study of anosognosia. New York: Oxford University Press; 2010. pp. 193–231. [Google Scholar]
- Della Sala S., Cocchini G., Beschin N., Cameron A. VATA-M: Visual-analogue test for anosognosia for motor impairment, A new test to assess awareness for motor impairment. The Clinical Neuropsychologist. 2009;23:406–427. doi: 10.1080/13854040802251393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham J. K., Sage K., Lambon Ralph M. A. The treatment of anomia using errorless learning. Neuropsychological Rehabilitation. 2006;16:129–154. doi: 10.1080/09602010443000254. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Emotional behavior and hemispheric side of the lesion. Cortex. 1972;8:41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- Gialanella B., Mattioli F. Anosognosia and extrapersonal neglect as predictors of functional recovery following right hemisphere stroke. Neuropsychological Rehabilitation. 1992;2:169–178. [Google Scholar]
- Heilman K. M. Anosognosia: Possible neuropsychological mechanisms. In: Prigatano G. P., Schacter D. L., editors. Awareness of deficit after brain injury. Oxford, UK: Oxford University Press; 1991. pp. 53–62. [Google Scholar]
- Huber W., Poeck K., Willmes K. The Aachen Aphasia Test. In: Rose F. C., editor. Progress in aphasiology, advances in neurology. New York: Raven Press; 1984. pp. 291–303. [PubMed] [Google Scholar]
- Kertesz A. Anosognosia in aphasia. In: Prigatano G., editor. The study of anosognosia. New York: Oxford University Press; 2010. pp. 113–122. [Google Scholar]
- Kertesz A., Benson D. F. Neologistic jargon: A clinicopathological study. Cortex. 1970;6:362–386. doi: 10.1016/s0010-9452(70)80002-8. [DOI] [PubMed] [Google Scholar]
- Kertesz A., Nadkarni N., Davidson W., Thomas A. W. The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. Journal of the International Neuropsychological Society. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M., Warrington E. K. Jargon aphasia. Neuropsychologia. 1963;1:27–37. [Google Scholar]
- Lebrun Y. Anosognosia in aphasics. Cortex. 1987;23:251–263. doi: 10.1016/s0010-9452(87)80035-7. [DOI] [PubMed] [Google Scholar]
- Levelt W. J. M. Monitoring and self-repair in speech. Cognition. 1983;14:41–104. doi: 10.1016/0010-0277(83)90026-4. [DOI] [PubMed] [Google Scholar]
- Levine N. Unawareness of visual and sensorimotor defects: A hypothesis. Brain and Cognition. 1990;13:233–281. doi: 10.1016/0278-2626(90)90052-p. [DOI] [PubMed] [Google Scholar]
- Luzzatti C., Willmes K., Bisiacchi P., De Bleser R., Faglia L., Mazzocchi A., et al. L'Aachener Aphasie Test (AAT). II: Proprietà psicometriche della versione italiana. Archivio di Psicologia, Neurologia e psichiatria. 1987;48:480–519. [Google Scholar]
- Maher L., Rothi L., Heilman K. Lack of error awareness in an aphasic patient with relatively preserved auditory comprehension. Brain and Language. 1994;46:402–418. doi: 10.1006/brln.1994.1022. [DOI] [PubMed] [Google Scholar]
- Marcel A., Tegnér R., Nimmo-Smith I. Anosognosia for plegia: Specificity, extension, partiality and disunity of bodily unawareness. Cortex. 2004;40:19–40. doi: 10.1016/s0010-9452(08)70919-5. [DOI] [PubMed] [Google Scholar]
- Marshall J., Robson J., Pring T., Chiat S. Why does monitoring fail in jargon aphasia? Comprehension, judgment, and therapy evidence. Brain and Language. 1998;63:79–107. doi: 10.1006/brln.1997.1936. [DOI] [PubMed] [Google Scholar]
- Marshall R., Rappaport B., Garcia-Bunuel L. Self monitoring behavior in a case of severe auditory agnosia with aphasia. Brain and Language. 1985;24:297–313. doi: 10.1016/0093-934x(85)90137-3. [DOI] [PubMed] [Google Scholar]
- Marshall R. C., Tompkins C. A. Verbal self-correction behaviors of fluent and nonfluent aphasic subjects. Brain and Language. 1982;15:292–306. doi: 10.1016/0093-934x(82)90061-x. [DOI] [PubMed] [Google Scholar]
- McGlynn S. M., Schacter D. L. Unawareness of deficits in neuropsychological syndromes. Journal of Clinical and Experimental Neuropsychology. 1989;11:143–205. doi: 10.1080/01688638908400882. [DOI] [PubMed] [Google Scholar]
- Miller N., Willmes K., De Bleser R. The psychometric properties of the English language version of the Aachen Aphasia Test (EAAT) Aphasiology. 2000;14(7):683–722. [Google Scholar]
- Orfei D., Caltagirone C., Spalletta G. The behavioral measurement of anosognosia as a multifaceted phenomenon. In: Prigatano G., editor. The study of anosognosia. New York: Oxford University Press; 2010. pp. 429–451. [Google Scholar]
- Orgass B. Der Token Test. Weinheim: Beltz; 1986. [Google Scholar]
- Poeck K. Stimmung und krankheitseinsicht bei aphasien. Archiv für Psychiatrie und Nervenkrankheiten. 1972;216:246–254. doi: 10.1007/BF00342645. [DOI] [PubMed] [Google Scholar]
- Prigatano G. P., Borgaro S., Baker J., Wethe J. Awareness and distress after traumatic brain injury: A relative's perspective. Journal of Head Trauma Rehabilitation. 2005;20:359–367. doi: 10.1097/00001199-200507000-00007. [DOI] [PubMed] [Google Scholar]
- Rubens A. B., Garrett M. F. Anosognosia of linguistic deficits in patients with neurological deficits. In: Prigatano G. P., Schacter D. L., editors. Awareness of deficit after brain injury. Oxford, UK: Oxford University Press; 1991. pp. 40–52. [Google Scholar]
- Rüsch N., Corrigan P. W. Motivational interviewing to improve insight and treatment adherence in schizophrenia. Psychiatric Rehabilitation Journal. 2002;26:23–32. doi: 10.2975/26.2002.23.32. [DOI] [PubMed] [Google Scholar]
- Schlenck K. J., Huber W., Willmes K. Prepairs and repairs: Different monitoring functions in aphasic language production. Brain and Language. 1987;30:226–244. doi: 10.1016/0093-934x(87)90100-3. [DOI] [PubMed] [Google Scholar]
- Shuren J. E., Hammond C. S., Maher L. M., Rothi L. J. G., Heilman K. M. Attention and anosognosia: The case of a jargon aphasic patient with unawareness of language deficit. Neurology. 1995;45:376–378. doi: 10.1212/wnl.45.2.376. [DOI] [PubMed] [Google Scholar]
- Smith G., Della Sala S., Logie H. R. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Nyentuis D. L., Yamamoto C., et al. Standardisation and validation for the visual analogue mood scale. The Clinical Neuropsychologist. 1997;11:407–415. [Google Scholar]
- Vuilleumier P. Anosognosia. In: Bogousslausky J., Cummings J. L., editors. Behavior and mood disorders in focal brain lesions. Cambridge, UK: Cambridge University Press; 2000. pp. 465–519. [Google Scholar]
- Weinstein E. A., Lyerly O. G., Cole M., Ozer M. N. Meaning in jargon aphasia. Cortex. 1966;2:165–187. [Google Scholar]
- Wernicke C. Der aphasische Symptomen komplex. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- Worrall L., Rose T., Howe T., Brennan A., Egan J., Oxenham D., et al. Access to written information for people with aphasia. Aphasiology. 2005;19:923–929. [Google Scholar]


