Abstract
Objective
Myofiber necrosis without prominent inflammation is a non-specific finding seen in patients with dystrophies and toxic or immune-mediated myopathies. However, the etiology of a necrotizing myopathy is often obscure and which patients would benefit from immunosuppression remains uncertain. We sought to identify novel autoantibodies in necrotizing myopathy patients.
Methods
Muscle biopsy and serum were available for 225 myopathy patients. Antibody specificities were determined by performing immunoprecipitations from 35S-methionine-labeled HeLa cell lysates. Selected biopsies were stained for membrane attack complex (MAC), major histocompatability complex I (MHC I), and endothelial cell marker CD31.
Results
38 of 225 patients had predominant myofiber necrosis on muscle biopsy. 12 of these had a known autoantibody association or other etiology for their myopathy. 16 of the remaining 26 sera immunoprecipitated 200 and 100 kDa proteins; this specificity was found in only 1/187 patients without necrotizing myopathy. Patients with anti-200/100 specificity had proximal weakness (100%), high creatine kinase (CK) levels (mean 10,333 IU/L), and an irritable myopathy on electromyography (EMG) (88%). 63% had exposure to statins prior to the onset of weakness. All patients responded to immunosuppressive therapy and many relapsed with medication tapering. Immunohistochemical studies showed MAC on small blood vessels in 6/8 and on the surface of non-necrotic myofibers in 4/8. 5/8 had abnormal capillary morphology and 4/8 expressed MHC I on the surface of non-necrotic myofibers.
Conclusion
An anti-200/100 kDa specificity defines a subgroup of necrotizing myopathy patients previously considered to be "autoantibody negative." We propose that these patients have an immune-mediated myopathy which is frequently associated with prior statin use and should be treated with immunosuppressive therapy.
Adults with proximal muscle weakness, elevated CK levels, myopathic features on electromyography, and evidence of muscle edema on magnetic resonance imaging have a broad differential diagnosis that includes autoimmune myopathies, toxic myopathies, paraneoplastic myopathies, and muscular dystrophies. Distinguishing between immune-mediated myopathies and other etiologies is crucial because only autoimmune muscle diseases routinely respond to immunosuppressive therapy.
In many cases, distinctive clinical features and/or a muscle biopsy can provide a definitive diagnosis. For example, perifascicular atrophy is pathognomic for dermatomyositis even in the absence of rash; vacuolar myopathy in a patient treated with colchicine strongly suggests a toxic myopathy; and reduced dystrophin staining in the muscle of a young man with calf hypertrophy is diagnostic for a dystrophinopathy.
However, in a substantial number of cases, muscle biopsies show degenerating and necrotic muscle fibers in the absence of disease-specific features. In these instances, the presence of myositis-specific autoantibodies (MSAs) may identify the disorder as belonging to the family of autoimmune myopathies (1). For example, patients with antibodies directed against the signal recognition particle (SRP) typically have a severe necrotizing myopathy responsive only to very aggressive immunosuppression (2–6). Unfortunately, clinical evaluation and currently available diagnostic tests do not always provide a definitive diagnosis and it may not be possible to determine whether a necrotizing myopathy is immune-mediated. This uncertainty can lead to under-treatment of autoimmune myopathies or inappropriate immunosuppression of patients who do not have an immune-mediated disease.
In this study, we identified 26 patients with necrotizing myopathies who, despite comprehensive evaluations, could not be diagnosed with a specific muscle disease. Sera from these patients were screened for the presence of novel autoantibodies and a unique autoantibody specificity against 200 and 100 kDa proteins was identified in 16 subjects. Further analysis of the clinical characteristics and muscle biopsy features of these anti-200/100 patients suggests they belong to the family of autoimmune myopathies responsive to immunosuppressive therapy.
MATERIALS AND METHODS
Patients
Two hundred twenty-five patients with banked sera, muscle biopsy specimens available for review, and a myopathy as defined by proximal muscle weakness, elevated CK levels, myopathic EMG findings, muscle edema on magnetic resonance imaging (MRI), and/or myopathic features on muscle biopsy were enrolled in a longitudinal study approved by the Johns Hopkins Institutional Review Board from March 2007 through December 2008. In addition to a history and physical examination at the Johns Hopkins Myositis Center, these patients underwent a comprehensive evaluation including some or all of the following: (i) EMG and nerve conduction studies, (ii) non-contrast bilateral thigh MRI, (iii) pulmonary function tests, (iv) malignancy screening including computed tomography (CT) scans of the chest, abdomen and pelvis, (v) a standard laboratory evaluation performed by several different commercial laboratories included CK levels, antinuclear antibody (ANA) screen, erythrocyte sedimentation rate (ESR), c-reactive protein (CRP) levels, anti-Ro and –La screen, and MSA screen, and (vi) when suspected based on clinical or biopsy features, testing for inherited muscle disease including limb-girdle muscular dystrophies (by Athena Diagnostics’ Limb Girdle Muscular Dystrophy Evaluation panel), acid maltase deficiency (by Athena Diagnostics’ Glycogen Storage Myopathy ‘A’ Profile and/or Genzyme’s dried blood spot test for alpha-glucosidase activity), and/or facioscapulohumeral dystrophy (by Athena Diagnostics’ FSHD DNA Test).
In order to determine whether statins were used at an increased frequency in patients with the anti-200/100 antibody, we also determined the frequency of statin use in patients from our cohort with definite or probable polymyositis (PM) and dermatomyositis (DM) (7, 8) as well as in those with possible inclusion body myositis (IBM) (9). The ages of the patients were compared by using two-tailed Student’s t-tests. The chi-square test was used to compare the frequency of statin use in the different groups.
Muscle biopsy analysis
Muscle biopsy specimens were obtained from the deltoid, biceps, or quadriceps muscle groups. In each case, the muscle selected was found to be weak by the examining physician. The slides from muscle biopsies were evaluated at the Johns Hopkins Neuromuscular Pathology Laboratory. These studies included H&E stained tissue as well as some or all of the following stains: modified Gomori trichrome, adenosine triphosphatase at pH 4.3, 4.6, and 9.4, nicotinomide adenosine dinucleotide - tetrazolium reductase, acid phosphatase, succinic dehydrogenase, cytochrome oxidase, esterase, alkaline phosphatase, Periodic acid-Schiff (PAS), PAS-diastase control, and Congo red. Both frozen and paraffin specimens were routinely screened for the presence of degenerating, regenerating, and/or necrotic fibers, primary endomysial inflammation, perivascular inflammation, rimmed vacuoles, perifascicular atrophy, and fibrosis. We identified “necrotizing myopathy” biopsy specimens based on the presence of necrotic muscle fibers as the predominant abnormal histological feature; with the exception of necrotic myofibers undergoing myophagocytosis, inflammatory cells were sparse, if present at all. Muscle biopsy specimens from patients with the anti-200/100 autoantibody specificity were stained with antibodies recognizing CD31 (an endothelial cell marker), C5b-9 (i.e., MAC), and MHC I. In brief, 7 micron-thick frozen muscle biopsy sections were fixed in ice-cold acetone. After 10 minutes in peroxidase-blocking reagent (DAKO) at room temperature, sections were incubated with 5% bovine serum albumin/phosphate buffered saline (BSA/PBS) for 1 hour at 37°C. Primary antibodies were prepared in 1% BSA/PBS at the following dilutions: 1:50 for MHC I(Santa Cruz Biotechnology), 1:20 for CD31 (DAKO), 1: 50 for Cb5–9 (Santa Cruz Biotechnology); primary incubations were overnight at 4 °C. After PBS washes, slides were incubated with horseradish-peroxidase-labeled goat anti-mouse secondary antibody (DAKO) in 1% BSA/PBS at 1:500 for 1 hour at room temperature. 3,3-diaminobenzidine chromagen (DAKO) was used to visualize each antibody and all sections were counterstained with hematoxylin. Normal muscle tissues were used as negative controls and muscle tissue from a Jo-1 positive myositis patient was used as a positive control for MHC I staining. For each primary antibody, all muscle sections were processed simultaneously under the same conditions.
Immunoprecipitations
Serum collected from each patient was stored at −80°C. HeLa cells cultured using standard procedures were radiolabeled for 2 hours with 100 µCi/mL [35S]-methionine and cysteine (MP Biomedicals) in methionine- and cysteine-free medium. The cells were subsequently lysed in Buffer A (50 mM Tris pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail). Each 10 cm dish was lysed in 1 mL Buffer A and was used for 10 immunoprecipitations. Immunoprecipitations were performed by adding 1 µL of patient sera to 100 µL radiolabeled lysate and bringing the volume to 1 mL with Buffer B (1/% nonidet P-40, 20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, and a protease inhibitor cocktail) and rotating the mix for 1 hour at 4°C. Protein A agarose beads (Pierce) were used to precipitate the antibody-antigen complexes which were subsequently electrophoresed on 10% SDS-polyacrylamide gels. The radiolabeled immunoprecipitates were visualized by fluorography.
RESULTS
Identification of necrotizing myopathy patients with uncertain diagnoses
Muscle biopsies from 225 patients who presented with proximal muscle weakness, elevated CK levels, myopathic EMGs, and/or other evidence of muscle disease were reviewed to identify those with a predominantly necrotizing myopathy. Subjects with biopsies notable for marked inflammatory cell infiltrates, rimmed vacuoles (characteristic of inclusion body myositis), perifascicular atrophy (pathognomonic for dermatomyositis), or other features characteristic of a specific diagnosis were not considered to have a predominantly necrotizing myopathy.
In all, 38 patients (17% of the total) were identified as having a predominantly necrotizing myopathy on muscle biopsy. Of these, 12 were definitively diagnosed with a specific muscle disease using existing testing methods. Ten had autoimmune myopathies as defined by the presence of anti-synthetase autoantibodies (one with anti-Jo-1, two with anti-PL-12, and one with anti-PL-7) or anti-signal recognition particle (SRP) autoantibodies (six patients); each of these patients also had a definite positive response to immunosuppressive therapy. In addition, one patient had a necrotizing myopathy associated with profound hypothyroidism and another had limb girdle muscular dystrophy type 2B (i.e., dysferlinopathy) confirmed by genetic testing. The remaining 26 patients (~10% of our cohort) had a predominantly necrotizing myopathy of unclear etiology.
Identification of a novel anti-200/100 kDa autoantibody specificity in patients with a necrotizing myopathy
Sera collected from the 26 patients identified above were screened for the presence of novel autoantibodies. Remarkably, we found that sera from 16 of these patients (62%) immunoprecipitated a pair of proteins from radioactively-labeled HeLa cell extracts with approximate sizes of 200 and 100 kDa (Figure 1). These proteins, with molecular weights that do not correspond to those of known myositis-specific autoantigens, were always immunoprecipitated as a pair. Although anti-200/100 immunoprecipitations were reproducible, no serum detected 200 or 100kDa proteins when used to immunoblots HeLa cell extracts (data not shown.)
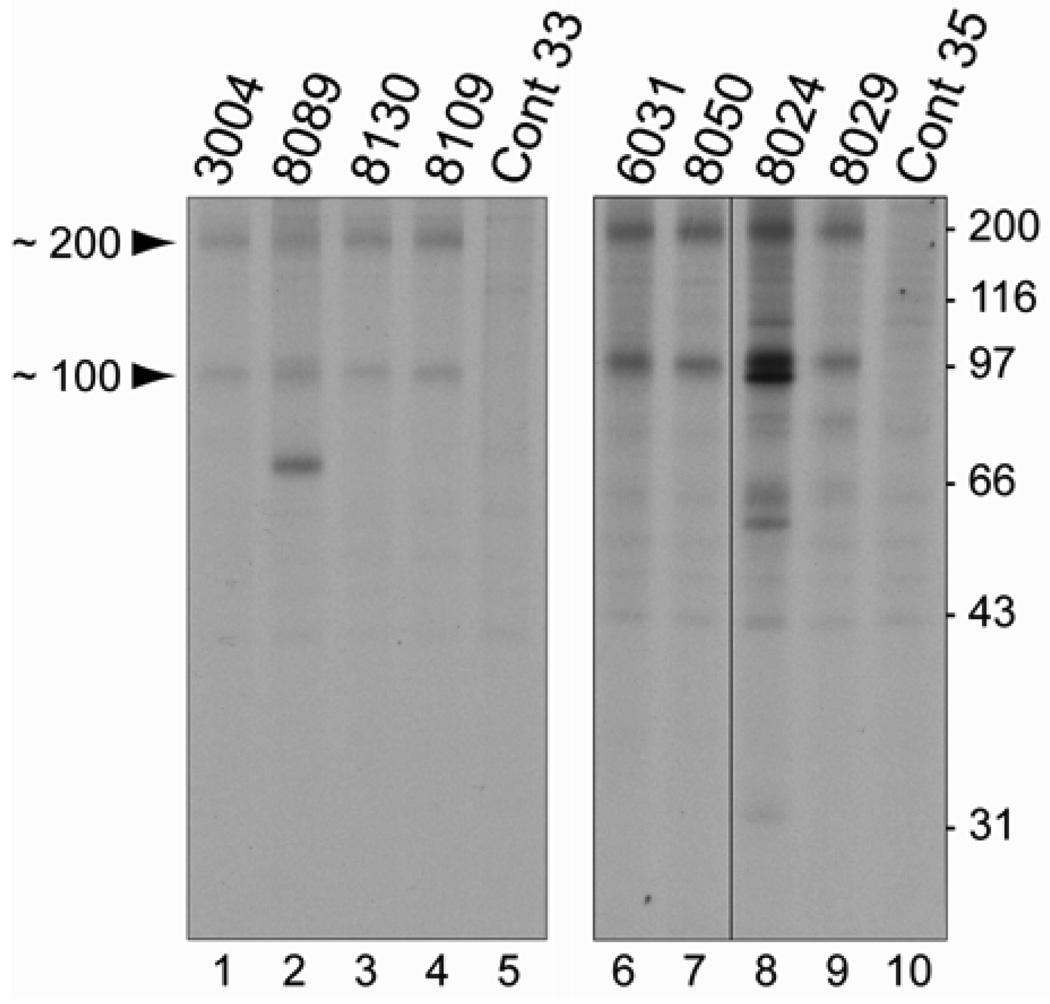
Figure 1. Sera from patients with a necrotizing myopathy immunoprecipitate ~200 and ~100 kDa proteins.
Patient sera were used to immunoprecipitate radioactively labeled proteins from HeLa cell extracts which had been incubated with 35S-methionine. Immunoprecipitated proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels. The left and right panels are autoradiographs from 2 separate experiments; data shown on the right panel is from a single autoradiograph which has been cropped between lanes 7 and 8 to exclude immunoprecipitations irrelevant to the current study. The numbers at the top of lanes 1–4 and 6–9 correspond to the patient numbers in Supplemental Table 1. Two different normal control sera (cont 33 and cont 35) were used in the immunoprecipitations shown in lanes 5 and 10. The migration of molecular weight marker standards is shown on the right side.
In order to evaluate the specificity of these antibodies for a necrotizing phenotype, we tested for anti-200/100 immunoreactivity in the remaining cohort. Among the 187 patients who did not have a predominant necrotizing myopathy, the serum from only one (0.5%) immunoprecipitated the 200 and 100 kDa proteins, demonstrating that this finding is highly specific for those patients with a necrotizing myopathy (p<10−15 by Fisher’s exact test). None of the sera from the 12 patients with necrotizing myopathies associated with previously known conditions, including the 6 anti-SRP cases, immunoprecipitated proteins with molecular weights of 200 or 100 kDa (data not shown).
Several of the anti-200/100 sera immunoprecipitated additional proteins. For example, the serum from patient 8089 immunoprecipitated a ~70 kDa protein as well as those of 200 and 100 kDa (figure 1, lane 2). Of note, each of the additional proteins was recognized by no more than one of the 16 anti-200/100 sera. Furthermore, none of the additional bands recognized by any of the anti-200/100 sera correspond in size to previously recognized myositis-specific autoantigens, including proteins with molecular weights of 72, 54, and/or 21 kDa as seen in patients with anti-SRP myopathy.
Clinical features of the anti-200/100 patient population
We analyzed the demographic information, laboratory findings, pattern of weakness, thigh MRIs, and other clinical features of the 16 anti-200/100 patients with a necrotizing myopathy (Table 1); the patient with anti-200/100 specificity who did not have a predominantly necrotizing myopathy was excluded from this analysis.
Table 1.
Clinical features of anti-200-100 patients
| (n = 16 patients) | |
|---|---|
| Demographic Information | |
| Average age at onset of disease | 54 years |
| Sex, % female | 63% |
| Race, % white | 56% |
| Race, % non-white | 44% |
| Deceased | none |
| Clinical Feature | |
| Subjective muscle weakness | 100% |
| Proximal weakness on exam | 100% |
| Wheelchair use | 25% |
| Interstitial lung disease | 0% |
| Malignancy | 13% |
| Raynaud's phenomenon | 13% |
| Rash | 44% |
| Myalgias | 75% |
| Arthralgias | 50% |
| Dysphagia | 63% |
| Statin use | 63% |
| Laboratory Findings | |
| Initial CPK, mean | 8,702 |
| Maximum CK, mean | 10,333 |
| Positive ANA (>1:160) | 6% |
| Elevated ESR | 38% |
| Elevated CRP | 6% |
| anti-RO positive | 0% |
| anti-LA positive | 0% |
| Thigh MRI Features | |
| Normal thigh MRI | 0% |
| Muscle edema | 100% |
| Atrophy | 75% |
| Fatty replacement | 67% |
| Fascial edema | 25% |
| EMG Findings | |
| Irritable myopathy | 88% |
| Non-Irritable myopathy | 13% |
| Normal EMG | 0% |
Men and women were represented in roughly equal numbers and had a mean age of 54 years at the onset of disease. All 16 patients reported previously normal strength with the acute or subacute onset of muscle weakness in adulthood. At initial evaluation, all patients had proximal muscle weakness, evidence of muscle edema on bilateral thigh MRI, and markedly elevated CK levels with a mean value of 10,333 IU/L (range 3,052–24,714). Each of the 16 EMGs available for review revealed myopathic features; 14/16 (88%) of these demonstrated an irritable myopathy, while the remaining two were non-irritable.
Other prominent clinical features included myalgias in 12/16 (75%), arthralgias in 8/16 (50%), and dysphagia in 10/16 (63%%). Only 2/16 (13%) patients had Raynaud’s phenomenon. Although 7/16 (44%) reported a non-specific rash, no patient had cutaneous features consistent with dermatomyositis on exam or by historical account.
None of these patients had antibodies against extractable nuclear antigens detected by clinical laboratories (including anti-Ro, anti-La, anti-RNP, and anti-Scl-70) and no patient met criteria for another connective tissue disease. Two patients had prior malignancies, one with non-recurrent ovarian cancer treated 5 years prior to the onset of muscle disease and another who was in clinical remission after treatment for prostate cancer.
None of the anti-200/100 patients had a family history of muscle disease. Furthermore, scapular winging, facial weakness, asymmetric weakness, or other distinctive features suggestive of inherited muscle disease were absent in each of these patients.
Of note, 10/16 subjects (63%) had an exposure to statin therapy prior to the onset of weakness. The mean duration of statin use prior to the onset of muscle symptoms was 31.3 +/− 27.4 months with a range 0–84 months (Supplementary Table 1). In each case, discontinuing the statin medication did not lead to clear clinical improvement and the mean length of time between statin discontinuation and muscle biopsy was 5.2 +/− 4.6 months with a range of 1–14 months (Supplementary Table 1). No other potential myotoxin exposures were identified on review of patient records.
To determine if the association with statin use was coincidental, we analyzed the frequency of statin use in other myositis groups evaluated at our clinic (Table 2). We found that 5/33 (15.2%) DM patients, 7/38 (18.4%) PM patients, and 11/31 (35.5%) IBM patients had used statins prior to undergoing a muscle biopsy; there was a significantly (P<0.05) increased frequency of statin use in the anti-200/100 group compared to both DM and PM groups. However, in this analysis, there was no significant difference in statin use between the anti-200/100 and IBM populations (P=0.08). Because older patients are more likely to use statins, we assessed the ages of patients with different forms of myositis. Compared to the anti-200/100 patients, who had a mean age of 57.8 +/− 14.8 years, the IBM patients were significantly older with a mean age of 67.7 +/− 9.9 years. When only those patients age 50 or older were included in the analysis, 10/12 (89%) anti-200/100 patients, 4/16 (25%) DM patients, 7/19 (36.8%) PM patients, and 10/30 (33.3%) IBM patients were exposed to statins (Table 2). In this age-matched comparison, statin use was significantly increased in the anti-200/100 population compared to DM (P = 0.002), PM (P = 0.011), and IBM (P = 0.003) populations.
Table 2.
Frequency of statin use in patients with different forms of muscle disease
| Group | Frequency of statin use | Mean age of patients |
|---|---|---|
| anti-200/100 | 10/16 (62.5%) | 57.8 +/− 14.8 |
| DM | 5/33 (15.2%)* | 51.0 +/− 12.2 |
| PM | 7/38 (18.4%)* | 49.1 +/− 14.1** |
| IBM | 11/31 (35.5%) | 67.7 +/− 9.9** |
| anti-200/100 (age > or = 50) | 10/12 (89%) | 64.4 +/− 9.2 |
| DM (age > or = 50) | 4/16 (25%)* | 61.0 +/− 8.3 |
| PM (age > or = 50) | 7/19 (36.8%)* | 60.4 +/− 7.6 |
| IBM (age > or = 50) | 10/30 (33.3%)* | 68.4 +/− 9.2 |
indicates frequency of statin use significantly different compared to the anti-200/100 group by use of the chi-square test (P<0.05)
indicates age significantly different compared to the anti-200/100 group by use of the Student's t-test (P<0.05)
There was a striking variation in clinical phenotype ranging from a chronically intubated, quadriplegic patient to several patients with only mild weakness. A unique feature in the majority of patients was their relative preservation of strength despite markedly elevated muscle enzymes. However, several patients reported an apparent threshold muscle enzyme level (usually between 3000–7000 IU/L) above which weakness ensued.
Medication regimens and treatment responses (based on objective improvements in strength) were variable and are summarized in Supplementary Table 1. Of the 14 patients who were followed longitudinally, 9/14 (64%) had a complete or near-complete response to immunosuppression and 5/14 (36%) had a partial response to immunosuppression; this included one patient whose progressive muscle weakness was stabilized – but did not improve – with immunosuppression. 6 /14 (43%) of patients relapsed with tapering or withdrawal of immunosuppressive medications. 7/14 (50%) are currently being tapered from immunosuppressive medications and have not relapsed to date. Only one patient was completely tapered from immunosuppressive medications without a relapse of weakness.
Most patients had a very modest initial response to prednisone and required combination immunosuppressive therapy. Rituximab and intravenous immunoglobulin appeared to be helpful adjuncts when added to prednisone and azathioprine or methotrexate. Most patients required some dose of prednisone for maintenance therapy and reported weakness with steroid taper even if their initial response to prednisone was only modest.
Muscle biopsy features of the anti-200/100 patient population
16 /17 (94%) patients with anti-200/100 autoantibodies had muscle biopsies with prominent myofiber necrosis; the remaining patient’s biopsy was notable for extensive inflammatory infiltrates and the subsequent analysis does not include this biopsy. Although close examination revealed endomysial and/or perivascular collections of inflammatory cells in 5/16 (31%) of these muscle biopsies, the degree of inflammation was mild compared to that seen in typical muscle biopsies from polymyositis and dermatomyositis patients. No anti-200/100 biopsy revealed evidence of more than mild denervation and no biopsy was positive for abnormal glycogen accumulation or amyloid deposition.
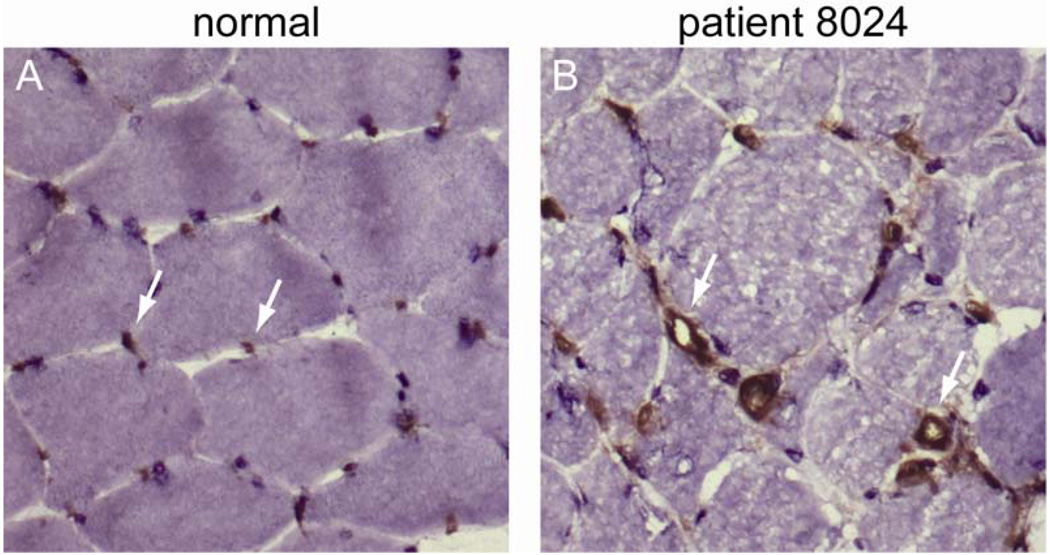
Of the 16 patients with necrotizing myopathies who were anti-200/100 positive, frozen muscle tissue from 8 was available for further analysis. To assess blood vessel morphology, sections were stained with anti-CD31 antibodies. Abnormally enlarged endomysial capillaries with thickened walls were found in 5/8 (63%) biopsy specimens (figure 2). However, the density of capillaries within muscle tissue was not noticeably reduced in any of the muscle biopsies.
Figure 2. Abnormal capillary morphology in anti-200/100 muscle biopsies.
A normal (A) and an anti-200/100 (B) muscle biopsy specimen were stained with anti-CD31, an endothelial cell marker. Arrows indicate endomysial capillaries with normal morphology in the control specimen (A) and those with thickened walls and dilated lumens in a patient with anti-200/100 autoantibodies (B). These biopsy specimens were processed simultaneously under identical conditions.
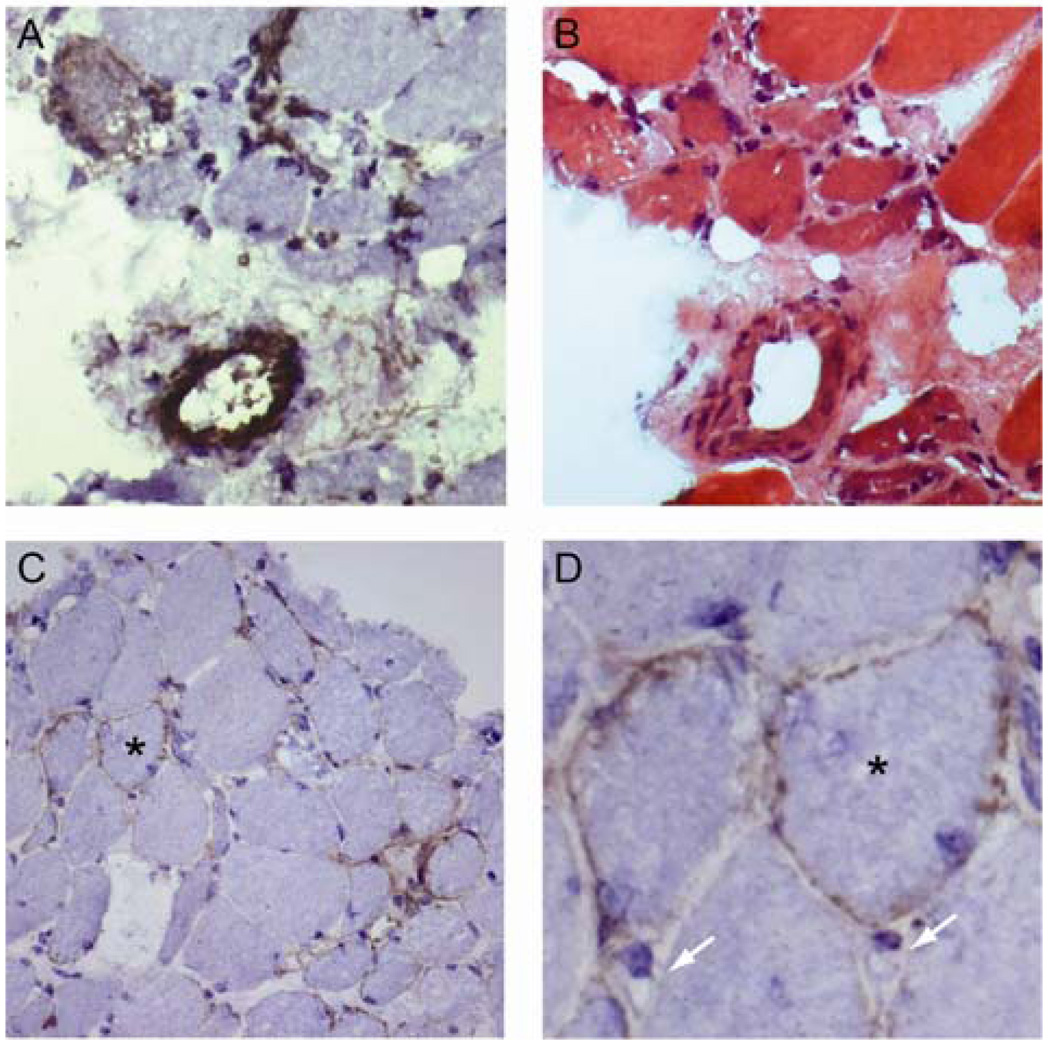
Complement deposition was evaluated by staining the available anti-200/100 muscle biopsy specimens with antibodies recognizing the MAC. Although endomysial capillaries were not definitively recognized by the antibody (figure 3D), in 6/8 (75%) muscle biopsy specimens, small perimysial vessels were stained (figure 3A and B.) In contrast, blood vessels from control muscles did not stain intensely with MAC antibodies (data not shown). As expected, MAC deposition was also present on necrotic and degenerating myofibers; this is considered a non-specific finding. However, in 4/8 (50%) of the anti-200/100 muscle biopsies, the sarcolemmal surfaces of scattered, non-necrotic muscle fibers stained positive for MAC (figure 3C and D); as shown, some of these muscle cells were relatively small, suggesting they could be regenerating fibers.
Figure 3. Membrane attack complex deposition on small blood vessels and non-necrotic myofibers.
Serial sections from an anti-200/100 necrotizing myopathy muscle biopsy (from patient 8076) stained with anti-MAC (A) or H&E (B) demonstrate a perimysial blood vessel with marked complement deposition. Another anti-200/100 muscle specimen (from patient 8024) shows MAC deposition on scattered non-necrotic fibers (C); a higher power detail from the same field is also shown (D; asterisks mark matching myofibers). Note the absence of MAC staining on endomysial capillaries (D; white arrows.)
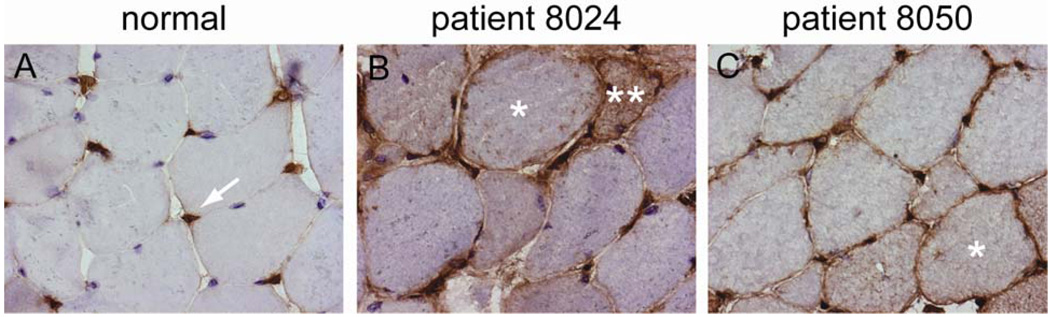
Staining of anti-200/100 muscle biopsies with antibodies recognizing MHC I showed that the sarcolemma of 4/8 (50%) were clearly MHC I positive (figure 4). Several others had borderline MHC I staining, but this appeared markedly less intense than that seen in the muscle biopsy specimens from Jo-1 positive polymyositis biopsies included as positive controls in the same experiment (data not shown).
Figure 4. MHC I deposition on non-necrotic fibers in anti-200/100 biopsies.
The endomysial capillaries of normal human muscle stain with anti-MHC I antibodies (arrow), but the sarcolemma do not (A). In contrast, the sarcolemma of scattered muscle fibers in two anti-200/100 patients (B and C; single asterisks) are stained by anti-MHC I. The cytoplasm of an anti-200/100 fiber also stains with anti-MHC I (B; double asterisk); this likely represents a regenerating fiber. These biopsy specimens were processed simultaneously under identical conditions.
DISCUSSION
The autoimmune myopathies (referred to collectively as “myositis”) are a family of conditions characterized clinically by symmetric proximal muscle weakness, elevated serum CK levels, and myopathic findings on EMG (10, 11). While other muscle conditions can cause similar clinical syndromes, diagnosing an autoimmune disorder carries important therapeutic and prognostic implications since only these routinely respond to immunosuppressive therapy.
As with other systemic autoimmune diseases, a strong association of autoantibodies with distinct clinical phenotypes is found in patients with autoimmune myopathy. For example, autoantibodies directed against aminoacyl-tRNA synthetases are the most frequent myositis-specific antibodies (MSAs) and are found in ~ 20% of myositis patients (12). These, and autoantibodies recognizing other tRNA synthetases, are associated with a specific constellation of clinical features including interstitial lung disease, Raynaud’s phenomenon, arthritis, and a characteristic cutaneous finding known as mechanic’s hands (13, 14). Although autoantibody screening can play a significant role in the diagnosis of immune-mediated muscle disease, such antibodies are not always found.
The presence of inflammatory infiltrates in muscle biopsy specimens is another well-recognized feature of the autoimmune myopathies (10). However, muscle biopsies from some patients with autoimmune myopathies contain few, if any, inflammatory cell infiltrates. For example, patients with MSAs directed against components of the SRP have biopsies notable for degenerating, necrotic, and regenerating muscle cells without extensive inflammatory cell infiltrates (3–6). We hypothesized that patients with otherwise undiagnosed necrotizing myopathies might also have unique autoantibodies that could be used for diagnosis.
Among a group of 225 myopathic patients, we found that 38 had muscle biopsies with predominantly necrotizing myopathies. Of these, 12 patients could be definitively diagnosed with specific conditions after extensive laboratory testing; these were largely anti-SRP or anti-synthetase myositis patients. We screened the sera of the remaining 26 patients for the presence of novel autoantibodies and found that 16 of these immunoprecipitated a pair of proteins with approximate molecular weights of 200 and 100 kDa. In addition, among the other 187, one patient with a biopsy showing abundant inflammatory cell infiltrates shared this immunospecificity. The patients with anti-200/100 antibodies did not have other known autoantibodies, including anti-SRP. Thus, anti-200/100 antibodies characterize a unique subset of myopathic patients, representing 16/26 (62%) of our patients with idiopathic necrotizing myopathies.
In many respects, the clinical features of patients with the anti-200/100 immunospecificity are similar to those with other forms of immune-mediated myopathy; they typically experienced the sub-acute onset of proximal muscle weakness with elevated CK levels, irritable myopathic findings on EMG, evidence of edema on MRI, and, in most cases, a clear response to immunosuppressive therapy. However, we did note a number of unique features of the anti-200/100 patients. First, several had very high CK levels (in the 3000–8000 range) with only minimal muscle weakness. This suggests either (a) an unusual capacity of these patients to regenerate muscle with sufficient efficiency to keep pace with extensive muscle destruction or (b) that these patients have a muscle membrane abnormality which allows a leakage of CK without causing weakness; such an abnormality could be consistent with the finding of MAC deposition on the sarcolemma of non-necrotic muscle fibers. Second, in more than 60% of these patients, exposure to statin therapy preceded the development of muscle symptoms and persisted long after the myotoxin was discontinued. Importantly, we found that this association was strongest in older patients; almost 90% of anti-200/100 positive patients age 50 or older had been exposed to statins. This was significantly higher than the rates of statin use in age-matched groups of PM, DM, and IBM patients.
Although the anti-200/100 patients share certain features with the well described anti-SRP population, two key findings distinguish these groups as distinct. First, anti-200/100 sera did not recognize any of the SRP subunits and anti-SRP sera did not recognize proteins with molecular weights at 200 or 100 kDa. These observations demonstrate that patients with the anti-200/100 specificity are immunologically distinct from the anti-SRP patient population. Second, we followed several anti-200/100 patients who had extremely high CK levels and only minimal weakness; in our experience and in other reports (3–6), anti-SRP patients with similarly high CK levels are uniformly very weak.
To further characterize the muscle disease in anti-200/100 patients, we stained muscle biopsy specimens with antibodies against MAC, endothelial cell markers, and MHC I. MAC deposition represents the end-stage of the complement cascade and may indicate that the tissue is targeted for destruction by the immune system. The deposition of MAC on endomysial capillaries has been shown in patients with dermatomyositis (15, 16) and in three of four analyses of anti-SRP biopsies (3–6); this does not occur in muscular dystrophies (17). Although we did not demonstrate MAC deposition on endomysial capillaries in anti-200/100 biopsies, in 5/8 endomysial capillaries were abnormally thickened and enlarged. Similar morphologic abnormalities have been described in both anti-SRP patients and in a group of patients with “necrotizing myopathy with pipestem capillaries.” Although the latter group shares some pathological features with anti-200/100 and anti-SRP patients, these patients differed in having either another connective tissue disease or active cancer (18).
Despite its absence on capillaries, 6/8 (75%) anti-200/100 biopsies had evidence of MAC deposition in small perimysial blood vessels. We hypothesize that deposition of complement in these cases may reflect a novel vascular target in our population. In addition, MAC localized to the surface of non-necrotic fibers was noted in 4/8 (50%) of the anti-200/100 biopsies we analyzed. Although the presence of MAC on non-necrotic fibers has previously been reported in immune-mediated myopathies (19), this is not general feature of these disorders; in multiple studies of anti-SRP myopathy, MAC was found on non-necrotic fibers in only 1/7 (3), 0/6 (5), and 1/3 (6) muscle biopsies. It should be noted that MAC deposition on non-necrotic myofibers has also been reported to occur in some dystrophies (17) and that MAC deposition on blood vessels and muscle fibers may be secondary to membrane damage rather than a primary pathologic event.
Finally, 4/8 of the available biopsy specimens included myofibers with sarcolemmal MHC I staining. This is a characteristic feature of immune-mediated myopathies and is rare or absent in biopsies from patients with muscular dystrophies and other muscle and nerve disorders (20, 21). By comparison, results of studies evaluating MHC I staining in anti-SRP patients have been mixed; one study noted MHC I positive fibers in 2/3 patients (6), a second in 3/6 patients (3), and a third in 0/6 patients (5).
Interestingly, two recent reports describe patients who developed a necrotizing myopathy with statin use that progressed despite discontinuation of the myotoxic medication (22, 23). In the larger of the two series, Grable-Esposito et al. described 25 patients who developed an apparently immune-mediated, statin-associated necrotizing myopathy that shares many clinical features with our cohort of anti-200/100 patients (23). For example, these patients had proximal muscle weakness, included men and women in almost equal numbers, had a mean CK level of 8203 IU/L, required multiple immunosuppressive medications to achieve improved strength, and relapsed with tapering of immunosuppressive medications. The muscle biopsies from 8 similar patients were analyzed in detail by Needham and colleagues (22). Whereas 8/8 of the biopsies they studied had increased MHC-I expression on the surface of non-necrotic muscle fiber, only 4/8 anti-200/100 biopsies were positive for MHC-I. Furthermore, in contrast to our findings in anti-200/100 patients, they did not find MAC deposition on the surface of non-necrotic muscle fibers in any of the cases they studied. Although the reasons for these discrepancies remain to be elucidated and the autoantibody profiles of the patients in these previous studies were not defined, we suspect that the cohorts of patients described by these two groups may represent a subset of the anti-200/100 population, the majority of whom also developed an immune-mediated myopathy after starting a statin medication.
In conclusion, we have identified a group of patients with a necrotizing myopathy and a novel anti-200/100 autoantibody specificity. Interestingly, development of this phenotype is associated with exposure to statin medications. In addition to the presence of autoantibodies, all of the patients responded to immunosuppression and many flared when this was tapered; this supports our hypothesis that this is an immune-mediated myopathy. The presence MHC I on the surface of non-necrotic fibers also suggests that this process is immune-mediated. Indeed, we propose that those patients with necrotizing myopathies and anti-200/100 autoantibodies most likely have an autoimmune disease that should be treated with immunosuppressive medication. Future work will be directed towards identifying the autoantigens targeted by the anti-200/100 antibodies; based on the observation that these proteins were always immunoprecipitated together, we anticipate they may be members of a protein complex.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Jennifer Mammen for critical review of the manuscript and Tonie Hines for expert technical assistance.
Dr. Christopher-Stine’s work is supported by the NIH (grant K23-AR-053197). Dr. Casciola-Rosen’s work was supported by the NIH (R01-AR-044684). Dr. Mammen’s work was supported by the Passano Foundation and the NIH (grant K08-AR-054783). These studies were also supported by the Dorothy and Donald Stabler Foundation.
REFERENCES
- 1.Targoff IN. Autoantibodies and their significance in myositis. Curr Rheumatol Rep. 2008 Aug;10(4):333–340. doi: 10.1007/s11926-008-0053-2. [DOI] [PubMed] [Google Scholar]
- 2.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990 Sep;33(9):1361–1370. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- 3.Miller T, Al-Lozi MT, Lopate G, Pestronk A. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002 Oct;73(4):420–428. doi: 10.1136/jnnp.73.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 2004 Jan;50(1):209–215. doi: 10.1002/art.11484. [DOI] [PubMed] [Google Scholar]
- 5.Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006 Dec;65(12):1635–1638. doi: 10.1136/ard.2006.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitri D, Andre C, Roucoules J, Hosseini H, Humbel RL, Authier FJ. Myopathy associated with anti-signal recognition peptide antibodies: clinical heterogeneity contrasts with stereotyped histopathology. Muscle Nerve. 2007 Mar;35(3):389–395. doi: 10.1002/mus.20693. [DOI] [PubMed] [Google Scholar]
- 7.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975 Feb 20;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 9.Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995 Nov;38(5):705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 10.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003 Sep 20;362(9388):971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 11.Mammen AL. Dermatomyositis and polymyositis: Clinical presentation, auto-antibodies, and pathogenesis. Ann N Y Acad Sci. doi: 10.1111/j.1749-6632.2009.05119.x. in press. [DOI] [PubMed] [Google Scholar]
- 12.Targoff IN. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002 Nov;28(4) doi: 10.1016/s0889-857x(02)00032-7. 859,90, viii. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Akizuki M, Mimori T, Yamagata H, Inada S, Homma M. The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. A marker for a subset of polymyositis with interstitial pulmonary fibrosis. Arthritis Rheum. 1983 May;26(5):604–611. doi: 10.1002/art.1780260505. [DOI] [PubMed] [Google Scholar]
- 14.Marguerie C, Bunn CC, Beynon HL, Bernstein RM, Hughes JM, So AK, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990 Oct;77(282):1019–1038. doi: 10.1093/qjmed/77.1.1019. [DOI] [PubMed] [Google Scholar]
- 15.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986 Feb 6;314(6):329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- 16.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990 Apr;27(4):343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- 17.Spuler S, Engel AG. Unexpected sarcolemmal complement membrane attack complex deposits on nonnecrotic muscle fibers in muscular dystrophies. Neurology. 1998 Jan;50(1):41–46. doi: 10.1212/wnl.50.1.41. [DOI] [PubMed] [Google Scholar]
- 18.Emslie-Smith AM, Engel AG. Necrotizing myopathy with pipestem capillaries, microvascular deposition of the complement membrane attack complex (MAC), and minimal cellular infiltration. Neurology. 1991 Jun;41(6):936–939. doi: 10.1212/wnl.41.6.936. [DOI] [PubMed] [Google Scholar]
- 19.Oxenhandler R, Hart MN, Bickel J, Scearce D, Durham J, Irvin W. Pathologic features of muscle in systemic lupus erythematosus: a biopsy series with comparative clinical and immunopathologic observations. Hum Pathol. 1982 Aug;13(8):745–757. doi: 10.1016/s0046-8177(82)80298-0. [DOI] [PubMed] [Google Scholar]
- 20.van der Pas J, Hengstman GJ, ter Laak HJ, Borm GF, van Engelen BG. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2004 Jan;75(1):136–139. [PMC free article] [PubMed] [Google Scholar]
- 21.Sundaram C, Uppin MS, Meena AK. Major histocompatibility complex class I expression can be used as a diagnostic tool to differentiate idiopathic inflammatory myopathies from dystrophies. Neurol India. 2008 Jul–Sep;56(3):363–367. doi: 10.4103/0028-3886.43457. [DOI] [PubMed] [Google Scholar]
- 22.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007 Feb;17(2):194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010 Feb;41(2):185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.