Summary
Toxic protein aggregation (proteotoxicity) is a unifying feature in the development of late-onset human neurodegenerative disorders. Reduction of insulin/IGF-1 signaling (IIS), a prominent lifespan, developmental and reproductive regulatory pathway, protects worms from proteotoxicity associated with the aggregation of the Alzheimer’s disease-linked Aβ peptide. We utilized transgenic nematodes that express human Aβ and found that late life IIS reduction efficiently protects from Aβ toxicity without affecting development, reproduction or lifespan. To alleviate proteotoxic stress in the animal, the IIS requires heat shock factor (HSF)-1 to modulate a protein disaggregase, while DAF-16 regulates a presumptive active aggregase, raising the question of how these opposing activities could be co-regulated. One possibility is that HSF-1 and DAF-16 have distinct temporal requirements for protection from proteotoxicity. Using a conditional RNAi approach, we found an early requirement for HSF-1 that is distinct from the adult functions of DAF-16 for protection from proteotoxicity. Our data also indicate that late life IIS reduction can protect from proteotoxicity when it can no longer promote longevity, strengthening the prospect that IIS reduction might be a promising strategy for the treatment of neurodegenerative disorders caused by proteotoxicity.
Keywords: Caenorhabditis elegans, insulin/IGF-1 signaling, longevity, proteotoxicity
Introduction
Aging is the major risk factor for the development of late-onset human neurodegenerative disorders, including Huntington’s disease (HD) and Alzheimer’s disease (AD) (Amaducci & Tesco, 1994), both linked to aberrant protein aggregation (Selkoe, 2003). HD is associated with the aggregation of expanded poly-glutamine stretches (polyQ) in the protein huntingtin (Bates, 2003), while AD is associated with the aggregation of the Aβ peptide. Although it is not entirely clear why these disorders emerge late in life, it is plausible that the aging process plays an active role in enabling their onset. One theory suggests that biological activities that defend against toxic protein aggregation (proteotoxicity) decline with age (Cohen et al., 2006). The insulin/IGF-1 signaling (IIS) pathway is a prominent aging regulator and lifespan determinant in worms (Kenyon et al., 1993; Kenyon, 2001), flies (Giannakou & Partridge, 2007) and mice (Bluher et al., 2003; Holzenberger et al., 2003; Taguchi et al., 2007). Reduced IGF signaling was recently shown to be linked to the regulation of human lifespan (Suh et al., 2008; Willcox et al., 2008; Flachsbart et al., 2009), suggesting that the longevity functions of this pathway are conserved from worms to humans. In the nematode Caenorhabditis elegans, the sole insulin/IGF-1 receptor, DAF-2, mediates the phosphorylation, via downstream kinases, of the forkhead-like transcription factor, DAF-16, prevents it from entering the nucleus and compromises DAF-16 target gene expression (Lee et al., 2001). This results in a shortened lifespan and elevated stress sensitivity. Thus, genetic knockdown of daf-2 enables DAF-16 to enter the nucleus and creates long-lived, stress-resistant worms (Kenyon, 2005). DAF-16 is critically required for reduced IIS to mediate longevity in worms, as daf-16 knockdown by RNAi or mutation abolishes the increased longevity of daf-2 mutant animals (Kenyon et al., 1993; Tissenbaum & Ruvkun, 1998; Lee et al., 2001).
The heat shock factor 1 (HSF-1) is also essential for lifespan extension facilitated by reduced IIS (Hsu et al., 2003; Morley & Morimoto, 2004). HSF-1 is predominantly regulated by trimer formation and nuclear entry upon heat shock induction (Sarge et al., 1993). DAF-16 and HSF-1 have shared and distinct regulation of downstream genes, especially those of the chaperone class. However, hitherto it is unknown whether hsf-1 activity is directly regulated by the IIS pathway.
Reduced IIS protects worms from various stress conditions, including thermal (Lithgow et al., 1995) and oxidative stress (Honda & Honda, 1999). Recent studies indicate that an IIS reduction can also protect worms from polyQ (Morley et al., 2002; Hsu et al., 2003) and Aβ (Cohen et al., 2006) aggregation associated proteotoxicity. These results indicate that the IIS coordinately regulates the aging process and protein homeostasis, suggesting that activities that defend against toxic protein aggregation decline with age (Cohen & Dillin, 2008).
To characterize the detailed mechanistic links between the IIS and toxic protein aggregation, we utilized transgenic worms that express the human Aβ1–42 minigene driven by a muscle specific promoter (Aβ worms) (Link, 1995), resulting in muscular dysfunction and age-dependent progressive paralysis of the worm population. We reported that IIS reduction protected animals from Aβ1–42 mediated paralysis in a DAF-16 and HSF-1 dependent manner when administered during development and adulthood [daf-2, daf-16 and hsf-1 RNAi treatments affects neither Aβ expression levels nor the Aβ total protein amounts (Cohen et al., 2006)]. HSF-1 and DAF-16 regulate opposing protective activities; HSF-1 influences disaggregation while DAF-16 mediates the formation of larger, plausibly less toxic aggregates (Cohen et al., 2006). Our findings suggest that an aging associated decline in these IIS regulated protective activities enables proteotoxicity to manifest late in life (Cohen & Dillin, 2008) and point to IIS reduction as a promising approach to develop neurodegenerative therapies (Morimoto, 2006). Recently, we have extended our studies to mammals and found that aging alteration by reducing IGF signaling protects mice from behavioral, pathological and biochemical phenotypes associated with AD-like disease (Cohen et al., 2009).
However, the IIS pathway controls multiple processes, including development, reproduction and longevity. The complexity of the physiological processes regulated by this pathway poses a significant hurdle utilizing and validating it as a bona fide target for neurodegenerative disease therapies. Most importantly, hitherto it is unknown whether IIS reduction late in life, the stage at which most human neurodegenerative disorders onset, could provide protection from proteotoxicity. To address this issue, we asked whether it was possible to separate the physiological requirements of the IIS pathway for protection against proteotoxicity from its developmental, reproductive and most importantly, its requirement for the determination of lifespan.
Results
IIS can regulate proteotoxicity independent of longevity
We have addressed this question using Aβ and DAF-16 reporter worm strains taking advantage of our understanding of the IIS pathway and its role in the highly conserved aging program of C. elegans. First, we tested whether the application of daf-2 RNAi late in life affects the intra-cellular localization of DAF-16, as it does early in life, using worms expressing green fluorescent protein (GFP) fused to functional DAF-16 [strain TJ356 (Henderson & Johnson, 2001)]. In worms at days 1, 5 and 9 of adulthood, DAF-16 efficiently enters the nucleus within 6 h after transferring the worms onto daf-2 RNAi expressing bacteria [Fig. 1A,B, daf-2 and daf-16 RNAi treatments effectively reduced target gene expression (Dillin et al., 2002)]. These observations indicate that daf-2 reduction by RNAi similarly affects DAF-16 localization whether applied in early adulthood (day 1) or late in adulthood (day 9) when it can no longer extend lifespan (Dillin et al., 2002).
Fig 1.
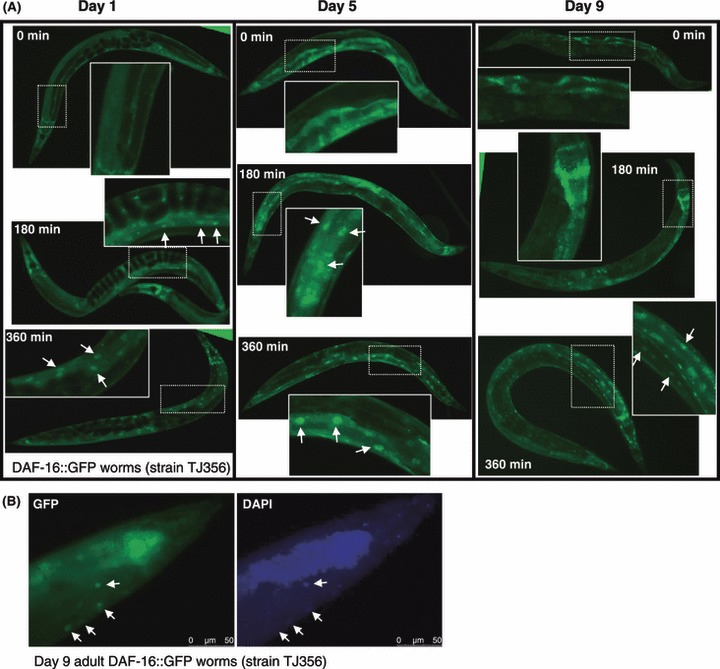
Late life insulin/IGF-1 signaling reduction promotes DAF-16 nuclear localization. (A) DAF-16::GFP expressing worms (strain TJ356) were grown on control bacteria (EV) to either day 1 or 9 of adulthood, and transferred onto daf-2 RNAi bacteria. Green fluorescent protein (GFP) signal was visualized 0, 3 or 6 h after the transfer. Six hours after transfer, GFP signal in worms that were treated during early and late adulthood were concentrated in the nuclei. (B) Day 9 DAF-16::GFP worms were placed on daf-2 RNAi for 6 h, fixed and stained with DAPI. Co-localization of the DAPI and GFP signals (arrows) confirmed the nuclear localization of DAF-16 in day 9 old worms that were fed daf-2 RNAi.
To test whether late life IIS reduction and the subsequent DAF-16 relocalization into the nucleus, could protect from age onset proteotoxicity associated with human Aβ1–42 peptide expression, we utilized the Aβ worm model. To temporally attenuate IIS, Aβ worms were hatched and developed on control bacteria harboring an empty vector (EV) and then transferred onto daf-2 RNAi bacteria at either day 1, 5 or 9 of adulthood (Fig. 2A, Fig. S1). daf-2 RNAi protected worms from paralysis associated with Aβ proteotoxicity when applied during early adulthood, days 1 and 5 of adulthood, the time window in which it can promote longevity (Dillin et al., 2002). Interestingly, application of daf-2 RNAi during late adulthood, day 9, also suppressed further Aβ proteotoxicity within the worm population (Fig. 2A,B). Importantly, this late life protective effect was observed even if daf-2 RNAi was applied relatively late in life beyond the time window in which it could extend lifespan (Fig. 2C blue, Supporting Fig. S2 and (Dillin et al., 2002). We also tested the effect on lifespan of transferring Aβ worms from EV bacteria onto daf-2 RNAi bacteria at day 5 of adulthood and found that the lifespan extension was relatively small (Fig. 2C red). This observation is consistent with the results published for wild-type worms transferred at the same time (Dillin et al., 2002). Thus, although the longevity and anti-proteotoxicity functions of reduced IIS overlap in early adulthood (days 1–5), they can be temporally dissociated late in life (day 9 of adulthood).
Fig 2.
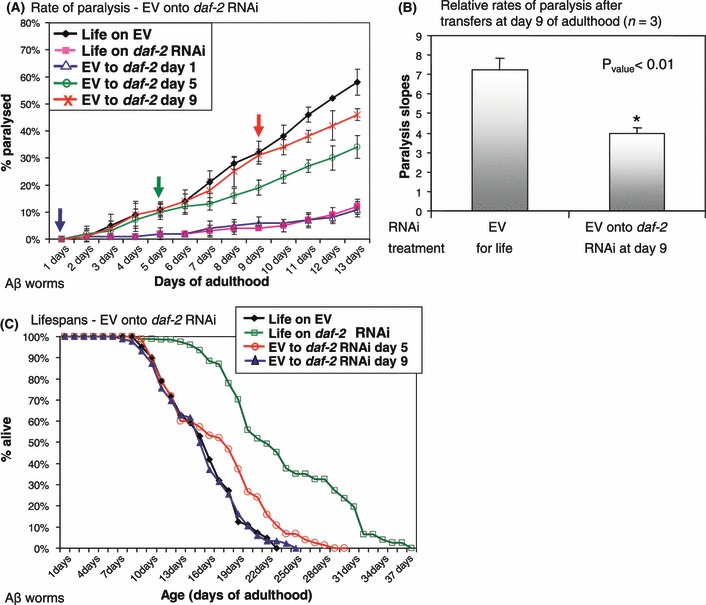
Timing requirements for daf-2 RNAi mediated protection from Aβ proteotoxicity. (A) Aβ worms were transferred from empty vector (EV) bacteria onto daf-2 RNAi bacteria at either day 1, 5 or 9 of adulthood. Paralysis rates decreased upon transfer to daf-2 RNAi compared to EV-grown control worms at all tested ages. (B) Three independent repeats of (A) indicate that the reduction of Aβ toxicity observed in worms transferred at day 9 is reproducible and significant. (C) Lifespan of control Aβ worms grown throughout life on EV bacteria and their counterparts which were transferred from EV onto daf-2 RNAi bacteria at day 9 of adulthood are undistinguishable (mean lifespan: 15.48 and 15.35 days respectively, Pvalue = 0.772). Lifespan of worms that were transferred from EV bacteria onto daf-2 RNAi at day 5 of adulthood were significantly shorter than these of their counterparts which were grown on daf-2 RNAi throughout life (mean lifespan: 17.04 and 23.74 respectively, Pvalue = 3.55E-09).
Late life IIS reduction promotes aggregation of Aβ
Previously, we found that protection from Aβ proteotoxicity by reduced IIS is associated with the accumulation of high molecular weight (high-MW) Aβ aggregates (Cohen et al., 2006). To examine whether a similar mechanism underlies the late life protection mediated by daf-2 RNAi treatment, we adopted a biochemical approach to measure the content of high-MW Aβ aggregates in worms that were treated either early or late in life with daf-2 RNAi.
Uniform length Aβ aggregates derived from the sonication of homogenized Aβ worms hasten an in-vitro Aβ1–40 polymerization reaction in a dose-dependent fashion (Hasegawa et al., 1999; Cohen et al., 2006). Therefore, the higher content of fibrillar Aβ aggregates in the worms shorten the lag phase associated with the initiation of Aβ aggregation in the test tube owing to the process of seeding or bypassing the requirement for nucleation. The dye thioflavin-T (ThT) selectively binds to Aβ fibrils, shifting the wave length of its fluorescent emission and enabling accurate measurement of the in-vitro Aβ fibrilization reaction. The in-vitro kinetic aggregation assay was utilized to measure the content of fibrillar Aβ aggregates within Aβ worms (Cohen et al., 2006). Aβ worms were hatched on control bacteria (EV) and transferred onto daf-2 RNAi bacteria at either early age (days 1–5 of adulthood) or late in life (days 9–13 of adulthood). In all cases, worms were cultured on the daf-2 RNAi bacteria for identical amounts of time, 4 days, prior to harvest for biochemical analysis. The worms were homogenized and separated by low-speed centrifugation (845 g, 5 min desktop centrifuge) into soluble [post debris supernatant (PDS)] and insoluble (debris) fractions. To provide robust quantification results independent from the initial distribution of fibril lengths, the PDS was sonicated for 10 min to break worm derived Aβ fibrils into unified length. Using the in-vitro kinetic aggregation assay, we compared the seeding efficiency of sonicated worm PDS fractions from animals treated with daf-2 RNAi early or late in life. Worms treated with daf-2 RNAi, whether early in life (days 1–5, Fig. 3A), or later in life (days 9–13, Fig. 3B) exhibited an increased amount of Aβ fibrils as reflected by the increased seeding efficiency of their sonicated PDS compared to untreated age-matched counterparts.
Fig 3.
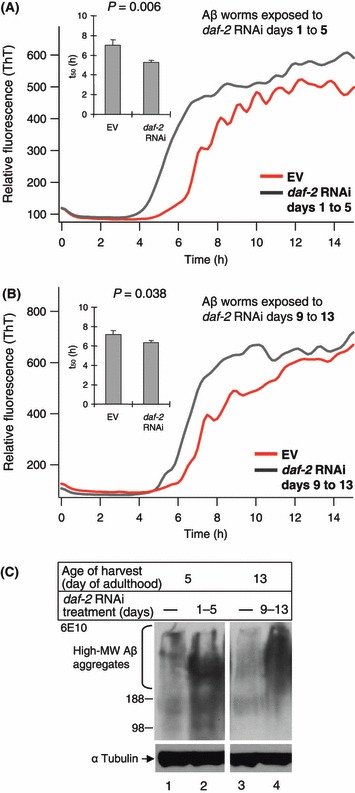
(A, B) In-vitro kinetic aggregation assay reveals that Aβ worm homogenates that were treated with daf-2 RNAi either early (days 1–5 of adulthood) (A) or late (days 9–13 of adulthood) (B) in life had higher Aβ seed content compared to their control untreated age-matched counterparts. (C) Western blot analysis using Aβ monoclonal antibody (6E10) indicated that high-MW Aβ aggregates contents in insoluble fractions (debris) of Aβ worm that were treated with daf-2 RNAi either early (lane 2) or late (lane 4) in life were higher compared to their control untreated age-matched counterparts (lanes 1 and 3 respectively).
In addition to the in-vitro kinetic assay, we also measured the high-MW Aβ aggregates in the insoluble fractions of the early and late-life treated worm groups using western blot analysis and an Aβ antibody (clone 6E10). We found that both early and late life daf-2 RNAi treatments afforded increased amounts of high-MW Aβ aggregates (Fig. 3C, lanes 2 and 4 respectively) relative to age-matched untreated controls (Fig. 3C, lanes 1 and 3).
Taken together, attenuation of daf-2 either early or late in life results in nuclear localization of DAF-16, protection from further Aβ associated proteotoxcity and increased amounts of high molecular weight Aβ aggregates. These observations suggest that IIS reduction either early or late in life, can protect from age onset proteotoxicity by invoking a mechanism that converts toxic aggregates into larger, less toxic high molecular weight aggregates.
DAF-16 is required during adulthood to protect from Aβ proteotoxicity
DAF-16 is essential for the counter-proteotoxic activity of reduced IIS (Morley et al., 2002; Hsu et al., 2003; Cohen et al., 2006). Thus, we asked whether daf-16 was required during the same time window as daf-2 RNAi (reduced IIS) to protect animals from age onset proteotoxicity. We tested whether daf-16 RNAi could directly eradicate the protection provided by IIS reduction by following the paralysis phenotype associated with expression of Aβ (Cohen et al., 2006). Aβ worms that were hatched and developed on daf-2 RNAi expressing bacteria transferred away from daf-2 RNAi onto daf-16 RNAi bacteria at either day 1, 5 or 9 of adulthood (daf-16 RNAi reduces mRNA levels within 3 h, Supporting Fig. S4A) and the rates of paralysis within the worm populations were recorded (Fig. 4A). Analogous to its role in lifespan determination (Dillin et al., 2002), daf-2 reduction during development did not protect from proteotoxicity, as worms transferred from daf-2 RNAi onto daf-16 RNAi at day 1 of adulthood paralyzed at similar rates as control animals [removal of the worms from daf-2 RNAi onto control bacteria did not abolish the counter-proteotoxic protective effect of daf-2 RNAi through day 13 of adulthood, most likely due to the stability of the RNAi (Supporting Fig. S3)]. Equivalent to its role in lifespan determination, the developmental functions of daf-2 (embryogenesis, larval development and reproductive timing) could be temporally separated from the anti-proteotoxicity function of reduced IIS. Therefore, daf-16 is required to protect from Aβ1–42 toxicity only during adulthood.
Fig 4.
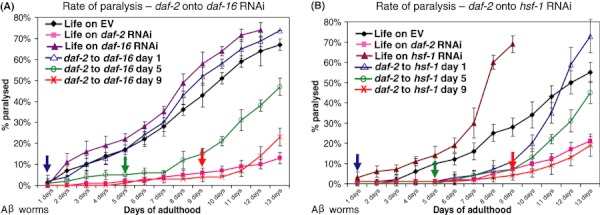
Timing requirements for daf-16 and hsf-1 RNAi mediated protection from Aβ proteotoxicity (A) Aβ worms were grown on daf-2 RNAi bacteria throughout life or were transferred to daf-16 RNAi bacteria on either day 1, 5 or 9 of adulthood. Development on daf-2 RNAi did not protect the worms from Aβ mediated paralysis compared to EV and daf-16 RNAi controls. Worm transferred from daf-2 onto daf-16 RNAi at either day 5 or 9 of adulthood were protected for 2 days after exposure to daf-16 RNAi. (B) Aβ worms developed on daf-2 RNAi were transferred onto hsf-1 RNAi at either day 1, 5 or 9 of adulthood. Development on daf-2 RNAi protected the worms from paralysis for 8 days while animals transferred at day 5 were protected for one additional day. All panels display one of three independent experiments.
Animals developed on daf-2 RNAi and transferred onto daf-16 RNAi at days 5 or 9 were temporarily protected, but eventually succumbed to proteotoxicity 2 days after the transfer (Fig. 4A). The 2-day phenotypic lag period might stem from the turnover of DAF-16 or protective proteins encoded by DAF-16 regulated genes. Alternatively, Aβ toxicity may take up to 2 days for full induction of paralysis in the worm model. Taken together, daf-2 and daf-16 have overlapping temporal requirements for protection from proteotoxicity that extend well into adulthood after the appearance of proteotoxic stress on the population.
HSF-1 is predominantly required during larval development for proteotoxicity protection
Heat shock factor-1 is also critical for the anti-proteotoxicity activity of reduced IIS (Hsu et al., 2003; Cohen et al., 2006). To determine the timing requirements for hsf-1, Aβ worms were grown on daf-2 RNAi and transferred onto hsf-1 RNAi bacteria at either day 1, 5 or 9 of adulthood (Fig. 4B). Surprisingly, and in stark contrast to the daf-16 RNAi experiments, worms transferred away from daf-2 RNAi onto hsf-1 RNAi at day 1 of adulthood did not exhibit paralysis until day 9 of adulthood, whereas animals developed and maintained on hsf-1 RNAi throughout life readily succumbed to proteotoxicity. In addition, animals transferred to hsf-1 RNAi at day 5 of adulthood were protected for only one additional day compared to their day 1 transferred counterparts. Animals transferred at day 9 showed no additional paralysis until termination of the experiment. Using quantitative PCR, we found that hsf-1 RNAi readily reduced hsf-1 gene expression 6 h after transferring the worms onto hsf-1 RNAi bacteria (Supporting Fig. S4B), similar to daf-2 and daf-16 RNAi shown previously (Dillin et al., 2002). hsf-1 RNAi treatment has similar effects on the expression of its target gene HSP-16.2 (Link et al., 1999) when applied early and late in life (Supporting Fig. S5). Thus, our observations indicate that unlike daf-16, hsf-1 is required foremost during larval development, yet it is also needed for a lesser extent during adulthood to counter Aβ proteotoxicity.
Discussion
Collectively, our findings indicate that daf-2, daf-16 and hsf-1 are required up to advanced age for protection against muscular Aβ proteotoxicity and that late life IIS attenuation can protect from further damage without extending lifespan (Fig. 5). Furthermore, the developmental and reproductive functions of daf-2 and daf-16 could be separated from the proteotoxicity function of the IIS, which acts during adulthood.
Fig 5.
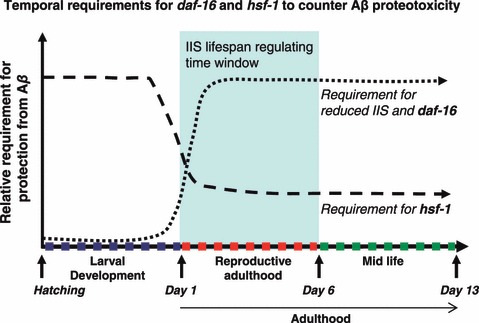
Timing requirement for reduced insulin/IGF-1 signaling (IIS), daf-16 and hsf-1 to counter Aβ proteotoxicity in the worm. IIS reduction during development has no effect on Aβ proteotoxicity if daf-16 is attenuated at day 1 of adulthood. In contrast, IIS attenuation during reproductive adulthood and midlife protect from Aβ. This protection is associated with Aβ hyper-aggregation and dependent in daf-16. hsf-1 is foremost required for protection from Aβ proteotoxicity during larval development but is also required for a lesser extent during early adulthood and midlife.
It was surprising to find that hsf-1 was predominantly required during development and again, to a lesser extent, during adulthood. The two-step requirement of hsf-1 suggests that there might be an initiation phase required by hsf-1 during development that is later acted upon during adulthood. Consistent with this idea, the histone deactylase SIRT1 is required to attenuate the heat shock response by directly deactylating HSF-1, enabling maintenance of HSF-1 for binding to its target genes (Westerheide et al., 2009). Furthermore, SIRT1 expression declines with age in accordance with the age-dependent decline of the heat shock response. In future, it will be imperative to determine the temporal requirements of SIRT1 for longevity and proteotoxic stress, especially given the fact that SIRT1 also regulates the activity of FOXO in addition to HSF-1 (Brunet et al., 2004).
The counter proteotoxic functions of the IIS are conserved from worms to mammals [Cohen et al. (in press)], suggesting that drugs directed to reduce IIS pathway, or its target genes, could delay neurodegeneration even if administered after diagnosis late in life, thereby possibly circumventing many of the potential deleterious effects of IIS reduction during development. The finding that hsf-1 can function early, as well as later in life, to protect animals towards proteotoxicity provides a unique opportunity to study proteotoxic diseases during development, such as the aggressive forms of Huntington’s, Spinocerebral Ataxic diseases and juvenile Parkinsonism (Giasson & Lee, 2001).
Experimental procedures
Worm and RNAi strains
CL2006 (Link, 1995), CL2070, TJ356 and N2 worm strains were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN). The worms were grown at 20 °C. To reduce gene expression, we used previously described (Dillin et al., 2002) bacterial strains expressing dsRNA: EV (pAD12), daf-2 (pAD48), daf-16 (pAD43). hsf-1 dsRNA expressing bacterial strain was from genomic RNAi library (J. Ahringer). Each RNAi bacterial colony was grown at 37 °C in LB with 100 μg mL−1 carbenicillin, and then seeded onto NG-carbenicillin plates supplemented with 100 mm Isopropyl β-D-1-thiogalactopyranoside (IPTG).
DAF-16 localization assay
Synchronized TJ356 worms were grown on the EV control bacteria. At the indicated ages (days 1 or 9 of adulthood), 25 worms were transferred onto daf-2 RNAi bacteria for the indicated time (0, 3 or 6 h). The worms were washed twice with M9, snap froze in liquid nitrogen and nuclei were labeled for 30 min using 4′,6-diamidino-2-phenylindole (DAPI) [200 ng mL−1; (Molecular Probes), Invitrogen, Carlsbad, CA, USA]. DAPI and GFP signals were visualized using a fluorescent microscope (Leica DM6000 B; Leica, Wetzlar, Germany).
Paralysis assay
Synchronous CL2006 worm populations were grown on (NG) plates containing 100 μg mL−1 carbenicillin, spotted with E. coli cultures expressing dsRNA as indicated. On the first day of adulthood, 100 worms were placed on ten plates (ten animals per plate). The plates were divided randomly to five sets (two plates, 20 worms per set). The worms were tested every day for paralysis by tapping their noses with a platinum wire. Worms that moved their noses but failed to move their bodies were scored as ‘paralyzed’ and removed from the plates. To avoid scoring of old animals as paralyzed, paralysis assay terminated at day 13 of adulthood.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from synchronized populations of approximately 12 000 sterile worms (strain CF512) grown at 20 °C for each time point. Total RNA was extracted using QIAzol reagent (Cat #79306; QIAGEN, Hilden, Germany) and purified using RNeasy kit (QIAGEN #74104). cDNA was created using QuantiTect Probe RT-PCR Kit (QIAGEN #204443). For quantitative PCR reactions, dilutions of 1:10 were used. SybrGreen real-time qPCR experiments were performed as described in the manual using ABI Prism7900HT (Applied Biosystems, Foster city, CA, USA). Quantification was completed using SDS2.1 software (Applied Biosystems), normalizing to control levels of act-1 cDNA.
hsf-1 primer set 1: forward: TTGACGACGACAAGCTTCCAGT; reverse: AAAGCTTGCACCAGAATCATCCC.
hsf-1 primer set 2: forward: GTCTCTGTCATGCAGCCAGG; reverse: TTGGGTCCGGCAGTTCC.
daf-16 primers: forward: CTTCAAGCCAATGCCACTACC reverse: GGAGATGAGTTGGATGTTGATAGC.
act-1 primer set: forward: GAGCACGGTATCGTCACCAA; reverse: TGTGATGCCAGATCTTCTCCAT.
Lifespan analysis
Synchronized worm eggs were placed on master NG-carbenicillin plates seeded with the indicated RNAi bacterial strain and supplemented with 100 mm IPTG. The eggs were incubated at 20 °C until the worms reached L4 larval stage and were transferred onto small NG-carbenicillin plates (ten animals per plate). The worms were transferred onto freshly seeded plates every 4 days. Dead worms were scored daily. Lifespan analyses were conducted at 20 °C.
In-vitro kinetic aggregation assay
Aβ worms were grown on RNAi bacterial strains as indicated. At the desired ages, the worms were washed twice with M9 and once more with phosphate-buffered saline (PBS) (RT), resuspended in 300 μL ice-cold PBS, transferred to a 2-mL tissue grinder (885482; Kontes, Vineland, NJ, USA) and homogenized. Crude homogenates were spun in a desktop microfuge (845 g, 3 min). Supernatants were transferred to new tubes and total protein concentrations were measured with BCA kit (Pierce, Rockford, IL, USA). Aβ1–40 peptide was diluted to a final concentration of 10 μm in phosphate buffer (150 mm NaCl, 50 mm Na-phosphate, pH 7.4) containing ThT (20 μm). PDS were sonicated for 20 min in a water bath sonicator (Model FS60; Fisher Scientific, Pittsburg, PA, USA). Proteinase K was added to final concentration of 200 ng mL−1, incubated for 2 h and supplemented with complete (EDTA free) protease inhibitors cocktail (Cat#1836170; Roche, Basel, Switzerland). The treated PDS solution was added to the assay at a final total protein concentration of 10 μg mL−1. Three aliquots (100 μL) of these solutions were transferred into wells of a 96-well microplate (Costar black, clear bottom) for each reaction. The plate was sealed and loaded into a Gemini SpectraMax EM fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA), where it was incubated at 37 °C. The fluorescence (excitation at 440 nm, emission at 485 nm) was measured from the bottom of the plate at 10 min intervals, with 5 s of shaking before each reading.
Aβ blotting and detection
Equal numbers of Aβ worms were grown on either EV or daf-2 RNAi bacteria as indicated. The worms were washed and homogenized as prepared for the in-vitro kinetic aggregation assay (see above). Total protein amounts were equalized using BCA kit as described above. Worm debris (insoluble fractions) were supplemented with 120 μL PBS, 40 μL LDS sample buffer and 10 μL, reducing agent (Invitrogen, Carlsbad, CA, USA) boiled for 10 min and separated on 4–12% Bis–Tris gels (Invitrogen; cat #NP0322). The proteins were transferred onto nitrocellulose membrane (Protean 0.2 μm; Whatman, Dassel Germany), blocked with 5% powdered milk in TBST (10 mm Tris, 150 mm NaCl, 0.15% Tween-20, pH 8.0). Aβ was detected using the 6E10 monoclonal antibody (SIG-39320; Covance Emeryville, CA, USA). ECL was developed using ECL system.
Early and late life hsf-1 RNAi efficiency assay
To compare the effects of hsf-1 RNAi early and late in life worms that express GFP under the regulation of the HSF-1 target gene HSP-16.2 were used (strain CL2070). Synchronized worm populations were developed and grown on EV up to either day 1 or 9 of adulthood and transferred onto hsf-1 RNAi bacteria for 4 days (hsf-1 RNAi treatments: either days 1–5 or days 9–13 of adulthood, control worm groups were grown on EV bacteria up to either day 5 or 13 of adulthood). At the last day of treatment, the worms were heat shocked for 6 h at 33 °C (to induce GFP expression), homogenized and cleared by centrifugation (2350 g, 5 min, desktop centrifuge). Total protein amounts in the PDS samples were normalized using BCA assay (Pierce, Rockford, IL, USA) and equal amounts were loaded onto 12% PAA gel, transferred onto Polyvinylidene Fluoride (PVDF) membrane and detected using an odyssey imager. Antibodies: affinity purified rabbit anti-GFP was a generous gift from Jill Meisenhelder, α tubulin (Cat # T5168; Sigma, St. Louis, MO, USA). Signal intensities were measured using imagej software.
Funding
This study was generously supported by the McKnight endowment for neuroscience (AD) and P01 AG031097 (AD and JWK).
Author contributions
EC and AD designed and initiated this study; EC performed fluorescent detection of DAF-16, paralysis assays, Western blot and quantitative RT-PCR. DD performed in-vitro kinetic aggregation assays. EK carried out lifespan experiments and DJ performed a paralysis assay and GFP detection. YV performed quantitative RT-PCR experiments. EC, AD and JWK wrote the manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 (A) Repeat of 1A.
Fig. S2 Statistical data of the lifespan experiment displayed in Figure 1C.
Fig. S3 Aβ worms that were grown ondaf-2 RNAi and transferred onto control bacteria (EV) ateither day 1, 5 or 9 of adulthood were notably protected fromAβ toxicity through day 13 of adulthood, indicating thatremoval of the worms from daf-2 RNAi does not
abolish the counter proteotoxic effect associated with reduced IIS.
Fig. S4 Quantitative RT-PCR analysis ofdaf-16 and hsf-1 expression in L4 wild-type larva(grown at 20°C): (A) daf-16 mRNA levelsare reduced to approximately 50% 3h after transferring the animalsonto daf-16 RNAi bacteria. (B) hsf-1mRNA levels are reduced by nearly 80% 6 hours after transferringworms onto hsf-1 RNAi bacteria.
Fig. S5 CL2070 worms were grown and developedon control bacteria and transferred onto hsf-1 RNAi ateither day 1 or 9 of adulthood. Both worm groups were grown onhsf-1 RNAi for identical time, four days, before harvesting. GFP contents in the PDS samples of both groups were analyzed using WB. Relative signal intensities were measured using ImageJ software.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Amaducci L, Tesco G. Aging as a major risk for degenerative diseases of the central nervous system. Curr. Opin. Neurol. 1994;7:283–286. doi: 10.1097/00019052-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Science. Vol. 299. New York, NY: 2003. Extended longevity in mice lacking the insulin receptor in adipose tissue; pp. 572–574. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Science. Vol. 303. New York, NY: 2004. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase; pp. 2011–2015. [DOI] [PubMed] [Google Scholar]
- Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat. Rev. Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Science. Vol. 313. New York, NY: 2006. Opposing activities protect against age-onset proteotoxicity; pp. 1604–1610. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, Von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl Acad. Sci. USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Lee VM. Parkin and the molecular pathways of Parkinson’s disease. Neuron. 2001;31:885–888. doi: 10.1016/s0896-6273(01)00439-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between A beta(1-42) and A beta(1-40) in Alzheimer’s beta-amyloid fibril formation in vitro. Biochemistry. 1999;38:15514–15521. doi: 10.1021/bi991161m. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Link C. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Stress, aging, and neurodegenerative disease. N. Eng. J. Med. 2006;355:2254–2255. doi: 10.1056/NEJMcibr065573. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl Acad. Sci. USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Science. Vol. 317. New York, NY: 2007. Brain IRS2 signaling coordinates life span and nutrient homeostasis; pp. 369–372. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Science. Vol. 323. New York, NY: 2009. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1; pp. 1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article:
Fig. S1 (A) Repeat of 1A.
Fig. S2 Statistical data of the lifespan experiment displayed in Figure 1C.
Fig. S3 Aβ worms that were grown ondaf-2 RNAi and transferred onto control bacteria (EV) ateither day 1, 5 or 9 of adulthood were notably protected fromAβ toxicity through day 13 of adulthood, indicating thatremoval of the worms from daf-2 RNAi does not
abolish the counter proteotoxic effect associated with reduced IIS.
Fig. S4 Quantitative RT-PCR analysis ofdaf-16 and hsf-1 expression in L4 wild-type larva(grown at 20°C): (A) daf-16 mRNA levelsare reduced to approximately 50% 3h after transferring the animalsonto daf-16 RNAi bacteria. (B) hsf-1mRNA levels are reduced by nearly 80% 6 hours after transferringworms onto hsf-1 RNAi bacteria.
Fig. S5 CL2070 worms were grown and developedon control bacteria and transferred onto hsf-1 RNAi ateither day 1 or 9 of adulthood. Both worm groups were grown onhsf-1 RNAi for identical time, four days, before harvesting. GFP contents in the PDS samples of both groups were analyzed using WB. Relative signal intensities were measured using ImageJ software.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.


