Abstract
When ejaculates from rival males compete for fertilization, there is strong selection for sperm traits that enhance fertilization success. Sperm quantity is one such trait, and numerous studies have demonstrated a positive association between sperm competition and both testes size and the number of sperm available for copulations. Sperm competition is also thought to favor increases in sperm quality and changes in testicular morphology that lead to increased sperm production. However, in contrast to sperm quantity, these hypotheses have received considerably less empirical support and remain somewhat controversial. In a comparative study using the Australian Maluridae (fairy-wrens, emu-wrens, grasswrens), we tested whether increasing levels of sperm competition were associated with increases in both sperm quantity and quality, as well as an increase in the relative amount of seminiferous tubule tissue contained within the testes. After controlling for phylogeny, we found positive associations between sperm competition and sperm numbers, both in sperm reserves and in ejaculate samples. Additionally, as sperm competition level increased, the proportion of testicular spermatogenic tissue also increased, suggesting that sperm competition selects for greater sperm production per unit of testicular tissue. Finally, we also found that sperm competition level was positively associated with multiple sperm quality traits, including the proportion of motile sperm in ejaculates and the proportion of both viable and morphologically normal sperm in sperm reserves. These results suggest multiple ejaculate traits, as well as aspects of testicular morphology, have evolved in response to sperm competition in the Australian Maluridae. Furthermore, our findings emphasize the importance of post-copulatory sexual selection as an evolutionary force shaping macroevolutionary differences in sperm phenotype.
Introduction
When females copulate with multiple males during a single reproductive episode, sperm from these males compete to fertilize the female's ova in a process known as sperm competition [1]. Sperm competition is a powerful selective force that favours male traits that maximize competitive fertilization success. Across a diverse range of taxa, comparative and experimental studies have demonstrated that a common evolutionary response to sperm competition is an increase in testes size [2]–[7], [ see also 8]. Indeed, relative testis size is often used as a measure of sperm competition [e.g. 9]–[12]. Furthermore, inter- and intra-specific studies suggest that sperm competition is positively associated with greater numbers of sperm (i.e. sperm reserves or ejaculate size) [5], [13]–[15]. This is at least partially because larger testes produce more sperm [16]–[20]. However, in addition to testes size, sperm competition may select for increases in sperm production: species under higher sperm competition have a greater proportion of sperm-producing tissue within the testes [21], [22]. Currently, however, there is limited empirical data concerning testis morphology and additional studies are clearly warranted in order to more fully understand the links between sperm numbers, sperm production and sperm competition.
Sperm competition is also thought to favor a range of sperm phenotypic traits that influence the fertilizing capability of an ejaculate [23], [24]. For example, sperm motility influences paternity success in a range of taxa (e.g. birds [25]–[27], fish [28], [29], mammals [30]) and, across species, there is a positive association between the intensity of sperm competition and sperm swimming speed (birds [31], [ but see 32], fish [33]). Additionally, sperm competition is associated with changes in sperm design (e.g. morphology) and function (e.g. sperm energetics) that influence swimming velocity [34]–[36]. More generally, sperm competition appears to be associated with sperm size, though comparative studies have found both positive [e.g. 12], [ 31], [ 37], [ 38] and negative [e.g. 5], [ 12] associations, as well as no association [e.g. 12], [ 39], between these traits [reviewed in 23]. Thus, in contrast to studies of testes size and sperm numbers, the effects of sperm competition on sperm phenotype remain relatively unresolved. In particular, studies on sperm viability are almost entirely lacking; though at least in insects, sperm viability has been shown to influence competitive fertilization success [40], and polyandrous species have been shown to have a greater proportion of viable sperm available for ejaculates relative to monadrous species [41]. Consequently, further studies of sperm quality traits are needed to determine how sperm competition shapes inter-specific variation in sperm phenotype.
Distributed throughout New Guinea and Australia, the Maluridae are a family of passerine birds comprised of 27 species across five genera. In Australia, the malurid genera include the fairy-wrens (Malurus), emu-wrens (Stipiturus) and grasswrens (Amytornis) [42], [43]. Australian malurids are relatively small passerines (5 to 40 grams) and species tend to be similar in general morphology, life-history and ecology [42], [44]–[46]. Importantly, all species of Australian Maluridae are known or believed to be socially monogamous, with males and females forming long-term social pair bonds, but engage in extra-pair copulations [42].
The Australian malurids have become model system for the study of reproductive promiscuity because some species in this group show extremely high rates of extra-pair paternity (EPP; e.g. M. cyaneus [47]). In addition, the five species for which paternity data are available show remarkable variation in EPP rate, with between 5.8% and 95% of broods in a population containing one or more extra-pair offspring, and EPP accounting for as little as 4.4% or as much as 76% of all young in a population [46]–[52]. These differences suggest that species experience a broad range of sperm competition levels. Consistent with this idea, relative testis size is highly variable in this group, ranging from less than 1% to more than 6% of male body mass [46], [53]. In birds, the incidence of EPP varies across species from 0 to ∼76% of offspring (with values <5% considered to be unusual [54], [55]) and testes mass ranges from 0.01% to more than 9% of male body mass [11]. Thus the range of sperm competition levels observed in the Australian malurids reflects those observed across avian species generally, making them an ideal system for studying the evolutionary consequences of sperm competition for male reproductive biology.
In this study, we tested whether increasing levels of sperm competition were associated with variation in testis morphology and sperm quantity and quality using data from eight species of Australian Maluridae. Specifically, we used a phylogenetically-controlled, comparative approach to determine the relationship between sperm competition and the number of sperm in sperm reserves, the number of sperm in ejaculates, the proportion of motile sperm in ejaculates, the proportion of morphologically normal sperm and the proportion of viable sperm in sperm reserves, and the proportion of spermatogenic tissue, relative to interstitial tissue, contained within the testes. Importantly, all samples were collected and analyzed by a single individual specifically for this study. Thus, our study avoids some of the problems that confound other studies that rely on data gleaned from the literature, where collection methods may vary and inter-individual differences in measurement may lead to uncertain and sometimes erroneous results.
Materials and Methods
Ethical statement
All work was undertaken with approval from the University of Chicago Animal Care and Use Committee (ACUP#71453), the Department of Environment and Heritage (South Australia) Wildlife Ethics Committee (Project No. 13/2004; Scientific Permit Q24832; AW licence No. 142), the Director-General of New South Wales Department of Primary Industries Animal Care and Ethics Committee (Trim File No. 06/3846; NSW NPWS scientific licence S12048), James Cook University Animal Ethics Review Committee (approval #A1004), and the Environmental Protection Agency (EPA) of Queensland. Finally, export of samples from Australia was approved by the Australian Government Department of Environment and Heritage (WT2005-10120 and WT2006-10958).
Study species and general field methods
Eight species of Australian Maluridae were studied over a three-year period (2004–2006). Species included the superb (M. cyaneus cyanochlamys), splendid (M. splendens melanotus), variegated (M. lamberti assimilis), blue-breasted (M. pulcherrimus), white-winged (M. leucopterus leuconotus), and red-backed fairy-wrens (M. melanocephalus), the southern emu-wren (S. malachurus malachurus), and the striated grasswren (A. striatus striatus). Populations were studied at several sites throughout southern and eastern Australia: superb fairy-wrens were studied at Murray River National Park, South Australia (140°32′E, 34°20′S); splendid, white-winged, and variegated fairy-wrens were studied at Brookfield Conservation Park, South Australia (139°29′E, 34°20′S); blue-breasted fairy-wrens were studied at Lincoln National Park, South Australia (135°52′E, 34°52′S); red-backed fairy-wrens were studied at Moomin Reservoir and Kalinvale Farm, near Herberton, Queensland (145°23′E, 17°23′S); southern emu-wrens were studied near Smith's Lake, New South Wales (152°28′E, 32°22′S); and striated grasswrens were studied at Pooginook and Cooltong Conservation Parks, near Berri. South Australia (140°35′E, 34°16′S).
Birds were captured in mist nets set on their home territory. Upon capture, birds were weighed to the nearest 0.1 g using a Pesola spring balance. Additionally, the length (L; measured from the anterior edge of the cloacal vent to the posterior edge of the protuberance, thus excluding the cloacal tip), width (W) and depth (D) of the cloacal protuberance was measured and cloacal protuberance volume was estimated as volume = π(D/2×W/2)×L [56]. All CP measurements were taken by one of us (MR) to minimize sampling error. We included data only from males in breeding condition, indicated by behavioral, morphological or physiological characteristics (e.g. courtship displays, breeding plumage, enlarged CP, active spermatogenesis).
Testes size and morphology
We collected three male striated grasswrens, 17 male red-backed fairy-wrens and six males of each of the following species: superb, splendid, variegated, blue-breasted, and white-winged fairy-wrens and the southern emu-wren. We quantified fresh testes weight (wet mass) for both the left and right testis to the nearest 0.01 g using an electronic balance (Ohaus Navigator) and calculated the combined testes mass (CTM) as the sum of the left and right testis mass. We also calculated the gonadosomatic index (GSI), where GSI = (combined gonad weight/body weight)×100 [57]. Testes were then fixed in 10% neutral buffered formalin and transferred to 70% ethanol for transport and later histological work.
We examined the relative proportion of sperm producing tissue, compared to interstitial tissue, in testes using standard histological techniques and image analysis. Following fixation, we dehydrated and cleaned each testis via a series of increasing alcohol concentrations (70%, 80%, 95%, 100%) and two changes of xylene, and then passed the tissue through four changes of infiltration paraffin (paraffin type 1, Richard-Allan Scientific) at 60°C. We then embedded testes in paraffin (paraffin type 9, Richard-Allan Scientific) and cut 5-µm thick sections using a microtome (HM315, Microm) and stained sections with haematoxylin-eosin. We captured digital images of four non-sequential sections of each testis using a Leitz Laborlux S compound light microscope (at 20× magnification), Spot insight camera (model 14.2) and Spot for mac (version 4.1.1) image capture software (Diagnostic Instruments, Inc). For each section, we measured the proportion of seminiferous tubule tissue (relative to interstitial tissue) using Image-J software and calculated the proportion of tissue per testis by averaging the values from the four sections. The total proportion of sperm producing tissue in the testes of an individual was calculated by averaging the values from the left and right testis.
Sperm quantity and quality
Sperm samples were collected using standard cloacal massage techniques [58]–[60]. Exuded semen was collected in 10 µl micro-capillary tubes, transferred to micro-centrifuge tubes containing a known volume of Lago Formulation Avian Semen Extender (Hygieia Biological Laboratories, Woodland, CA, USA), which is formulated to maintain sperm membrane integrity for a period of six hours or more, and mixed thoroughly. All ejaculate samples were collected and analyzed within three hours of collection using standard techniques by a single person (MR).
We measured sperm quantity as both as the number of sperm in ejaculates (i.e. sperm collected via cloacal massage) and the total number of sperm in the seminal glomera (i.e. sperm reserves). In the first instance, sperm density was determined in two aliquots of diluted semen using a calibrated Makler counting chamber (repeatability of counts within ejaculates: r = 0.93, P<0.0001; sensu [61]) and total sperm number was calculated by taking into account the sample dilution and the volume of semen recovered. Next, to quantify the number of sperm stored by each male, we first isolated the seminal glomera from males collected for testis size examination, measured the fresh weight (wet mass) of the left and right seminal glomerus to the nearest 0.01 g using an electronic balance (Ohaus Navigator), and calculated the combined seminal glomera mass as the sum of the left and right glomerus mass. Following this, sperm was flushed from each glomerus into a known volume of Lago Formulation Avian Semen Extender and sperm density, based on two aliquots (repeatability of counts within glomera: r = 0.95, P<0.0001; sensu [61]), and total count quantified using a Makler counting chamber. The total number of stored sperm was then calculated by summing the sperm count from the left and right glomerus and, when ejaculate samples were collected prior to dissection, the ejaculate sample sperm count. Finally, because CP size reflects sperm numbers [14], [55] and has been used as a proxy for sperm production in passerines [62], we include both CP volume and the mass of the seminal glomera as indirect measures of sperm quantity.
Measures of sperm quality were based on three sperm phenotypic traits. First, the proportion of motile sperm in ejaculates was estimated by visual examination [26], [63], [64]. Specifically, 10 µl of the diluted ejaculate was placed on a glass slide and the percentage of motile sperm (0–100%, in steps of 5%) assessed by examining several fields of view under phase contrast optics at 20 times magnification. Next, the viability and morphology of sperm from sperm reserves was assessed by examination of eosin-nigrosin stained sperm smears. Sperm viability was determined by recording the percentage of live (i.e. those excluding eosin) sperm [65]–[67], [ see also 68], [ 69]. For each male, we examined 100 sperm cells on each of two replicate smears (repeatability of viability counts: r = 0.83, P<0.0001; sensu [61]), for a total of 200 spermatozoa examined, and averaged these values to obtain a single metric of sperm viability. At the same time, sperm were categorized as either normal or abnormal (e.g. abnormal morphology of the head, midpiece or tail) to quantify the percentage of morphologically normal sperm in the sperm reserves of males [30], [59]. As for sperm viability, two replicate smears were examined and values averaged to provide a single measure of sperm morphology.
The measures we used were chosen instead of more elaborate assessment techniques (e.g. CASA) because many of our field sites lacked the necessary infrastructure for these methods. Furthermore, we chose metrics of sperm quality that appear to be less susceptible to change due to time since collection and temperature because many of our samples were collected from localities that prevented immediate assessment (i.e. birds were trapped long distances from roads and other locations were measures could be performed). For example, the percentage of motile sperm in ejaculates is reported to be stable for several hours after collection [26], [70], across a wide range of temperatures (e.g. 24–37°C, [71]).
Statistical analysis
We collected a total of 349 ejaculate samples from eight species over three years. Males that did not produce an ejaculate sample were not included in the analysis and, because individual males were often sampled over consecutive years or more than once within a season, each male is included only once in the analysis (3–87 males per species, for details of sample size for all traits see Supplementary Table 1). However, as not all parameters were successfully sampled for all individuals, sample sizes vary slightly for the different metrics of sperm quantity and quality. Non-normal data distributions were normalized using log (ln)-transformations (CTM, body mass), and all proportion data (proportion of interstitial tissue, proportion of motile, viable and morphologically normal sperm) were arcsine-transformed prior to analysis. All statistics were performed using the R (2.12.0) software package [72].
Because comparative studies can be confounded by non-independence of data as a result of common ancestry [73], we used a generalized least-squares approach in a phylogenetic framework [74], [75] implemented in the APE package [76]. Additionally, we tested for phylogenetic dependence of traits by estimating the phylogenetic scaling parameter λ, where values of λ close to 0 indicate phylogenetic independence, while values close to 1 indicate phylogenetic dependence. Finally, likelihood-ratio testes were used to compare the model with the maximum likelihood value of λ differed from models with λ values of 0 or 1. We used a phylogeny based on allozyme data [77], which has been recently confirmed using DNA evidence [78]. The striated grasswren, which was not present on this tree, was replaced with its congener the black grasswren (A. housei). Because branch length information was unknown, we assumed a punctuated model of evolution (i.e. set all branch lengths equal) for all analyses. We estimated sperm competition level by including both CTM and male body mass (both log-transformed) as independent variables in the multiple regression models.
Results
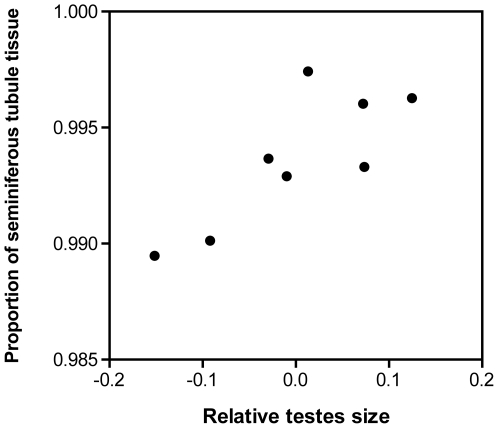
Across the eight species of Maluridae, mean CTM ranged from 0.05 to 0.34 g, or, when expressed as the gonadosomatic index, from 0.62 to 4.45% of male body mass (Table 1). Combined testes mass was not related to male body mass (r2 = 0.21, F = 1.60, P = 0.25, λ = 0.99). The testes were predominately comprised of densely packed spermatogenic tissue, with interstitial tissue generally accounting for less than 1% (range 0.26 to 1.01%) of total testicular material. The proportion of spermatogenic tissue in the testes was positively associated with the level of sperm competition (Fig. 1). Specifically, using a GLS multiple regression corrected for phylogeny, we found a significant association between the proportion of seminiferous tubule tissue in the testes and both CTM (t = 3.39, P = 0.019) and body mass (t = −3.88, P = 0.012). The estimated λ value for this model was 0.999, and the model including the maximum-likelihood estimate of λ was not significantly different from either the model with λ set to 0 (P = 0.12) or the model with λ set to 1 (P = 1).
Table 1. Descriptive statistics of testis morphology and sperm quantity in eight species of Australian Maluridae.
| Superb Fairy-wren | Splendid Fairy-wren | Variegated Fairy-wren | Blue-breasted Fairy-wren | White-winged Fairy-wren | Red-backed Fairy-wren | Southern Emu-wren | Striated Grasswren | |
| Body mass (g) | 8.93±0.22 (6) | 9.45±0.06 (94) | 8.29±0.12 (33) | 9.27±0.14 (15) | 7.7±0.12 (13) | 7.57±0.05 (76) | 7.32±0.11 (6) | 19.23±0.27 (3) |
| CTM (g) | 0.29±0.04 | 0.30±0.02 | 0.18±0.01 | 0.13±0.02 | 0.33±0.02 | 0.21±0.01 | 0.045±0.004 | 0.34±0.009 |
| GSI | 3.39±0.48 | 3.56±0.25 | 2.38±0.15 | 1.49±0.24 | 4.45±0.23 | 2.95±0.18 | 0.62±0.06 | 1.81±0.04 |
| Sperm tissue | 99.60±0.11 | 99.33±0.09 | 99.37±0.05 | 99.01±0.25 | 99.63±0.06 | 99.74±0.06 | 98.99±0.12 | 99.29±0.03 |
| CP volume (mm3) | 105.08±11.8 (6) | 96.99±3.27 (94) | 36.44±3.15 (34) | 31.39±3.36 (14) | 86.96±8.63 (13) | 128.24±3.36 (76) | 0 (6) | 94.15±9.47 (3) |
| SG mass (g) | 0.09±0.007 (6) | 0.09±0.01 (6) | 0.05±0.006 (5) | 0.04±0.01 (5) | 0.09±0.006 (6) | 0.05±0.004 (16) | 0.01±0.002 (6) | 0.05±0.01 (3) |
| Sperm reserves (×106) | 318.69±53.3 (6) | 276.47±9.48 (6) | 161.82±29.4 (5) | 94.55±23.45 (5) | 276.66±16.7 (6) | 195.0±0.01 (16) | 12.58±4.59 (6) | 42.46±6.14 (3) |
| Ejaculate size (×106) | 33.36±8.96 (6) | 48.37±3.82 (87) | 8.54±1.64 (24) | 15.16±2.49 (15) | 45.69±5.15 (13) | 36.13±3.0 (76) | 0* (6) | 6.56±2.18 (3) |
CTM = combined testes mass, GSI = gonadosomatic index.
Cloacal protuberance (CP) volume and seminal glomera (SG) mass represent indirect measures of sperm quantity, while the number of sperm in sperm stores (sperm reserves) and the number of sperm in ejaculate samples (ejaculate size) represent direct measures of sperm quantity.
Species differed significantly in all four measures of sperm quantity: CP volume (ANOVA: F 7,238 = 43.66, P<0.001), SG mass (ANOVA, F 7,45 = 20.96, P<0.001), sperm reserves (ANOVA, F 7,46 = 18.94, P<0.001), and ejaculate size (ANOVA, F 6,217 = 8.87, P<0.001).
*Ejaculate samples were not collected from the southern emu-wren due to logistical problems during the 2006 field season.
Sample sizes for all traits are shown in Supplementary Table 1.
Figure 1. Relationship between relative testes size and the proportion of spermatogenic tissue contained within the testes in eight species of Australian Maluridae.
Figure is not controlled for phylogeny (unlike analysis) and relative testes mass indicates the use of residual values from a linear regression of testis mass on body mass. Each data point represents a species. For further statistical details see main text.
The four measures of sperm quantity differed significantly across the species (Table 1). After controlling for phylogeny, we found a significant association between sperm competition level and both indirect measures of sperm quantity: CP volume and seminal glomera mass. In a model including both CTM and body mass, both CP volume and the mass of the seminal glomera were independent of body mass, but covaried positively with CTM (Table 2a). Similarly, we found a positive association between the number of sperm in the seminal glomera (i.e. sperm reserves) and testes size, but not body size, of males (Table 2a). Finally, there was also a trend towards a positive association between the number of sperm in ejaculate samples and CTM across species, whereas there was no relationship between sperm numbers in the ejaculate and body mass of males (Table 2a).
Table 2. Multiple regression analyses controlling for phylogeny (GLS) of sperm quantity and quality in relation to combined testis mass and body mass across eight species of Australian Maluridae.
| predictor | slope | t | P | λ | |
| (a) Sperm quantity | |||||
| CP volume | testis mass | 58.37 | 2.89 | 0.03 | <0.001 1.0; 0.09 |
| body mass | −45.39 | −1.34 | 0.24 | ||
| Seminal glomera mass | testis mass | 0.04 | 4.32 | 0.007 | <0.001 1.0; 0.04 |
| body mass | −0.03 | −1.94 | 0.11 | ||
| Sperm stores | testis mass | 152.33 | 3.14 | 0.02 | <0.001 1.0; 0.07 |
| body mass | −172.23 | −2.11 | 0.09 | ||
| Ejaculate sperm count | testis mass | 31.05 | 2.44 | 0.07 | <0.001 1.0; 0.04 |
| body mass | −40.69 | −2.23 | 0.09 | ||
| (b) Sperm quality | |||||
| Motile sperm in ejaculates | testis mass | 0.24 | 2.94 | 0.04 | <0.001 1.0; 0.02 |
| body mass | 0.05 | 0.45 | 0.67 | ||
| Viable sperm in sperm reserves | testis mass | 0.15 | 2.99 | 0.03 | <0.001 1.0; 0.007 |
| body mass | −0.04 | −0.47 | 0.66 | ||
| Morphologically normal sperm in sperm reserves | testis mass | 0.18 | 4.23 | 0.008 | <0.001 0.14; 0.5 |
| body mass | −0.36 | −4.95 | 0.004 |
The model including the maximum-likelihood value of λ was compared against the models including λ = 0 and λ = 1, and superscripts following the λ estimates indicate significance levels of the likelihood-ratio testes (first position: against λ = 0; second position: against λ = 1).
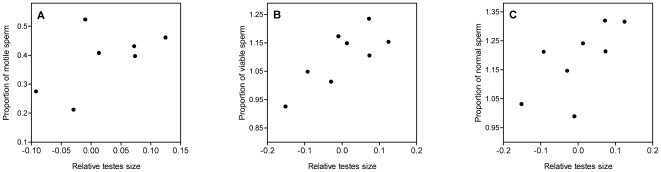
As for sperm quantity, measures of sperm quality varied significantly across species (ANOVA: motility: F 6,211 = 3.42, P = 0.003; viability: F 7,217 = 4.96, P<0.001; morphology: F 7,218 = 6.75, P<0.001). We found a positive relationship between the percent of motile sperm in an ejaculate and the level of sperm competition as measured by relative testes size (CTM with body mass as a covariate; Table 2b, Fig. 2a). Additionally, both the percent of viable sperm and the percent of morphologically normal sperm in sperm stores increased with increasing relative testes size (Table 2b, Fig. 2b, 2c). Neither the proportion of motile sperm or the proportion of viable sperm were associated with body mass, but the proportion of morphologically normal sperm was negatively correlated with male body mass (Table 2b). We obtained similar result in all analyses using raw species values (i.e. no phylogenetic control; see Supplementary Table 2).
Figure 2. Relationship between the level of sperm competition (measured as relative testes size) and a) the proportion of motile sperm in ejaculates, b) the proportion of viable sperm in sperm stores, and c) the proportion of morphologically normal sperm in sperm stores.
Unlike all analyses, figures are not controlled for phylogeny. The values for relative testes mass are the residuals obtained from a linear regression of testes mass on body mass. Proportion data are arcsine-transformed. Each data point represents a species. See main text for further statistical details.
Discussion
For males, a general evolutionary response to sperm competition is an increase in testes size [2]–[7], such that relative testes mass is a common measure of sperm competition in many taxa, including birds [e.g. 9]–[12]. In the current study, we found a more than seven-fold variation in relative testis size across species of Australian Maluridae, which suggests that these species experience a broad range of sperm competition levels: from low in the southern emu-wren, low to intermediate in the striated grasswren and some species of fairy-wrens, to high in other species of fairy-wren. Our results also revealed a positive association between the level of sperm competition and both the quantity and quality of sperm. Furthermore, we found that increasing levels of sperm competition were associated with a relatively greater proportion of seminiferous tubule tissue in the testes. Thus, across species of Australian Maluridae, post-copulatory sexual selection appears to select for a suite of male traits that may influence competitive fertilization success.
Under conditions of sperm competition, male fertilization success is commonly determined by the number of sperm, relative to rival males, transferred during copulation [62], [79]–[81]. We showed that across species the number of sperm in both sperm reserves and in ejaculates was positively associated with the level of sperm competition. Generally, large sperm reserves are thought to secure paternity success via frequent copulation (i.e. few sperm per copulation/many copulations; [14], [82]). In the fairy-wrens, however, copulation is believed to be relatively infrequent and under female control [41], [83], [84]. Furthermore, in some fairy-wrens (i.e. splendid, superb, white-winged and red-backed fairy-wrens), the number of sperm in ejaculates is very large (ca. 33–48×106 sperm) compared with data available for a few other species. For example, in the zebra finch (Taeniopygia guttata), the number of sperm in ejaculates ranged from 0.17–5.29×106 [85]; and in the much larger Japanese quail (Coturnix coturnix; mass ∼120 g), the mean number of sperm in ejaculates was 12×106 [86]. Even in the promiscuous red-winged blackbird (Agelaius phoeniceus), sperm numbers do not match those observed in fairy-wrens (mean sperm number: 12.5×106 [87]). The large sperm reserves and ejaculate size of fairy-wrens may therefore represent an alternate paternity strategy, whereby males maximize paternity success via the transfer of large numbers of sperm in one or a few ejaculates (i.e. many sperm per copulation/few copulations).
While the fitness benefits of increased sperm quantity are well understood, the mechanisms of sperm production and the potential selective forces operating on these mechanisms remain relatively unexplored. High sperm production can be achieved via an increase in relative testis size, an increase in the efficiency of sperm production (daily sperm production per unit of testes tissue, [16], [88]), or a combination of these factors. Sperm production efficiency may be increased if the proportion of spermatogenic tissue (relative to interstitial tissue) in the testes increases. In the current study, we demonstrated that increasing levels of sperm competition are accompanied by an increase in the relative proportion of seminiferous tubule tissue in the testes of Australian malurid species, which is consistent with recent results from two other avian families [22]. The congruent results of these two studies suggests that selection for sperm production rates via an increase in the amount of sperm-producing tissue may be a common evolutionary response to sperm competition, at least in passerine birds. However, the amount of spermatogenic tissue in the testes varies over a very small range in the Maluridae, and whether these small differences translate into significant differences in sperm production is unknown. An alternative, and perhaps equally likely, explanation of these results is that a correlation between testis size and spermatogenic tissue arises as an artefact of testicular structure scaling: if interstitial tissue does not scale isometrically with testicular size, larger testes will show a proportionally greater amount of sperm-producing tissue simply because the proportion of interstitial tissue decreases. These hypotheses are not necessarily mutually exclusive, however, and it is clear that additional studies are needed in order to understand the factors shaping testicular morphology.
The efficiency of sperm production may also be influenced by the duration of the cycle of the seminiferous epithelium (and consequently the rate of spermatogenesis). As Lüpold and coworkers [22] suggest, data regarding spermatogenesis in birds is limited in scope and focused on a few domesticated species (e.g. japanese quail [86], [89], turkey [90]). In mammals however, elevated levels of sperm competition are associated with a shorter seminiferous epithelium cycle length [91]–[93]. These studies highlight the need to integrate data on relative testis size and the kinetics of spermatogenesis in order to understand selection on sperm production in males. Finally, as sperm production may also be shaped by the number of mitotic divisions of spermatogonia, the capacity of sperm to survive and complete spermatogenesis, and the duration of sperm transport, future studies should also aim to investigate these aspects of spermatogenesis.
In addition to selection for more sperm, postcopulatory sexual selection appears to select for higher quality sperm in the Maluridae. In birds, fertilization success is determined by sperm mobility [25], [94] and the percentage of motile sperm in an ejaculate [26]. Thus, selection at the intraspecific level appears to translate to macroevolutionary patterns of increased swimming speed [31] and a greater percentage of motile sperm (this study) with increasing levels of sperm competition. There are two main reasons to assume that the percent of viable sperm and morphologically normal sperm may also influence fertilization success in birds. First, only viable and morphologically normal sperm enter the sperm storage tubules of females [95], [96], and second, morphologically abnormal sperm appear less effective at reaching the infundibulum (the site of fertilization) and penetrating an egg to achieve fertilization [69]. In addition, these traits have been shown to influence fertility in other taxa (e.g. sperm viability, insects, [40]; morphologically normal sperm, deer, [30]). We found significant interspecific variation in the proportion of viable and morphologically normal sperm. Specifically, our results showed that species experiencing higher levels of sperm competition had a greater proportion of morphologically normal and viable sperm available for copulation, suggesting sperm viability and morphology are favored under conditions of sperm competition.
The occurrence of dead or morphologically abnormal sperm is generally attributed to production errors during spermatogenesis [97], [98], or may result from increased replication-dependent mutations associated with increased sperm production [99] if these mutations alter sperm phenotype. Our results suggest that postcopulatory sexual selection may favor mechanisms that minimize sperm production errors and maintain sperm integrity during transport and storage. In the testes, oxidative stress results in a reduced capacity to differentiate normal sperm [100]. Additionally, oxidative stress reduces both sperm motility and viability [101], [102]. Consequently, selection may target mechanisms underlying sperm function aimed at avoiding oxidative stress and preventing oxidative damage to sperm structures (see [103]). Alternatively, males may be able to allocate substances to their testes and semen that protect sperm integrity and influence sperm quality (see [104], [105]); as has been observed in Drosophila (e.g. protease inhibitors, anti-microbial peptides [106]).
Regardless of the underlying cause(s) of extra-pair copulation, female promiscuity has significant evolutionary consequences for the reproductive biology of male Australian malurids. In the current study, we show that postcopulatory sexual selection is associated with an increase in the quantity and quality of sperm in sperm reserves and ejaculates. Furthermore, we demonstrate that increased sperm quantity is likely achieved, not only through an increase in testes size, but through selection for greater sperm production via an increase in the relative amount of sperm-producing tissue contained within the testes. Future intraspecific studies that investigate the relative importance of these sperm traits on male paternity success will provide a more comprehensive understanding of how these macroevolutionary patterns in sperm traits have evolved.
Supporting Information
Number of males contributing to the analysis of male reproductive traits (testicular morphology, sperm quantity and sperm quality) for all fairy-wren (F-W), emu-wren and grasswren species.
(DOC)
Results of all analyses using raw species values (i.e. λ set to 0 in GLS analysis, no phylogenetic control).
(DOC)
Acknowledgments
We are most grateful to the many field assistants that participated in this project, though special thanks go to Christina Rockwell, Wen Shen, and Veronica Yoyovich. Additionally, our fieldwork could not have happened without the help of a number of people across Australia: thanks to Dave Armstrong, Chris Baxter, Jane Cooper, Win Filewood, Jody Gates, Emma Greig, David Hair, Philippa Horton, Jordan Karubian, Willow Lindsay, David Paton, Marcus Pickett, David Turner, Claire Varian, Mike Webster and Peter Wilkins. We also wish to express our thanks to The Lincoln Marine Science Centre, especially Toby Bolton and Bob Delaine, for generously allowing us to use their lab space while in Port Lincoln, Andrew Beattie for loaning us equipment, and R. P. Freckleton for providing R script for the comparative analysis. Finally, this work was greatly improved by discussion with Murray Bakst, Albert Phillimore, Trevor Price and Jill Mateo and comments received from Arild Johnsen, Lewis George Halsey, and two anonymous reviewers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant to SP-J from the National Science Foundation (IOB-051697, 2005–2008) and by a grant to MR from the Hinds Fund from the University of Chicago (2005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol Rev. 1970;45:525–567. [Google Scholar]
- 2.Møller AP. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am Nat. 1991;137:882–906. [Google Scholar]
- 3.Harcourt AH, Purvis A, Liles L. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct Ecol. 1995;9:468–476. [Google Scholar]
- 4.Hosken DJ. Sperm competition in bats. Proc Roy Soc B. 1997;264:385–392. doi: 10.1098/rspb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockley P, Gage MJG, Parker GA, Møller AP. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- 6.Hosken DJ, Ward PI. Experimental evidence for testis size evolution via sperm competition. Ecol Lett. 2001;4:10–13. [Google Scholar]
- 7.Byrne PG, Roberts JD, Simmons LW. Sperm competition selects for increased testes mass in Australian frogs. J Evol Biol. 2002;15:347–355. [Google Scholar]
- 8.Simmons LW, García-González F. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung bettles. Evolution. 2008;62:2580–2591. doi: 10.1111/j.1558-5646.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- 9.Briskie JV, Montgomerie R. Sperm size and sperm competition in birds. Proc Roy Soc B-Biol Sci. 1992;247:89–95. doi: 10.1098/rspb.1992.0013. [DOI] [PubMed] [Google Scholar]
- 10.Dunn PO, Whittingham LA, Pitcher TE. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 11.Pitcher TE, Dunn PO, Whittingham LA. Sperm competition and the evolution of testes size in birds. J Evol Biol. 2005;18:557–567. doi: 10.1111/j.1420-9101.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 12.Immler S, Birkhead TR. Sperm competition and sperm midpiece size: no consistent pattern in passerine birds. Proc Roy Soc B. 2007;274:561–568. doi: 10.1098/rspb.2006.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage MJ. Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit fly. An Behav. 1991;42:1036–1037. [Google Scholar]
- 14.Birkhead TR, Briskie JV, Møller AP. Male sperm reserves and copulation frequency in birds. Behav Ecol Sociobiol. 1993;32:85–93. [Google Scholar]
- 15.Firman RC, Simmons LW. Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution. 2010;64:1245–1256. doi: 10.1111/j.1558-5646.2009.00894.x. [DOI] [PubMed] [Google Scholar]
- 16.Amann RP. Sperm Production Rates. In: Johnson AD, Gomes WR, Vandemark NL, editors. The Testis. Academic Press; 1970. pp. 433–482. [Google Scholar]
- 17.de Reviers M, Williams JB. Testis development and production of spermatozoa in the cockerel (Gallus domesticus). In: Cunningham FJ, Lake PE, Hewitt D, editors. Reproductive Biology of Poultry. Edited by British Poultry Science Ltd; 1984. pp. 183–202. [Google Scholar]
- 18.Møller AP. Testes size, ejaculate quality and sperm competition in birds. Biol J Linn Soc. 1988;33:273–283. [Google Scholar]
- 19.Møller AP. Ejaculate quality, testes size and sperm competition in primates. J Human Evol. 1988;17:479–488. [Google Scholar]
- 20.Møller AP. Ejaculate quality, testes size and sperm production in mammals. Funct Ecol. 1989;3:91–96. [Google Scholar]
- 21.Schultz AH. The relative weight of the testes in primates. Anat Rec. 1938;72:387–394. [Google Scholar]
- 22.Lüpold S, Linz GM, Rivers JW, Westneat DF, Birkhead TR. Sperm competition selects beyond relative testes size in birds. Evolution. 2009;63:391–402. doi: 10.1111/j.1558-5646.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 23.Snook RR. Sperm in competition: not playing by the numbers. Trends Ecol Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Pizzari T, Parker GA. Sperm competition and sperm phenotype. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Academic Press; 2009. pp. 205–244. In by. [Google Scholar]
- 25.Birkhead TR, Martinez JG, Burke T, Froman DP. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc Roy Soc B-Biol Sci. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denk AG, Holzmann A, Peters A, Vermeirssen ELM, Kempenaers B. Paternity in mallards: effects of sperm quality and female sperm selection for inbreeding avoidance. Behav Ecol. 2005;16:825–833. [Google Scholar]
- 27.Pizzari T, Worley K, Burke T, Froman DP. Sperm competition dynamics: ejaculate fertilising efficiency changes differentially with time. BMC Evol Biol. 2008;8:332–338. doi: 10.1186/1471-2148-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, et al. Spermatozoal traits and sperm competition in Atlantic Salmon: relative sperm velocity is the primary determinant of fertilization success. Curr Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- 29.Gasparini C, Simmons LW, Beveridge M, Evans JP. Sperm swimming velocity predicts competitive fertilization success in the Green Swordtail Xiphophorus helleri. PLoSone 2010. 2010;5:e12146. doi: 10.1371/journal.pone.0012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malo AF, Garde JJ, Soler AJ, García AJ, Gomendio M, et al. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol Reprod. 2005;72:822–829. doi: 10.1095/biolreprod.104.036368. [DOI] [PubMed] [Google Scholar]
- 31.Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, et al. Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution. 2009;63:2466–2473. doi: 10.1111/j.1558-5646.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 32.Lüpold S, Calhim S, Immler S, Birkhead TR. Sperm morphology and sperm velocity in passerine birds. Proc Roy Soc B. 2009;276:1175–1181. doi: 10.1098/rspb.2008.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, et al. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. PNAS. 2009;106:1128–1132. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson MJ, Dixson AF. Motility and the midpiece in primates. Nature. 2002;416:496. doi: 10.1038/416496a. [DOI] [PubMed] [Google Scholar]
- 35.Gomendio M, Roldan ERS. Sperm competition influences sperm size in mammals. Proc Biol Sci. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MJ, Chapman SJ, Videan EN, Evans E, Fritz J, et al. Functional evidence for differences in sperm competition in humans and chimpanzees. Am J Phys Anth. 2007;134:274–280. doi: 10.1002/ajpa.20674. [DOI] [PubMed] [Google Scholar]
- 37.Balshine S, Leach BJ, Neat F, Werner NY, Montgomerie R. Sperm size of African cichlids in relation to sperm competition. Behav Ecol. 2001;12:726–731. [Google Scholar]
- 38.Lüpold S, Linz GM, Birkhead TR. Sperm design and variation in the New World blackbirds (Icteridae). Behav Ecol Sociobiol. 2009;63:899–909. [Google Scholar]
- 39.Gage MJ, Freckleton RP. Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc Roy Soc B. 2003;270:625–632. doi: 10.1098/rspb.2002.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-González F, Simmons LW. Sperm viability matters in insect sperm competition. Curr Biol. 2005;15:271–275. doi: 10.1016/j.cub.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Hunter FM, Birkhead TR. Sperm viability and sperm competition in insects. Curr Biol. 2002;12:121–123. doi: 10.1016/s0960-9822(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 42.Rowley I, Russell E. Fairy-wrens and grasswrens: Maluridae. 1997. Oxford University Press, Oxford.
- 43.Christidis L. Evolution and biogeography of the Australian grasswrens, Amytornis (Aves: Maluridae): Biochemical perspectives. Aust J Zool. 1999;47:113–124. [Google Scholar]
- 44.Harrison CJO. The affinities of the blue wren genus Malurus and related genera with special reference to the grass wren genus Amytornis. Emu. 1969;69:1–8. [Google Scholar]
- 45.Maguire GS, Mulder RA. Breeding biology and demography of the southern emu-wren (Stipiturus malachurus). Aust J Zool. 2004;52:583–604. [Google Scholar]
- 46.Kingma SA, Hall ML, Segelbacher G, Peters A. Radical loss of an extreme extra-pair mating system. BMC Ecol. 2009;9:15–25. doi: 10.1186/1472-6785-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulder RA, Dunn PO, Cockburn A, Lazenby-Cohen KA, Howell MJ. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc Roy Soc London B- Biol Sci. 1994;255:223–229. [Google Scholar]
- 48.Brooker MG, Rowley I, Adams M, Baverstock PR. Promiscuity an inbreeding avoidance mechanism in a socially monogamous species. Behav Ecol Sociobiol. 1990;26:191–200. [Google Scholar]
- 49.Karubian J. Costs and benefits of variable breeding plumage in the red-backed fairy-wren. Evolution. 2002;56:1673–1682. doi: 10.1111/j.0014-3820.2002.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 50.Webster MS, Tarvin KA, Tuttle EM, Pruett-Jones S. Reproductive promiscuity in the splendid fairy-wren: effects of group size and auxiliary reproduction. Behav Ecol. 2004;15:907–915. [Google Scholar]
- 51.Maguire GS, Mulder RA. Low levels of extra-pair paternity in southern emu-wrens (Aves: Maluridae). Aust J Zool. 2008;56:79–84. [Google Scholar]
- 52.Webster MS, Varian CW, Karubian J. Plumage color and reproduction in the red-backed fairy-wren: Why be a dull breeder? Behav Ecol. 2008;19:517–524. [Google Scholar]
- 53.Rowe M, Pruett-Jones S. Reproductive biology and sperm competition in Australian fairy-wrens. Avian Poultry Biol Rev. 2006;17:21–37. [Google Scholar]
- 54.Møller AP, Briskie JV. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behavioral Ecology and Sociobiology. 1995;36:357–365. [Google Scholar]
- 55.Griffith SC, Owens IPF, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 56.Tuttle EM, Pruett-Jones S, Webster MS. Cloacal protuberances and extreme sperm production in Australian fairy-wrens. Proc Roy Soc B. 1996;263:1359–1364. [Google Scholar]
- 57.Taborsky M. Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol Evol. 1998;13:222–227. doi: 10.1016/s0169-5347(97)01318-9. [DOI] [PubMed] [Google Scholar]
- 58.Wolfson A. The cloacal protuberance - a means for determining breeding condition in live male passerines. Bird-Banding. 1952;23:159–165. [Google Scholar]
- 59.Gee GF, Bertschinger H, Donoghue AM, Blanco J, Soley J. Reproduction in nondomestic birds: Physiology, semen collection, artificial insemination and cryopreservation. Avian Poult Biol Rev. 2004;15:47–101. [Google Scholar]
- 60.Rowe M, Swaddle JP, Pruett-Jones S, Webster MS. Plumage coloration, ejaculate quality and reproductive phenotype in the red-backed fairy-wren. An Behav. 2010;79:1239–1246. [Google Scholar]
- 61.Lessells CM, Boag PT. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- 62.Laskemoen T, Kleven O, Fossøy F, Robertson RJ, Rudolfsen G, et al. Sperm quantity and quality effects on fertilization success in a highly promiscuous passerine, the tree swallow Tachycineta bicolor. Behav Ecol Sociobiol. 2010;64:1473–1484. [Google Scholar]
- 63.Wishart GJ, Wilson YI. Bakst MR, Cecil HC, editors. Sperm motility and metabolism I. Visual scoring of motility using the hanging drop method. Techniques for Semen Evaluation, Semen Storage, and Fertility Determination The Poultry Science Association. 1997.
- 64.Penfold LM, Wildt DE, Herzog TL, Lynch W, Ware L, et al. Seasonal patterns of LH, testosterone and semem quality in the Northern pintail duck (Anas acuta). Reprod Fert Devel. 2000;12:229–235. doi: 10.1071/rd00093. [DOI] [PubMed] [Google Scholar]
- 65.Cooper DM, Rowell JG. Relations between fertility, embryonic survival and some semen characteristics in the chicken. Poultry Science. 1958;37:699–707. [Google Scholar]
- 66.Bakst MR, Cecil HC. Bakst MR, Cecil HC, editors. Sperm viability I. Nigrosin/eosin stain for determining live/dead and abnormal sperm counts. Techniques for Semen Evaluation, Semen Storage, and Fertility Determination The Poultry Science Association. 1997. pp. 29–34.
- 67.Tuttle EM, Pruett-Jones SG. Estimates of extreme sperm production: morphological and experimental evidence from reproductively promiscuous fairy-wrens (Malurus). An Behav. 2004;68:541–550. [Google Scholar]
- 68.Birkhead TR, Petrie M. Ejaculate features and sperm utilization in peafowl Pavo cristatus. Proc Roy Soc Lond B-Biol Sci. 1995;261:153–158. [Google Scholar]
- 69.Wishart GJ, Lindsay C, Staines HJ, McCormick P. Semen quality in captive Houbara bustard, Chlamydotis undulata undulata. Reprod Fert Devel. 2002;14:401–405. doi: 10.1071/rd02014. [DOI] [PubMed] [Google Scholar]
- 70.Ciereszko A, Rybnik PK, Horbańczuk JO, Dietrich GJ, Deas A, et al. Biochemical characterization and sperm motility parameters of ostrich (Stuthio camelus) semen. An Reprod Sci. 2010;122:222–228. doi: 10.1016/j.anireprosci.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Sontakke SD, Umapathy G, Sivaram V, Kholkute SD, Shivaji S. Semen characteristics, cryopreservation, and successful artificial insemination in the Blue rock pigeon (Columba livia). Theriogenology. 2004;62:139–153. doi: 10.1016/j.theriogenology.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 72.R Development Core Team. R: A language and environment for statistical computing. 2010. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org.
- 73.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 74.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 75.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 76.Paradis E, Claude J, Strimmer K. APE: analysis of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 77.Christidis L, Schodde R. Relationships within the Australo-Papuan fairy-wrens (Aves: Malurinae): An evaluation of the utility of allozyme data. Aust J Zool. 1997;45:113–129. [Google Scholar]
- 78.Driskell AC, Norman JA, Pruett-Jones S, Mangall E, Sonsthagen S, et al. A multigene phylogeny examining evolutionary and ecological relationships in the Australo Papuan wrens of the subfamily Malurinae (Aves). Mol Phylo Evol. 2011 doi: 10.1016/j.ympev.2011.03.030. in press. [DOI] [PubMed] [Google Scholar]
- 79.Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. The effect of ratios and numbers of spermatozoa mixed from two males on proportions of offspring. J Reprod Fert. 1974;39:251–258. doi: 10.1530/jrf.0.0390251. [DOI] [PubMed] [Google Scholar]
- 80.Parker GA. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J Theor Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. [DOI] [PubMed] [Google Scholar]
- 81.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Gene. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 82.Briskie JV. Anatomical adaptations to sperm competition in Smith's Longspurs and other polygynandrous passerines. Auk. 1993;110:875–888. [Google Scholar]
- 83.Mulder RA, Cockburn A. Sperm competition and the reproductive anatomy of male superb fairy-wrens. Auk. 1993;110:588–593. [Google Scholar]
- 84.Double M, Cockburn A. Pre-dawn infidelity: Females control extra-pair mating in superb fairy-wrens. Proc Roy Soc Lond B-Biol Sci. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birkhead TR, Fletcher F, Pellatt EJ, Staples A. Ejaculate quality and the success of extra-pair copulation in the zebra finch. Nature. 1995;377:422–424. [Google Scholar]
- 86.Clulow J, Jones RC. Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix. J Reprod Fert. 1982;64:259–266. doi: 10.1530/jrf.0.0640259. [DOI] [PubMed] [Google Scholar]
- 87.Westneat DF, McGraw LA, Fraterrigo JM, Birkhead TR, Fletcher F. Patterns of courtship behavior and ejaculate characteristics in male red-winged blackbirds. Behav Ecol Sociobiol. 1998;43:161–171. [Google Scholar]
- 88.Amann RP. A critical review of methods for evaluation of spermatogenesis from seminal characteristics. J Andrology. 1981;2:37–58. [Google Scholar]
- 89.Lin M, Jones RC, Blackshaw AW. The cycle of the seminiferous epithelium in the Japanes quail (Coturnix coturnix japonica) and estimation of its duration. J Reprod Fert. 1990;88:481–490. doi: 10.1530/jrf.0.0880481. [DOI] [PubMed] [Google Scholar]
- 90.Noirault J, Brillard J-P, Bakst MR. Spermatogenesis in the turkey (Meleagris gallopavo): Quantitative approach in immature and adult males subjected to various photoperiods. Theriogenology. 2006;65:845–859. doi: 10.1016/j.theriogenology.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Peirce EJ, Breed WG. A comparative study of sperm production in two species of Australian arid zone rodents (Pseudomys australis, Notomys alexis) with marked differences in testis size. Reproduction. 2001;121:239–247. doi: 10.1530/rep.0.1210239. [DOI] [PubMed] [Google Scholar]
- 92.Parapanov R, Nusslé S, Hausser J, Vogel P. Relationships of basal metabolic rate, relative testis size and cycle length of spermatogenesis in shrews (Mammalia, Soricidae). Reprod Fert Devel. 2008;20:431–439. doi: 10.1071/rd07207. [DOI] [PubMed] [Google Scholar]
- 93.Ramm SA, Stockley P. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol Lett. 2010;6:219–221. doi: 10.1098/rsbl.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Froman DP, Wardell JC, Feltmann AJ. Sperm mobility: deduction of a model explaining phenotypic variation in roosters (Gallus domesticus). Biol Reprod. 2006;74:487–491. doi: 10.1095/biolreprod.105.046755. [DOI] [PubMed] [Google Scholar]
- 95.Allen TE, Grigg GW. Sperm transport in the fowl. Aust J Ag Res. 1958;8:788–799. [Google Scholar]
- 96.Lake P. Gamete production and the fertile period with particular reference to domesticated birds. Symp Zool Soc Lond. 1975;35:225–244. [Google Scholar]
- 97.Cohen J. Correlation between sperm “redundancy” and chiasma frequency. Nature. 1967;215:862–863. doi: 10.1038/215862a0. [DOI] [PubMed] [Google Scholar]
- 98.Cohen J. Cross-overs, sperm redundancy and their close association. Heredity. 1973;31:408–413. doi: 10.1038/hdy.1973.96. [DOI] [PubMed] [Google Scholar]
- 99.Blumenstiel JP. Sperm competition can drive a male-biased mutation rate. J Theo Biol. 2010;249:624–632. doi: 10.1016/j.jtbi.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevity. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujihara N, Howarth B. Lipid peroxidation in fowl spermatozoa. Poult Sci. 1978;57:1766–1768. doi: 10.3382/ps.0571766. [DOI] [PubMed] [Google Scholar]
- 102.Wishart GJ. Effects of lipid peroxide formation in fowl semen on sperm motility, ATP content and fertilizing ability. J Reprod Fert. 1984;71:113–118. doi: 10.1530/jrf.0.0710113. [DOI] [PubMed] [Google Scholar]
- 103.Costantini D, Rowe M, Butler MW, McGraw KJ. From molecules to living systems: historical and contemporary issues in oxidative stress and antioxidant ecology. Funct Ecol. 2010;24:950–959. [Google Scholar]
- 104.Froman DP, Pizzari T, Feltman AJ, Castillo-Juarez H, Birkhead TR. Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc Roy Soc B. 2002;269:607–612. doi: 10.1098/rspb.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rowe M, McGraw KJ. Carotenoids in the seminal fluid of wild birds: interspecific variation in fairy-wrens. Condor. 2008;110:694–700. [Google Scholar]
- 106.Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of males contributing to the analysis of male reproductive traits (testicular morphology, sperm quantity and sperm quality) for all fairy-wren (F-W), emu-wren and grasswren species.
(DOC)
Results of all analyses using raw species values (i.e. λ set to 0 in GLS analysis, no phylogenetic control).
(DOC)