Abstract
We previously reported that global deletion of insulin receptor substrate protein 1 (Irs1) extends lifespan and increases resistance to several age-related pathologies in female mice. However, no effect on lifespan was observed in male Irs1 null mice. We suggested at the time that the lack of any effect in males might have been due to a sample size issue. While such lifespan studies are essential to our understanding of the aging process, they are generally based on survival curves derived from single experiments, primarily due to time and economic constraints. Consequently, the robustness of such findings as a basis for further investigation has been questioned. We have therefore measured lifespan in a second, separate cohort of Irs1 null female mice, and show that, consistent with our previous finding, global deletion of Irs1 significantly extends lifespan in female mice. In addition, an augmented and completed study demonstrates lifespan extension in male Irs1 null mice. Therefore, we show that reduced IRS1-dependent signalling is a robust mechanism through which mammalian lifespan can be modulated.
Introduction
The precise mechanisms underlying the aging process in multicellular organisms are currently unknown. However, the insulin/insulin-like growth factor-1 (IGF-1) signalling (IIS) pathway and mammalian target of rapamycin (mTOR) signalling pathway are highly conserved candidates for protection against the effects of the ageing process [1], [2], [3], [4], [5], [6]. Reduced IIS extends lifespan in both invertebrate and vertebrate model organisms [2], [3], [7]. In addition, extended lifespan in growth hormone (GH)/GH-receptor deficient dwarf mice may act through IIS attenuation [8], and polymorphisms in several IIS and GH-related genes have been shown to correlate with human lifespan [9], [10], [11].
Recently, we reported that global deletion of insulin receptor substrate protein 1 (Irs1), a key downstream mediator of IIS, increased lifespan in female mice relative to wild type (WT) controls [12]. In addition, we demonstrated that this long life was accompanied by a resistance to several age-related pathologies including skin, bone and motor dysfunction and protection against age-related glucose intolerance [12]. However, no difference was reported in male Irs1 null (Irs1−/−) mice compared to wild-type (WT) controls. In our earlier paper [12] we suggested that this lack of a lifespan effect in males may be partly explained by the relatively small number of male Irs1 −/− mice used (n = 10), and/or by the fact that 3 individuals from this group were still alive at the time of publication. Interestingly, despite our previously reported lack of any lifespan effect in male Irs1 −/− mice, these animals, like females Irs1 −/− mice, were protected against late life pathologies including complete protection against the ulcerative dermatitis seen in aged male WT controls [12]. Male Irs1 −/− mice also were protected against an age-associated alteration in T cell populations (increase in memory T cells, decrease in naive T cells) [12].
As the majority of IIS mutant mouse aging studies are based on lifespan curves derived from single experimental cohorts, several authors (e.g. [13], [14]) have suggested that replication and validation of these experiments is necessary in order to use these models with confidence in our quest to understand the aging process. Indeed, significant effort and resources may be wasted if any model subsequently shows no repeatable retardation in aging. Ladiges et al. [14] recently reported that only 3 (Ames (Prop1df/df), Snell (Pit1dw/dw), growth hormone receptor knockout (GHR-KO)) out of 20 published long-lived mouse models showed consistent lifespan extension across separate studies. In pure IIS mutants the situation appears more ambiguous, as mice heterozygous for a null allele of insulin receptor substrate protein 2 (Irs2) were reported as being long-lived [15], but this finding was not replicated in a second study [16]. The reasons for this discrepancy between these two studies are currently unclear [16], but may be due to an atypical WT survival curve in the earlier study [15].
In the current study we examined lifespan in a second, independent cohort of female WT and Irs1−/− mice to determine whether our original finding was indeed repeatable. In addition, we present data from our completed male lifespan study which has been further augmented by 2 additional WT and 2 additional Irs1−/− littermates that were not included in the original study, but were derived from the same group of breeders as that of our original study.
Results and Discussion
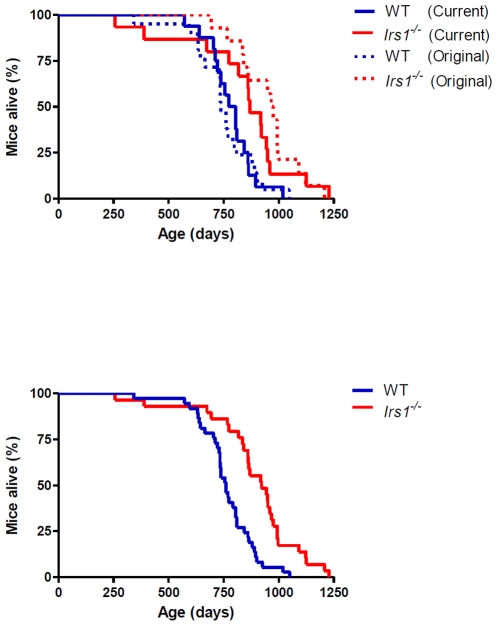
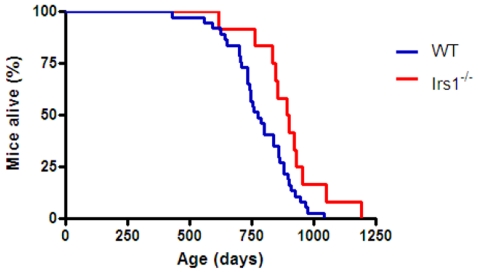
In agreement with our previous study [12], median lifespan was significantly increased (Log rank X2 = 4.916, P<0.05) in female Irs1−/− mice relative to WT controls (Fig. 1A (indicated by solid lines), Table 1). No difference in lifespan was observed within each genotype (i.e. WT vs. WT; Irs1−/− vs. Irs1−/−) between the current study and our original study [12] (Fig. 1A; WT, X2 = 0.020, P>0.05; Irs1−/−, X2 = 0.805, P>0.05). Cox regression analysis on combined data from both independent female studies (current vs. original study [12]) indicated that date of birth (Z = 1.522, P>0.05), parental identity (Z = 0.313, P>0.05) or experimental cohort (Z = 1.233, P>0.05) did not have any significant impact on lifespan. However, the effect of genotype on lifespan was highly significant (Z = 14.557, P<0.001). When the mortality data from both studies were combined (Fig. 1B; Table 1), median lifespan was again significantly higher (X2 = 16.480, P<0.0001) in female Irs1−/− mice compared to WT controls. Maximum longevity was also increased in this combined data set: the lifespan of the longest lived 10% of mice was significantly greater (X2 = 5.629, P<0.05) in Irs1−/− mice (1187±31 days) relative to WT animals (973±36 days). Median lifespan was also significantly increased (X2 = 5.059, P<0.05) in male Irs1−/− mice relative to WT controls (Fig. 2; Table 2).
Figure 1. Female Irs1−/− mice are significantly long-lived.
Kaplan-Meier survival curves for female WT and Irs1−/− mice from this current study (Solid lines; under husbandry conditions exactly as previously described [12], [16]) and our original study (Stippled lines; [12]). Blue and red lines indicate WT and Irs1−/− mice respectively (A). Kaplan-Meier survival curves for the combined data for female WT and Irs1−/− mice from this current study and our original study ([12]). Blue and red lines indicate WT and Irs1−/− mice respectively (B).
Table 1. Comparative survival characteristics of female wild type (WT) and Irs1−/− mice.
| Genotype | Median | Mean | Range | N | |
| Current study | WT | 789 | 782±27 | 572–1019 | 16 |
| Irs1−/− | 869 | 837±64 | 257–1228 | 15 | |
| Original study | WT | 738 | 748±32 | 343–1049 | 21 |
| Irs1−/− | 971 | 950±38 | 693–1207 | 14 | |
| Combined | WT | 760 | 763±21 | 343–1049 | 37 |
| Irs1−/− | 922 | 891±39 | 257–1228 | 29 |
Lifespan is reported in days (± s.e.m., where appropriate) for WT and Irs1−/− mice from this current study and our original study [12]. Combined = Combined lifespan data derived from current study and original study. N = sample size.
Figure 2. Male Irs1−/− mice are significantly long-lived.
Kaplan-Meier survival curves for male WT and Irs1−/− mice. Blue and red lines indicate WT and Irs1−/− mice respectively.
Table 2. Comparative survival characteristics of male wild type (WT) and Irs1−/− mice.
| Genotype | Median | Mean | Range | N |
| WT | 775 | 786±21 | 432–1042 | 37 |
| Irs1−/− | 896 | 897±41 | 619–1192 | 12 |
Lifespan is reported in days (± s.e.m., where appropriate) for WT and Irs1−/− mice. N = sample size.
In conclusion, we replicate and validate our previous finding [12] that global deletion of Irs1 induces a robust lifespan extension in female mice. Therefore, we now clearly show that, in addition to the GH deficient dwarf mice [17], [18], [19], [20], [21], [22], female Irs1−/− mice also show a highly consistent and repeatable lifespan extension across distinct studies. In common with the Ames dwarf mouse lifespan studies [17], [18], the two separate lifespan studies in female Irs1−/− mice were undertaken in the same vivarium, albeit at different times. We suggest that replication of survival data, such as ours and those of the GH dwarfs [17], [18], [19], [20], [21], [22], will significantly help increase confidence in exactly what genetically modified mice are valid models of retarded aging [13], [14]. In turn, this confidence should focus time and resources on those interventions that will ultimately help us to understand what mechanisms underlie healthy aging in humans. It should be noted that one additional limitation in mouse longevity studies is that they are generally performed in a single genetic background, e.g. C57BL/6. We suggest that it will be highly informative for future studies to examine longevity in mice, such as Irs1 nulls, across different genetic backgrounds.
We also show that, in contrast to our earlier study [12], a completed and augmented lifespan study in male mice indicates that median lifespan is extended in male Irs1−/− mice by ∼16%. This new finding suggests that the lack of any effect was probably due to an incomplete and slightly underpowered earlier study in males [12], with larger sample sizes undoubtedly helping to detect relatively subtle changes between genotypes [23]. This robust lifespan extension across genders in Irs1−/− mice is consistent with findings reported for other manipulations including dietary restriction [24], [25], rapamycin treatment [6] and GH/GH-receptor deficiency [8], [23], but is not seen in other sufficiently powered longevity studies (e.g. [26]). We suggest that these new findings indicate that global deletion of IRS1 is a ‘replicable and robust phenomenon’ [13] that extends lifespan in both female and male mice. Therefore, we suggest that this model can be used with confidence to explore the mechanisms underlying healthy ageing in mammals.
Materials and Methods
Ethics statement
All efforts were made to ameliorate suffering following previously described husbandry protocols (see [12]). The experimental procedures described were carried out following local animal ethical committee review (University College London, London UK.), following guidelines set out by 1986 UK Home Office Animal Procedures Act under the Home Office Licence PPL70/6648.
Animal husbandry
Mice were maintained under exactly the same experimental conditions and protocols as previously described [12], [16], [26]. In brief, Irs1 −/− and wild-type (WT; Irs1+/+) control littermates were generated from heterozygote parents maintained on a C57BL/6 background following 10 backcrosses. Mice were housed in groups of three to eight same-sex littermates at ∼22°C and on a 12-h light/dark cycle (lights on from 0700–2100hrs) under specific pathogen-free conditions [12], [16], [26]. Mice had ad libitum access to chow [2018 Teklad Global (5% fat, 18% protein, 57% carbohydrate, and 20% other components) Rodent Diet; Harlan Teklad, Bicester, Oxfordshire, UK] and water. Kaplan-Meier survival curves were constructed using known birth and death dates (see [12], [16], [26]) and the log-rank test used to evaluate statistical differences between genotypes. Cox regression analysis was employed to determine whether date of birth, parental identity, genotype or experimental cohort (experiment 1 [12] or current experiment) influenced lifespan significantly in female mice. Maximum life span was calculated as mean age of the oldest 10% of mice per genotype. The three Irs1 −/− male mice that were alive and censored at 756, 756 and 828 days of age in the original study [12] lived until 995, 933 and 1192 days of age respectively. The 2 WT control males alive and censored at 756 and 993 days of age in the original study [12] lived until 968 and 1042 days of age respectively. The age at death of the two additional Irs1 −/− male littermates used in this current study were 763 and 1051 days of age and their WT control littermates were 734 and 736 days of age.
Acknowledgments
We are grateful to Steve Lingard for his help and input throughout this project. We also thank Scott Pletcher and Alex Douglas for statistical advice, and thank the Biological Services Unit staff, University College London, U.K. for animal care.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Wellcome Trust (Functional Genomics and Strategic) awards to L.P. and D.J.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Piper MD, Selman C, McElwee JJ, Partridge L. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J Intern Med. 2008;263:179–191. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 3.Selman C, Withers DJ. Mammalian models of extended healthy lifespan. Philos Trans R Soc Lond B Biol Sci. 2010 (in press) doi: 10.1098/rstb.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: The role of the insulin/Igf and TOR signalling network. Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 8.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonafe M, Olivieri F. Genetic polymorphism in long-lived people: cues for the presence of an insulin/IGF-pathway-dependent network affecting human longevity. Mol Cell Endocrinol. 2009;299:118–123. doi: 10.1016/j.mce.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. Faseb J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 13.Austad S. Advances in vertebrate aging research 2007. Aging Cell. 2008;7:119–124. doi: 10.1111/j.1474-9726.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 14.Ladiges W, Van Remmen H, Strong R, Ikeno Y, Treuting P, et al. Lifespan extension in genetically modified mice. Aging Cell. 2009;8:346–352. doi: 10.1111/j.1474-9726.2009.00491.x. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 16.Selman C, Lingard S, Gems D, Partridge L, Withers DJ. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science. 2008;320:1012; author reply 1012. doi: 10.1126/science.1152366. [DOI] [PubMed] [Google Scholar]
- 17.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, et al. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 18.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 19.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1 (dw) can increase lifespan in mice. Mech Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 20.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, et al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 23.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, et al. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]