Summary
The mechanism by which CD4 T-cells are depleted in HIV-infected hosts remains poorly understood. In ex vivo cultures of human tonsil tissue, CD4 T cells undergo a pronounced cytopathic response following HIV infection. Strikingly, >95% of these dying cells are not productively infected but instead correspond to bystander cells. We now show that the death of these “bystander” cells involves abortive HIV infection. Inhibitors blocking HIV entry or early steps of reverse transcription prevent CD4 T-cell death while inhibition of later events in viral life cycle does not. We propose that the nonpermissive state exhibited by the majority of resting CD4 tonsil T-cells leads to accumulation of incomplete reverse transcripts. These cytoplasmic nucleic acids activate a host defense program that elicits a coordinated proapoptotic and proinflammatory response involving caspase-3 and caspase-1 activation. While this response likely evolved to protect the host, it centrally contributes to the immunopathogenic effects of HIV.
Introduction
Despite extensive efforts over the past quarter century, the precise mechanism by which HIV-1 causes progressive depletion of CD4 T cells remains debated. Both direct and indirect cytopathic effects have been proposed. When immortalized T-cell lines are infected with laboratory-adapted HIV-1 strains, direct CD4 T-cell killing predominates. Conversely, in more physiological systems, such as infection of lymphoid tissue with primary HIV-1 isolates, the majority of dying cells appear as uninfected “bystander” CD4 T cells (Finkel et al., 1995; Jekle et al., 2003).
Various mechanisms have been proposed to contribute to the death of these bystander CD4 T cells including the action of host-derived factors like tumor necrosis factor-α, Fas ligand and TRAIL (Gandhi et al., 1998; Herbeuval et al., 2005), and viral factors like HIV-1 Tat, Vpr, and Nef released from infected cells (Schindler et al., 2006; Westendorp et al., 1995). Considerable interest has also focused on the role of gp120 and gp41 Env protein in indirect cell death, although it is not clear whether death signaling involves gp120 binding to its chemokine receptor or gp41-mediated fusion. It is also unclear whether such killing is caused by HIV-1 virions or by infected cells expressing Env.
Most studies have focused on death mechanisms acting prior to viral entry. Less is known about the fate of HIV-1-infected CD4 T cells that do not express viral genes, in particular naive CD4 T cells in tissue that are refractory to productive HIV infection (Glushakova et al., 1995; Kreisberg et al., 2006). In these cells, infection is aborted after viral entry, as reverse transcription is initiated but fails to reach completion (Kamata et al., 2009; Swiggard et al., 2004; Zack et al., 1990; Zhou et al., 2005).
Human lymphoid aggregated cultures (HLACs) prepared from tonsillar tissue closely replicate the conditions encountered by HIV in vivo and thus form an attractive, biologically relevant system for studying HIV-1 infection (Eckstein et al., 2001). Lymphoid organs are the primary sites of HIV replication and contain more than 98% of the body's CD4 T cells. Moreover, events critical to HIV disease progression occur in lymphoid tissues, where the network of cell-cell interactions mediating the immune response deteriorates and ultimately collapses. Primary cultures of peripheral blood cells do not fully mimic the cytokine milieu, the cellular composition of lymphoid tissue, nor the functional relationships that are undoubtedly important in HIV pathogenesis. Finally, HLACs can be infected with a low number of viral particles in the absence of artificial mitogens, allowing analysis of HIV cytopathicity in a natural and preserved environment. In this study, we used the HLAC system to explore the molecular basis for HIV-induced killing of CD4 T cells.
Results
Selective Depletion of CD4 T Cells by X4-Tropic HIV-1
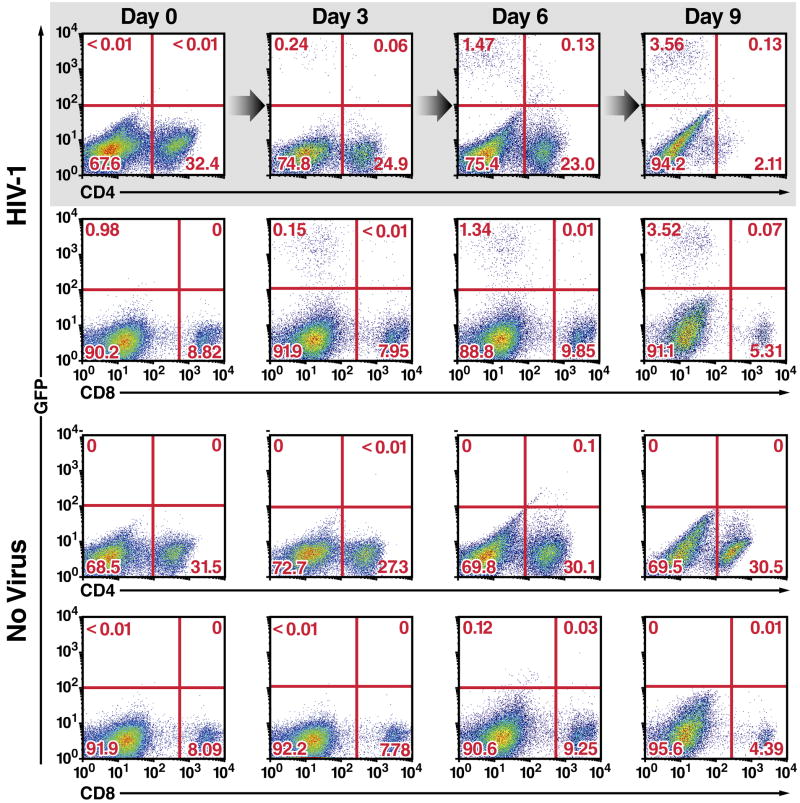
To explore depletion of CD4 T cells by HIV-1, HLACs made from freshly dissected human tonsillar tissues were infected with a GFP reporter virus (NLENG1), prepared from the X4-tropic NL4-3 strain of HIV-1. This reporter produces fully replication-competent viruses. An IRES inserted upstream of the Nef gene preserves Nef expression and supports LTR-driven GFP expression (Levy et al., 2004), allowing simultaneous quantification of the dynamics of HIV-1 infection and T-cell depletion. NL4-3 was selected because tonsillar tissue contains a high percentage of CD4 T cells expressing CXCR4 (90–100%). Productively infected GFP-positive cells appeared in small numbers 3 days after infection, peaked on days 6–9, and decreased until day 12, when few CD4 T cells remained in the culture (Figure 1). Fluorescence-linked antigen quantification (FLAQ) assay of HIV-1 p24 (Hayden et al., 2003) confirmed the accumulation of viral particles in the medium between day 3 and days 8–9, when a plateau was reached (data not shown). Interestingly, when HIV-1 p24 levels plateaued no more than 1.5% of all cells (about 5% of CD4 T cells) were GFP-positive. However, although the number of CD4 T cells was not markedly altered in infected cultures through six days, the culture was almost completely devoid of CD4 T cells by day 9. CD8 T cells were not depleted in infected cultures, and CD4 T cells were not depleted in uninfected cultures. These findings reveal marked and selective depletion of CD4 T cells in HLAC cultures. However, due to the nature of the assay, we could not definitely conclude whether the principal mechanism of depletion involved direct or indirect effects of HIV-1.
Figure 1. Massive Depletion of CD4 T Cells in HLACs Containing Small Number of Productively Infected Cells.
(A) Kinetics of spreading viral infection versus depletion of CD4 T cells after infection of HLACs with a replication-competent HIV reporter virus encoding GFP. CD4 downregulation in GFP-positive cells likely represents the combined action of the HIV Nef, Vpu, and Env proteins expressed by this virus. Ratios of viable CD4 versus CD8 T cells in HIV-infected and uninfected cultures are also shown. Flow cytometry plots represent live-gated cells, based on the forward-scatter versus side-scatter profile of the complete culture. These data are the representative results of six independent experiments utilizing tonsil cells from six different donors.
Extensive Depletion of Non-Productively Infected CD4 T Cells in HLACs
To determine if indirect killing (formerly indicated as “bystander”) of CD4 T cells accounted for most of the observed cellular depletion, we took advantage of a reported experimental strategy (Jekle et al., 2003) that unambiguously distinguishes between the death of productively and non-productively infected cells (Figure 2A). After 6 days of co-culture, survival analysis of CFSE-labeled cells by flow cytometry (Figure 2B) showed extensive depletion of CD4 T cells in cultures mixed with HIV-infected cells but not in those mixed with uninfected cells (Figure 2C). The relative proportion of CD8 T cells was not altered. CD3+/CD8− T cells were similarly depleted, indicating that the loss was not an artifact of downregulated surface expression of CD4 following direct infection. Loss of CFSE-labeled CD4 T cells was prevented by AMD3100, which blocks the engagement of gp120 with CXCR4, but not by the reverse transcriptase inhibitor AZT. Thus, productive viral replication is not required for CD4 T-cell death.
Figure 2. CD4 T-cell Depletion in HIV-1-Infected HLACs Predominantly Involves Non-productively Infected Cells.
(A) Experimental strategy to assess indirect cell killing in HIV-1-infected human lymphoid cultures. Fresh human tonsil tissue from a single donor is processed into HLAC, and then separated into two fractions. One fraction is challenged with HIV-1 and cultured for 6 days, allowing viral spread. On day 5, the uninfected fraction is treated with AZT (5 μM) and labeled with CFSE (1 μM). On day 6, the infected and CFSE-labeled cultures are mixed and co-cultured in the presence of AZT. Because of its site of action, AZT does not block viral output from the HIV-infected cells but prevents productive infection of CFSE-labeled cells. After 6 days of co-culturing, the number of viable CSFE-positive cells is determined by flow cytometry.
(B) Flow cytometry analysis of the mixed HLACs. Indirect killing is determined by gating on live CFSE-positive cells in the mixed cultures. Effector cells are either infected or uninfected cells.
(C) Extensive depletion of non-productively infected CD4 T cells by HIV-1. CFSE-labeled cells mixed with uninfected or infected cells were cultured in the presence of 5 μM AZT alone or together with 250 nM AMD3100. Data represent live CFSE-positive cells 6 days after co-culture with infected or uninfected effector cells. The absence of productive infection in the CFSE-positive cells was confirmed by internal p24 staining and monitoring GFP expression following infection with the NLENG1 HIV-1 reporter virus (not shown).
(D) Preferential depletion of non-productively infected CD4 T cells by HIV-1. The absolute numbers of viable CFSE-positive CD4 and CD8 T cells and B cells were determined. Percentages are normalized to the number of viable CFSE-positive cells co-cultured with uninfected cells in the presence of AZT, as depicted by (*). Error bars represent standard deviations of three samples from the same donor. This experiment is the representative of more than 10 independent experiments with more than 10 donors of tonsillar tissues.
See also Figure S1.
To estimate the absolute numbers of all CFSE-labeled cell subsets, we added a standard number of fluorescent beads to the cell suspensions (Figure 2D). In contrast to the sharp decline in CD4 T cells, the absolute numbers of CD8 T and B-cells were unaltered. Separating the HLAC into distinct cell types revealed that cell death occurred in purified populations of CD4 T cells suggesting that other cell types did not mediate the killing. (Figure S1). In all instances, CD4-specific killing was prevented by AMD3100 but not AZT. Importantly, the extent of CD4 T-cell depletion in the presence of AZT was similar to that observed when no antiviral drugs were added (Figure 2C and Figure 1, respectively). Together, these results suggest that indirect killing is the predominant mechanism for CD4 T-cell depletion in HIV-infected HLACs.
HIV gp41-mediated Fusion Is Necessary for Depletion of Non-Productively Infected CD4 T Cells
Studies with AMD3100 and AZT indicated that indirect CD4 T-cell killing is mediated by events occurring between gp120-CXCR4 binding and reverse transcription. Engagement of the chemokine coreceptor induces conformational changes in gp41, resulting in insertion of viral fusion peptide on gp41 into the target T-cell membrane. To determine if the gp120-CXCR4 interaction alone or later events involving viral fusion are required for indirect killing, we evaluated the effects of enfuvirtide (T20), a fusion inhibitor that blocks six-helix bundle formation by gp41, a prerequisite for virion fusion and core insertion.
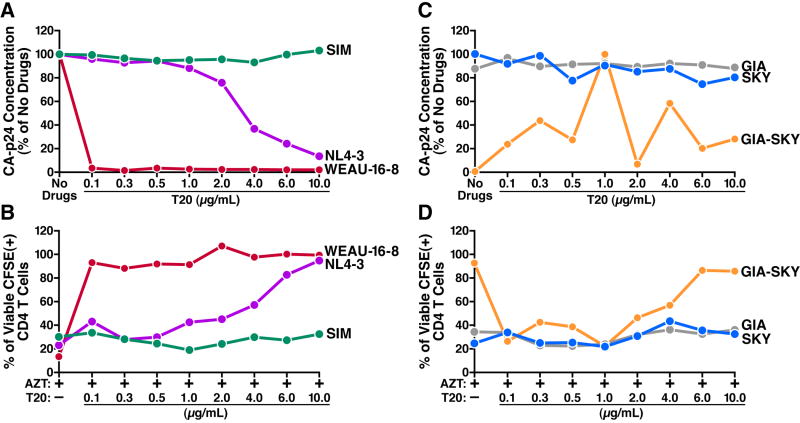
We first determined the optimal concentrations of T20 that block viral infection (Figure 3A). In NL4-3-infected cells, T20 began to inhibit infection at concentrations >2 μg/ml; complete inhibition required 10 μg/ml. In cells infected with a primary viral isolate, WEAU 16-8 (Figure S2), infection was completely inhibited by 0.1 μg/ml of T20. T20 did not inhibit infection with a T20-resistant mutant, SIM (Rimsky et al., 1998), regardless of concentration.
Figure 3. HIV-1 Fusion Is Necessary to Induce Killing of Non-Productively Infected Cells.
(A and C) Concentrations of T20 that block viral infection. HLACs were infected with the indicated clones of HIV-1 in the presence of the indicated concentrations of T20 or no drugs. One hour before incubation with the virus, cells were pretreated with T20 or left untreated. At 12 hours, cells were washed extensively and cultured under the same conditions. On day 9, the viral concentration was determined using a p24gag FLAQ assay. The amount of p24gag accumulated in the absence of drugs by each viral clone (A) or by SKY (C) was defined as 100%.
(B and D) Effect of T20 on indirect killing. CFSE-labeled cells were co-cultured with cells infected with the indicated viral clones in the presence of 5 μM AZT and the indicated concentrations of T20. After 6 days, indirect killing in the mixed cultures was assessed. The number of viable CFSE-positive CD4 T cells co-cultured with uninfected cells in the presence of AZT was defined as 100% (not shown). To allow successful initial infection we pseudotyped the GIA-SKY mutants with the VSV-G envelope. NL4-3, WT lab-adapted virus; WEAU 16-8, primary virus; SIM, T20-resistant virus; GIA-SKY, T20-dependent virus; GIA and SKY, single-domain mutant viruses. Representative data from three independent experiments with different donors are shown.
See also Figure S2.
Next, we investigated the effect of T20 on indirect CD4 T-cell killing (Figure 3B). In the absence of T20, high levels of indirect killing were observed. T20 concentrations that blocked infection also greatly inhibited indirect killing. T20 did not inhibit indirect killing in cultures containing SIM-infected cells. Thus, blocking gp41-mediated fusion prevents indirect killing.
We then examined a T20-dependent mutant, GIA-SKY (Baldwin et al., 2004), which fuses only when T20 is present, but cannot initiate a spreading infection in the absence of T20 (Figure 3C). Consistent with its T20 dependency, in the presence of 1 μg/ml T20, the GIA-SKY mutant readily replicated while growth was inhibited at higher or lower T20 concentrations. The single-domain mutants GIA and SKY exhibited a T20-resistance phenotype similar to that of SIM.
GIA-SKY-infected cells did not induce indirect killing of CD4 T cells in the absence of T20 (Figure 3D). Indirect killing was observed in cultures treated with 1 μg/ml T20 but was inhibited at higher or lower concentrations. Since T20-dependent viruses were bound to CXCR4 before T20 was added, these findings argue that CXCR4 signaling is not sufficient to elicit indirect CD4 T-cell killing.
Indirect Killing Requires a Close Interaction between Uninfected and HIV-Infected Cells
Next we examined whether indirect killing requires close contact with HIV-infected cells or instead can be fully supported by virions accumulating in the supernatants of the infected histocultures. We found that cell-free supernatants from HIV-infected histocultures were much less efficient at inducing indirect killing (Figure 4A). To exclude the possibility that the concentration of virions in the supernatants was too low, we repeated this experiment using a 20-fold concentrated virion supernatants (1 μg p24/ml) but failed to detect indirect CD4 T cell killing (Figure 4B). Together, these findings suggest that close cell-cell contact is likely required for indirect killing.
Figure 4. Killing of Non-Productively Infected CD4 T Cells Requires Fusion of Virions from Nearby HIV-1-Producing Cells.
(A) Supernatants from HIV-infected HLACs are less efficient at inducing indirect killing than mixing of HIV-infected and uninfected HLACs.
(B) HIV-1 virions released into the medium do not participate in indirect killing. Replacing the mixed culture with fresh RPMI every 24 hours did not impair indirect killing. Challenging HLACs with supernatants containing 20-fold more histoculture-derived virions (1 μg p24/ml) than normally accumulated in mixed cultures containing infected cells (50 ng p24/ml) did not induce indirect killing. Percentages are normalized to the number of viable CFSE-positive cells depicted by (*).
(C) CFSE-labeled cells are not killed when HIV-infected HLAC is physically separated by a 1 μm –pore transwell system that allows free diffusion of HIV-1 particles. Values represent the levels of viable CFSE-positive cells after 6 days of culture in the presence of the indicated drugs. Red, HIV-infected cells; blue, uninfected cells; green, CFSE-labeled cells.
(D) Mature and immature viruses carry equivalent amounts of envelope protein and Blam-Vpr, but differ in their content of capsid and Gag precursor. NL4-3 and TR712 viruses were generated in 293T cells with or without amprenavir, lysed and subjected to SDS-PAGE immunoblotting analysis for gp120, p55 Gag, p24 CA, Blam-Vpr, and free Blam.
(E) Immature viruses have reduced capacity to enter cells. SupT1 cells were mock infected or infected with mature or immature NL4-3 or TR712 virions containing Blam-Vpr. After loading of cells with CCF2 dye, fusion was analyzed by flow cytometry. Percentages are the fraction of cells displaying increased cleaved CCF2 fluorescence, indicating virion fusion.
(F) Protease inhibitors inhibit indirect killing. CFSE-labeled cells were co-cultured with NL4-3-infected or uninfected cells in the presence of AZT (5 μM) alone or together with AMD3100 (250 nM). To the indicated cultures were added 5 μM of Amprenavir, Saquinavir, or Indinavir. Percentages are normalized to the number of viable CFSE-positive cells depicted by Error bars represent the SD obtained with three independent samples from the same donor.
See also Figure S3.
To further explore the potential requirement of close cell-cell contact for indirect killing (Sherer et al., 2007; Sourisseau et al., 2007), we repeated these assays using cells that had been washed daily with fresh RPMI to prevent accumulation of HIV-1 virions and soluble factors. Such cell washing did not affect the ability of the resultant infected cells to mediate indirect CD4 T-cell killing (Figure 4B), suggesting that virions released into the medium do not participate in indirect killing. We confirmed these findings using a transwell culture system. CSFE-labeled cells and HIV-infected cells were mixed or physically separated by a transwell insert with 1 μm pores, which allows free diffusion of virions but not cells. Indirect killing was substantial in the mixed cultures but not in the transwell cultures (Figure 4C). Together, these findings indicate that indirect killing requires close interaction between CFSE-labeled and HIV-1-infected cells, consistent with in vitro (Garg et al., 2007; Holm and Gabuzda, 2005) and in vivo studies showing that apoptotic non-productively infected cells in human lymph nodes often cluster near productively infected cells (Finkel et al., 1995).
Indirect Killing Requires Fusion of Virions from Nearby HIV-Producing Cells
Indirect killing required gp41-mediated fusion and close interaction with HIV-infected cells, suggesting that cell death may be caused by the fusion of HIV-1 virions to CD4 T cells, syncytia formation, or hemifusion (mixing of lipids in the absence of fusion pore formation) mediated by Env present on HIV-infected cells interacting with neighboring CD4 T cells. HIV-1 virions (Holm et al., 2004; Jekle et al., 2003; Vlahakis et al., 2001), cell-mediated fusion (LaBonte et al., 2000; Margolis et al., 1995), and hemifusion (Garg et al., 2007) have been proposed to be involved in indirect killing. Therefore, the requirement for cell-cell interaction in indirect killing may be mediated either by effective delivery of HIV-1 virions or by cell-associated Env.
To discriminate between virion-mediated and cell-associated Env induction of indirect killing, we tested the effects of HIV protease inhibitors. These inhibitors act during the budding process, resulting in immature viral particles that cannot fuse with target cells (Wyma et al., 2004). We first assessed the effect of protease inhibitors on viral maturation. NL4-3 viruses carrying a β-lactamase-Vpr (BlaM-Vpr) reporter protein were produced in 293T cells in the presence or absence of the HIV protease inhibitor amprenavir. We also produced a mutant virus, TR712, encoding a form of gp41 lacking 144 of the 150 amino acids in the C-terminal cytoplasmic tail. This deletion largely relieves the impaired fusogenic properties of immature HIV-1 particles (Wyma et al., 2004). Protein analysis of viral lysates showed that the NL4-3 and TR712 virions appropriately cleaved gp160 to generate gp120 in the presence and absence of amprenavir. However, in the presence of amprenavir, an uncleaved form of p55 Gag polypeptide rather than the mature p24 CA protein accumulated in both NL4-3 and TR712 virions (Figure 4D). These results confirm that amprenavir treatment of virus producing cells results in the accumulation of immature particles containing normal levels of incorporated Env proteins.
To test the ability of these viruses to fuse with target cells, we used an HIV virion-based fusion assay that measures β-lactamase (BlaM) activity delivered to target cells upon the fusion of virions containing BlaM fused to the Vpr protein (BlaM-Vpr) (Cavrois et al., 2002). Immunoblotting for BlaM confirmed that NL4-3 and TR712 virions incorporated Blam-Vpr in the presence or absence of amprenavir (Figure 4D).
Next, SupT1 cells were infected with mature or amprenavir-treated immature NL4-3 or TR712 virions containing BlaM-Vpr. Immature NL4-3 viruses displayed a 90% decline in fusogenic properties (Figure 4E). In contrast, immature TR712 retained 40% fusion capacity, indicating that the impaired fusion is not a result of a defective BlaM enzyme. Thus, immature virions generated in the presence of amprenavir display greatly reduced ability to fuse with target cells. Importantly, protease inhibitors did not affect the function of Env proteins expressed on infected cells and did not block cell-cell fusion (Figure S3–C).
We next investigated the effect of protease inhibitors on indirect killing. Remarkably, three different protease inhibitors inhibited indirect killing as efficiently as AMD3100 (Figure 4F). These results indicated that HIV-1 virions, not HIV-infected cells, are responsible for indirect CD4 T cell killing. Additionally, recapitulating the efficient viral delivery of close cell-cell interactions by spinoculation of free virions resulted in extensive and selective indirect killing of CD4 T cells while sparing CD8 T cells and B cells (Figure S3 A-B). Thus, although indirect killing in lymphoid cultures requires a close interaction between non-productively and productively infected cells, this killing involves virions rather than cell-associated Env.
Non-Permissive CD4 T Cells Die from Abortive Infection
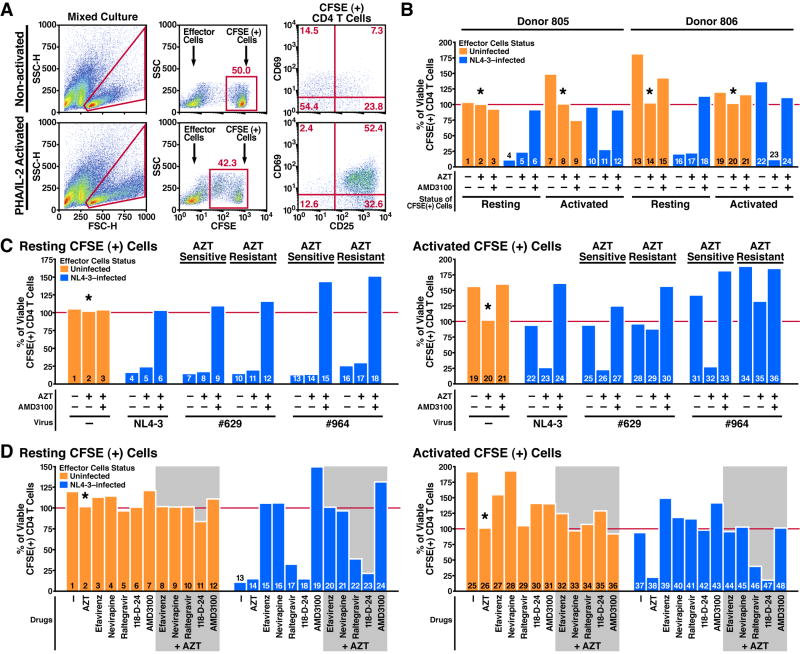
Based on these findings, we hypothesized that “indirect killing” involves an abortive form of infection, like that which occurs in nonpermissive resting CD4 T cells. These naive CD4 T cells exhibit an early post-entry block to HIV-1 infection that can be relieved by activation with phytohemagglutinin (PHA) and interleukin-2 (IL-2) (Kreisberg et al., 2006; Santoni de Sio and Trono, 2009; Unutmaz et al., 1999; Zack et al., 1990). To test this hypothesis, we compared the killing of activated and non-activated CFSE-labeled cells in HLACs.
CFSE-labeled cells were activated with PHA and IL-2 two days before mixing with effector cells, and contained a large percentage of dividing CD25 and CD69 positive cells. Non-activated (resting) CFSE-labeled cells did not divide and typically contained a small percentage of cells expressing CD25 and CD69 (Figure 5A). Either in the presence or absence of AZT, killing of resting CFSE-labeled CD4 T cells was robust (Figure 5B, columns 4+5 and 16+17). In sharp contrast, activated CFSE-labeled CD4 T cells were not depleted in the absence of AZT, but were extensively depleted in cultures containing AZT (Figure 5B, columns 10+11 and 22+23). Addition of AMD3100 prevented the AZT-induced killing of activated CFSE-labeled cells, excluding non-specific toxic effects of AZT in the activated cells (Figure 5B, columns 12 and 24).
Figure 5. Death of Abortively Infected CD4 T Cells Is Due to Impaired Reverse Transcription.
(A) Status of mixed HLACs containing either resting or activated CFSE-labeled cells, 4 days after co-culturing with effector cells. Activated CFSE-labeled cells were stimulated with PHA and IL-2 48 hours before mixing, but not during co-culturing with effector cells. To avoid direct killing of activated CFSE-labeled cells in cultures with no drugs, cell killing was terminated and analyzed 4 days after co-culturing.
(B) AZT renders activated CFSE-labeled CD4 T cells sensitive to indirect killing. Resting or activated CFSE-labeled cells were co-cultured with effector cells in the presence of no drugs, AZT (5 μM) alone, or AZT+AMD3100 (250 nM). Data are from two independent experiments performed with tonsil cells from two different donors.
(C) AZT-induced killing is lost when AZT-resistant viruses are tested. Resting or activated CFSE-labeled cells were co-cultured with cells infected with NL4-3 or HIV-1 clones #629 and #964 in the presence of no drugs, AZT (0.5 μM) alone, or AZT+AMD3100 (250 nM). AZT-sensitive and AZT-resistant sub-clones are depicted. Data are representative of three independent experiments with three different donors.
(D) NNRTIs prevent killing of abortive infected CD4 T cells. Resting or activated CFSE-labeled cells were co-cultured with infected or uninfected effector cells, in the presence of no drugs, AZT (5 μM), AMD3100 (250 nM), the NNRTIs Efavirenz (100 nM), and Nevirapine (1 μM), or the integration inhibitors Raltegravir (30 μM) and 118-D-24 (60 μM). Killing of resting CFSE-labeled CD4 T cells was blocked with equal efficiency by NNRTIs and AMD3100 (columns 15, 16), but not by integration inhibitors (columns 17, 18). In combination, NNRTIs prevented cell death induced by AZT in activated CFSE-labeled cells (compare column 38 to 44 and 45). Data are representative of four independent experiments with four different donors.
The absolute numbers of CFSE-labeled CD8 T cells and B cells was unaltered in these experiments (data not shown). Percentages are normalized to the number of viable CFSE-positive cells depicted by (*).
See also Figure S4.
The ability of AZT to promote indirect killing of activated CD4 T cells suggested that cell death is triggered by impaired reverse transcription. To investigate this possibility, we repeated the experiment with two pairs of AZT-resistant HIV-1 clones, 629 and 964 (Larder et al., 1989). We first determined that concentrations of 0.5 μM AZT block viral replication in NL4-3-infected and AZT-sensitive clones and achieve half maximal inhibitory effect in AZT-resistant clones (Figure S4 A–B).
When resting CFSE-labeled cells were used, the extent of killing by the AZT-resistant HIV-1 viruses was similar to that obtained with NL4-3 with or without AZT (Figure 5C resting CFSE-positive cells), demonstrating a redundant function for endogenous termination of reverse transcription and AZT. Alternatively, when activated CFSE-labeled cells were tested, AZT-resistant HIV-1 clones did not deplete CFSE-labeled CD4 T cells in the presence of AZT (Figure 5C, columns 29 and 35).
Death of Abortively Infected CD4 T Cells Is Triggered By Premature Termination of Viral DNA Elongation
We next asked what stage of reverse transcription triggers abortive infection cell death. AZT inhibits DNA elongation but not early DNA synthesis (Arts and Wainberg, 1994). We therefore examined whether blocking early DNA synthesis with non-nucleoside reverse transcriptase inhibitors (NNRTIs) would have the same effect as AZT. Impaired reverse transcription may also lead to abortive integration, causing chromosomal DNA breaks and a genotoxic response. To exclude this possibility, we used integrase inhibitors. To discriminate between the cytopathic response induced by endogenous termination of reverse transcription and the response induced by AZT, we separately assessed resting and activated CFSE-labeled cells.
Remarkably, the NNRTIs, efavirenz and nevirapine, blocked indirect killing of resting CD4 T cells as efficiently as AMD3100 (Figure 5D, columns 15 and 16). These findings suggested that allosteric inhibition of reverse transcriptase induced by these NNRTI's interrupts reverse transcription sufficiently early to abrogate the death response. In contrast, the integrase inhibitors raltegravir and 118-D-24 did not prevent abortive infection killing (Figure 5D, columns 17 and 18), suggesting that cell death involves signals generated prior to viral integration. NNRTIs also protected activated CFSE-labeled cells from death induced by AZT (Figure 5D, column 38 vs. columns 44 and 45), demonstrating that a certain degree of DNA synthesis is required to elicit the cytopathic response.
This notion was further strengthened in findings obtained with vif-deficient (Δvif) HIV-1 particles where reverse transcription is inhibited during strong-stop DNA synthesis due to incorporated APOBEC3G (A3G) (Bishop et al., 2008; Li et al., 2007). Abortively infected CD4 T cells were not depleted by Δvif NL4-3-infected cells (Figure S4 C–D), indicating that termination of reverse transcription before the completion of strong-stop DNA synthesis is not sufficient to generate a cytopathic response. Other HIV-1 mutants containing substitutions in RNase H and nucleocapsid that promote early defects in reverse transcription failed to elicit indirect CD4 T cell killing (Figure S4 E–F). Together, these findings indicate that accumulation of reverse-transcribed DNA, rather than any inherent activity of the HIV-1 proteins, is the key factor that triggers the death response.
Abortively Infected CD4 T Cells Commence But Do Not Complete Reverse Transcription
We next examined the status of HIV-1 reverse transcription in tonsillar CD4 T cells after infection. Specifically, we investigated the effect on reverse transcription after treatment with NNRTIs, such as efavirenz and nevirapine, which prevent the death of abortively infected CD4 T cells, or with AZT or integrase inhibitor (raltegravir) that do not prevent CD4 T-cell death. Taqman-based quantitative real-time PCR (QPCR) was used to quantify the synthesis of reverse transcription products in isolated CD4 T cells from HLAC 16 hours after infection with NL4-3. We designed specific QPCR primers and probes (Table S1) to monitor sequential steps in reverse transcription including generation of strong-stop DNA, first template exchange (Nef), and DNA strand elongation (Env) (Figure 6A). Reverse transcription products corresponding to strong-stop DNA were similar in untreated CD4 T cells or cells treated with AZT, NNRTIs, or raltegravir but were greatly reduced in cells treated with AMD3100 or in cultures infected with Δvif NL4-3 where arrest occurs prior to the completion of strong-stop DNA synthesis (Figure 6B columns 1–8). In contrast, the accumulation of later reverse transcription products detected by the Nef and Env probes were dramatically inhibited by the NNRTIs but not by raltegravir. Levels of Nef (Figure 6B, columns 10+11) and Env (columns 18+19) DNA products were similar in untreated cells and cells treated with AZT, indicating that reverse transcription in most tonsillar CD4 T cells naturally terminates during DNA chain elongation, coinciding with the block induced by AZT. The minor inhibition detected by AZT is likely due to a small number of permissive CD4 T cells in the culture. These results show that abortively infected CD4 T cells accumulate incomplete reverse transcription products representative of DNA strand elongation. Blocking earlier steps of reverse transcription by NNRTIs or by genetic mutations like deletion of Vif or mutation of RNase H restricts accumulation of such products, and prevents abortive infection-induced cell death (Figure S6–A).
Figure 6. Cytoplasmic HIV-1 DNA Triggers Proapoptotic and Proinflammatory Responses in Abortively Infected CD4 T Cells.
(A) Critical reactions in HIV-1 reverse transcription as detected by probes monitoring different regions within the Strong stop, Nef, and Env DNA fragments. RDDP, RNA-dependent DNA polymerase. Adapted from S.J. Flint et al., Principles Of Virology, 2000 ASM Press, Washington DC, with permission.
(B) NNRTIs prevent accumulation of DNA elongation products. The amount of viral DNA detected by a particular probe was calculated as a fold change relative to cells treated with no drugs (i.e. calibrator). A β–actin probe was used as an internal reference. Mean cycle threshold (Ct) values of calibrator samples are depicted. CD4 T cells were infected with WT NL4-3 produced in 293T cells, or with a Δvif NL4-3 collected from supernatants of infected HLAC, as described in Figure S4–C. Data are representative of two independent experiments performed with cells from two different donors.
(C and D) Abortive HIV-1 infection generates a coordinated proapoptotic and proinflammatory response involving caspase-3 and caspase -1 activation. HLACs were spinoculated with no virus or with NL4-3 and AZT (5 μM), Efavirenz (100 nM), and T20 (10 μg/ml), as indicated (see Figure S3 A–B). After 3 days, cells were assessed by flow cytometry for intracellular levels of proinflammatory cytokines, serine 37 phosporylated p53, and activated caspases as indicated. Ethidium monoazide was used to exclude dead and necrotic cells from the annexinV binding analysis. Data are representative of three independent experiments with three different donors.
(E) Death of abortively infected CD4 T cells requires caspase activation. CSFE-labeled cells were co-cultured with effector cells in the presence of 20 μM of Z-VAD-FMK, a general caspase inhibitor, or Z-FA-FMK, a negative control for caspase inhibitors. AZT (5 μM); AMD3100 (250 nM). Percentages are normalized to the number of viable CFSE-positive cells depicted by (*). Error bars represent standard error of the mean of three experiments from three different HLAC donors.
(F) Abortive HIV infection promotes the maturation and secretion of IL-1β in tonsillar CD4 T cells. Isolated tonsillar CD4 T cells were either untreated, or stimulated with PMA (Phorbol-12-myristate-12-acetate, 0.5 μM) and the potassium ionophore nigericin (10 μM), or spinoculated with or without NL4-3 in the presence of AZT (5 μM), AMD3100 (250 nM), and efavirenz (100 nM) as indicated. After 3 days, half of the cells were lysed and subjected to SDS-PAGE immunobloting analysis. On day 5, the supernatants from the rest of the cells were collected and subjected to SDS-PAGE immunobloting analysis. The IL-1β antibody detects the pro-IL-1β (37kD) and the mature cleaved form (17kD). Data are the representative results of five independent experiments using tonsillar CD4 T cells isolated from five different donors.
(G) DNA reverse transcription intermediates induce an IFN-stimulatory antiviral innate immune response (ISD). ISRE-GFP reporters were transfected with 1μg of HIV-1 reverse transcription intermediate products as indicated by numbers (detailed description in Figure S5–E), empty DNA plasmid, or polyinosinic:polycytidylic acid [poly(I:C)], and were analyzed by flow cytometry after 48 hours. Data are representative of three independent experiments; error bars show the SD for three independent samples from the same experiment.
See also Figure S5 and Figure S6.
DNA Reverse Transcription Intermediates Elicit a Coordinated Proapoptotic and Proinflammatory Response in Abortively Infected CD4 T Cells
We next evaluated whether HIV-mediated indirect killing of CD4 T cells is associated with deregulation of cytokine production or a DNA damage response. To facilitate a vigorous and synchronized killing effect, HLACs were spinoculated with NL4-3 virions in the presence of various antiviral drugs. Interestingly, based on immunostaining after cytokine capture, abortively infected CD4 T cells expressed IFN-β, and high levels of the proinflammatory interleukin 1β (IL–1β), but not tumor necrosis factor (TNFα) (Figure 6C). Phosphorylation of S37 p53 was not observed, suggesting that abortive HIV-1 infection does not induce a DNA damage cascade. Abortively infected CD4 T cells also displayed Caspase-1 and Caspase-3 activity along with appearance of annexin V (Figure 6D). T20 and efavirenz but not AZT prevented activation of these caspases, indicating that apoptosis was induced by abortive HIV-1 infection. Cell death was completely prevented by Z-VAD-FMK, a pan-caspase inhibitor, suggesting that caspase activation is required for the observed cytopathic response (Figure 6E). Such mode of cytokine production and caspase activation was not observed in CD8 T or B cells (Figure S5 B–C).
We next examined whether abortive HIV-1 infection signals for the maturation and secretion of IL-1β. In cells IL–1β activity is rigorously controlled. Cells can be primed to express inactive pro-IL-1β by various proinflammatory signals. However, the release of bioactive IL-1β requires a second signal leading to activation of inflammasomes, cleavage of pro-IL-1β by caspase 1 and secretion of the bioactive 17 kDa form of IL-1β (Schroder and Tschopp, 2010). Interestingly, western blot analysis revealed high amounts of intracellular pro-IL-1β in untreated CD4 T cells, suggesting that tonsillar CD4 T cells are primed to release proinflammatory mediators (Figure 6F). Stimulating the CD4 T cells with PMA and nigericin induced further accumulation of pro-IL-1β and promoted the maturation and release of the bioactive 17 kDa IL-1β into the supernatant. Remarkably, infection of CD4 T cells with NL4-3 in the presence of AZT similarly resulted in maturation and release of the bioactive 17 kDa IL-1β into the supernatant. This response was completely prevented by efavirenz and AMD3100, suggesting that abortive HIV-1 infection signals the maturation and release of bioactive IL-1β in these CD4 T cells.
To identify the nature of the nucleic acid species that trigger these responses, we used a recently described H35 rat hepatocyte cell line containing an IFN-sensitive response element (ISRE) linked to GFP (Patel et al., 2009). H35 cells were first infected with pseudotyped VSV-G HIV-1 virions. These virions induced GFP expression and cell death in the presence or absence of AZT. Importantly, the expression of GFP and cell death response were blocked by efavirenz but not raltegravir (Figure S5–D). Thus, the H35 system successfully reconstitutes the cytokine and cytopathic response observed in tonsillar CD4 T cells. We next synthesized the various HIV-1 reverse transcription intermediates and tested their ability to activate the ISRE-GFP reporter. Interestingly, none of the RNA-containing oligonucleotides stimulated the ISRE-GFP reporter expression above baseline. In sharp contrast, ssDNA and dsDNA oligonucleotides longer than 500 bases in length, which corresponded to reverse transcription intermediates produced during DNA elongation, evoked a potent ISRE-GFP activation (Figure 6G). Similarly, when cells were stimulated with poly(I:C), a synthetic double-stranded RNA known to activate IRF3 via the RIG-I pathway elicited a comparable ISRE-GFP response. Taken together, these findings indicate that reverse transcription intermediates generated during DNA chain elongation induce a coordinated proapoptotic and proinflamatory innate immune response involving caspase-3 and caspase-1 activation in abortively infected CD4 T cells.
Discussion
The mechanism through which HIV-1 kills CD4 T cells, a hallmark of AIDS, has been a topic of vigorous research and one of the most pressing questions for the field over the last 28 years (Thomas, 2009). In this study, we investigated the mechanism of HIV-1-mediated killing in lymphoid tissues, which carry the highest viral burdens in infected patients. We used HLACs formed with fresh human tonsil cells and an experimental strategy that clearly distinguishes between direct and indirect mechanisms of CD4 T-cell depletion. We now demonstrate that indirect cell killing involving abortive HIV infection of CD4 T-cells accounts for the vast majority of cell death occurring in lymphoid tissues. No more than 5% of the CD4 T cells are productively infected, but virtually all the remaining CD4 T cells are abortively infected ultimately leading to caspase-mediated cell death. Equivalent findings were observed in HLACs formed with fresh human spleen (Figure S6 B–C), indicating this mechanism of CD4 T-cell depletion can be generalized to other lymphoid tissues.
The massive depletion of non-productively infected CD4 T cells is in contrast to their survival after infection of intact blocks of tonsillar tissue in human lymphoid histoculture (HLH) (Grivel et al., 2003). This result probably reflects differences between the HLH and the HLAC experimental systems. In HLH, the complex three-dimensional spatial cellular organization of lymphoid tissue is preserved, but cellular movement and interaction are restricted, both of which are required for indirect killing. In HLAC, the tissue is dispersed, and cells are free to interact, resulting in a rapid and robust viral spread. While the mechanism triggering indirect CD4 T-cell death is certainly identical in both settings, HLH allows only a slow, nearly undetectable progression of indirect CD4 T-cell death. In HLAC, this process is accelerated, allowing the outcome to be detected in a few days. Interestingly, indirect killing was also less efficient when peripheral blood cells were tested (data not shown). It is possible that cellular factors specifically produced in lymphoid organs are required to accelerate indirect killing of peripheral blood CD4 T cells.
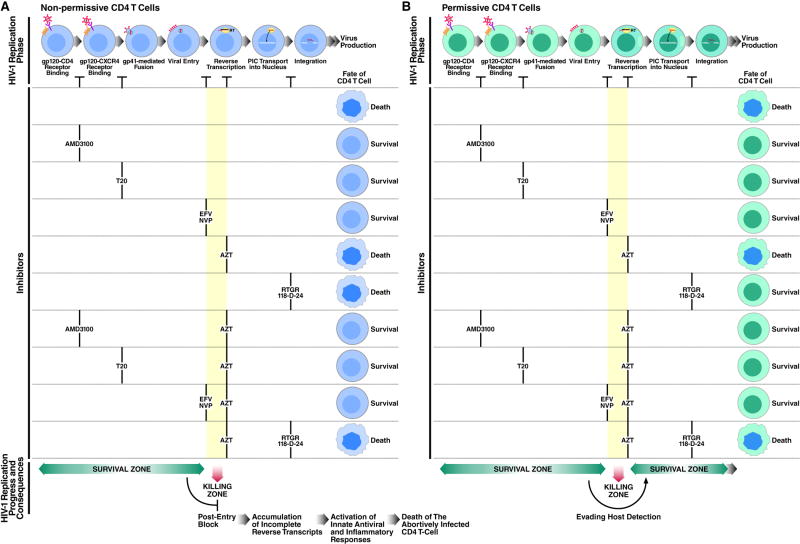
Several mechanisms have been proposed to explain indirect CD4 T-cell killing during HIV infection. Our finding that CD4 T-cell death is blocked by entry and fusion inhibitors but not by AZT, strongly suggested that such killing involves non-productive infection of CD4 T cells. Therefore, we focused on events that occur after HIV-1 entry. Our investigations demonstrate that abortive viral DNA synthesis occurring in nonpermissive, quiescent CD4 tonsil T cells, plays a key role in the cell death response. Conversely, in the small subset of permissive target cells, reverse transcription is not interrupted, minimizing the accumulation and subsequent detection of such reverse transcription intermediates (Figure 7).
Figure 7. Consequences of Inhibiting Early Steps of HIV-1 Infection on CD4 T-cell Death.
(A) The nonpermissive state of most CD4 T cells in lymphoid tissue leads to endogenous termination of reverse transcription during DNA chain elongation (i.e. “killing zone”). As a result, DNA intermediates accumulate in the cytoplasm and elicit a multifaceted proapoptotic and proinflammatory innate immune defense programs, coordinated by IFN-stimulatory DNA (ISD) response, Caspase-3, Caspase-1, and IL-1β, to restrict viral spread. Different classes of antiretroviral drugs act at different stage of the HIV life cycle. NNRTIs like efavirenz and nevirapine inhibit early steps of DNA synthesis and therefore prevent such response and the consequence CD4 T-cell death. AZT is less efficient at blocking DNA synthesis and therefore unable to abrogate this response.
(B) In permissive CD4 T cells reverse transcription proceeds, allowing HIV-1 to bypass the “killing zone” and move on to productive (or latent) infection. Interrupting reverse transcription by AZT traps the virus in the “killing zone” and induces cell death. EFV, Efavirenz; NVP, Nevirapine, RTGR, Raltegravir.
See also Figure S6.
Interrupted or slowed reverse transcription may create persistent exposure to cytoplasmic DNA products that elicit an antiviral innate immune response coordinated by activation of type I IFNs (Stetson and Medzhitov, 2006). Such activation, termed IFN-stimulatory DNA (ISD) response, may be analogous to the type I IFN response triggered by the RIG-I-like receptor (RLR) family of RNA helicases that mediate a cell-intrinsic antiviral defense (Rehwinkel and Reis e Sousa, 2010). Our results suggest that abortive HIV-1 infection also stimulates activation of caspase-3, which is linked to apoptosis, and caspase-1, which promotes the processing and secretion of the proinflammatory cytokines like IL–1β. It is certainly possible that pyroptosis elicited in response to caspase-1 activation also contributes to the observed cytopathic response (Schroder and Tschopp, 2010). The release of inflammatory cytokines during CD4 T-cell death could also contribute to the state of chronic inflammation that characterizes HIV infection. This inflammation may fuel further viral spread by recruiting uninfected lymphocytes to the inflamed zone. While this innate response was likely designed to protect the host, it is subverted in the case of HIV infection and importantly contributes to the immunopathogenic effects characteristic of HIV infection and AIDS.
Such antiviral pathways comprise an unrecognized cell-intrinsic retroviral detection system (Manel et al., 2010; Stetson et al., 2008). Viral RNA in infected cells is recognized by members of the RIG-I-like family of receptors that detect specific RNA patterns like uncapped 5′ triphosphate (Rehwinkel and Reis e Sousa, 2010). Although uncapped RNA intermediates are generated by the HIV-1 RNase H, they contain a 5′ monophosphate and therefore may be not recognized by the RIG-I system (Figure 6G). In contrast to RNA receptors, intracellular sensing of viral DNA remains poorly understood. Consequently, it is unclear how HIV-1 DNA intermediates are detected in the cytoplasm of abortively infected CD4 T cells. AIM2 (absent in melanoma 2) was recently identified as a cytoplasmic dsDNA receptor that induces cell death in macrophages through activation of caspase-1 in imflammasomes (Hornung et al., 2009). Our preliminary investigations have not supported a role for AIM2 in cell death induced by abortive HIV infection (not shown) suggesting the potential involvement of a different DNA-sensing mechanism. We also have not identified a role for TLR9 and MYD88 signaling in this form of cell death. Additional candidate sensors recognizing cytoplasmic HIV-1 DNA are now under study.
In summary, both productive and nonproductive forms of HIV infection contribute to the pathogenic effects of this lentivirus. The relative importance of these different cell death pathways might well vary with the stage of HIV infection. For example, direct infection and death might predominate during acute infection where CCR5-expressing memory CD4 T cells in gut-associated lymphoid tissue are effectively depleted. Conversely, the CXCR4-dependent indirect killing we describe in tonsil tissue may reflect later stages of HIV-induced disease where a switch to CXCR4 coreceptor usage occurs in approximately 50% of infected subjects. The current study demonstrates how a cytopathic response involving abortive viral infection of resting nonpermissive CD4 T cells can lead not only to CD4 T-cell depletion but also to the release of proinflammatory cytokines. The ensuing recruitment of new target cells to the site of inflammation may fuel a vicious cycle of continuing infection and CD4 T cell death centrally contributing to HIV pathogenesis.
Experimental Procedures
Culture and Infection of HLACs
Human tonsil or splenic tissues were obtained from the National Disease Research Interchange and the Cooperative Human Tissue Network and processed as previously described (Jekle et al., 2003). For a detailed description see supplemental experimental procedures.
FACS Analysis and Gating Strategy, Preparation of HIV-1 Virions, and Virion-based Fusion Assay
Data were collected on a FACS Calibur (BD Biosciences) and analyzed with Flowjo software (Treestar). HIV-1 viruses were generated by transfection of proviral DNA into 293T cells by the calcium phosphate method. Virion-based fusion assay was performed as previously described (Cavrois et al., 2002). Detailed protocols are provided in the supplemental experimental procedures.
Spinoculation, and Taqman-Based QPCR Analysis of HIV-1-Infected CD4 T Cells
The spinoculation method is described in detail in supplemental Figure S3 A–B. Isolation of HLAC CD4 T cells and QPCR protocol are described in detail in supplemental experimental procedures. Primers and probes sequences used to detect reverse transcription products are provided in Table S1. QPCR reactions were performed in an ABI Prism 7900HT (Applied Biosystems).
ISRE-GFP H35 Reporter Cells, Microscopy, and Generation of Synthetic HIV-1 Reverse Transcription Intermediates
H35 rat hepatic cells containing an ISRE-GFP reporter were maintained as described (Patel et al., 2009). For microscopy imaging, ISRE-GFP reporter H35 cells were infected with a replication competent VSV-G pseudotyped NL4-3 and analyzed using an Axio observer Z1 microscope (Zeiss). Transfections and generation of synthetic HIV-1 reverse transcription intermediates are described in detail in supplemental Figure S5–E and supplemental experimental procedures.
Supplementary Material
Acknowledgments
We thank David N. Levy for the NLENG1 plasmid, David Fenard for the NL4-3 variant plasmids SIM, GIA, GIA-SKY, and SKY, George M. Shaw for the WEAU 16-8 env clone, and Suraj J. Patel, Kevin R. King and Martin L. Yarmush for the H35 ISRE-GFP reporter cell line. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: AMD3100, T-20, Saquinavir, Amprenavir, Indinavir, Nevirapine, Efavirenz, and AZT-resistant HIV-1 clones #629 and #964. Special thanks to Dr. Eva Herker for assistance with fluorescence microscopy, to Dr. Stefanie Sowinski for help with assessing inflammatory responses in primary immune cells, and to Jason Neidleman for stimulating discussions and technical advice. We also thank Marty Bigos for assistance with the flow cytometry, Stephen Ordway and Gary Howard for editorial assistance, and Robin Givens and Sue Cammack for administrative assistance. Dr. Gilad Doitsh was funded by the Universitywide AIDS Research Program (F04-GIVI-210). We also received the following funding NIH/NIGMS T32 GM007618-32 (O.Z.) and NIH/NIAID P30 AI027763 (support for M.C.). The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arts EJ, Wainberg MA. Preferential incorporation of nucleoside analogs after template switching during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1994;38:1008–1016. doi: 10.1128/aac.38.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CE, Sanders RW, Deng Y, Jurriaans S, Lange JM, Lu M, Berkhout B. Emergence of a drug-dependent human immunodeficiency virus type 1 variant during therapy with the T20 fusion inhibitor. J Virol. 2004;78:12428–12437. doi: 10.1128/JVI.78.22.12428-12437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15:671–682. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H, Joshi A, Freed EO, Blumenthal R. Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion. J Biol Chem. 2007;282:16899–16906. doi: 10.1074/jbc.M701701200. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- Grivel JC, Biancotto A, Ito Y, Lima RG, Margolis LB. Bystander CD4+ T lymphocytes survive in HIV-infected human lymphoid tissue. AIDS Res Hum Retroviruses. 2003;19:211–216. doi: 10.1089/088922203763315713. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Palacios EH, Grant RM. Real-time quantitation of HIV-1 p24 and SIV p27 using fluorescence-linked antigen quantification assays. Aids. 2003;17:629–631. doi: 10.1097/00002030-200303070-00021. [DOI] [PubMed] [Google Scholar]
- Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, Yagita H, Lifson JD, Shearer GM. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm GH, Gabuzda D. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J Virol. 2005;79:6299–6311. doi: 10.1128/JVI.79.10.6299-6311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm GH, Zhang C, Gorry PR, Peden K, Schols D, De Clercq E, Gabuzda D. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J Virol. 2004;78:4541–4551. doi: 10.1128/JVI.78.9.4541-4551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. Epub 2009 Jan 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata M, Nagaoka Y, Chen IS. Reassessing the role of APOBEC3G in human immunodeficiency virus type 1 infection of quiescent CD4+ T-cells. PLoS Pathog. 2009;5:e1000342. doi: 10.1371/journal.ppat.1000342. Epub 1002009 Mar 1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med. 2006;203:865–870. doi: 10.1084/jem.20051856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonte JA, Patel T, Hofmann W, Sodroski J. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J Virol. 2000;74:10690–10698. doi: 10.1128/jvi.74.22.10690-10698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder BA, Darby G, Richman DD. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A. 2004;101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Guo F, Zhang L, Kleiman L, Cen S. APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J Biol Chem. 2007;282:32065–32074. doi: 10.1074/jbc.M703423200. [DOI] [PubMed] [Google Scholar]
- Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis LB, Glushakova S, Baibakov B, Zimmerberg J. Syncytium formation in cultured human lymphoid tissue: fusion of implanted HIV glycoprotein 120/41-expressing cells with native CD4+ cells. AIDS Res Hum Retroviruses. 1995;11:697–704. doi: 10.1089/aid.1995.11.697. [DOI] [PubMed] [Google Scholar]
- Patel SJ, King KR, Casali M, Yarmush ML. DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci U S A. 2009;106:12867–12872. doi: 10.1073/pnas.0809292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Reis e Sousa C. RIGorous detection: exposing virus through RNA sensing. Science. 2010;327:284–286. doi: 10.1126/science.1185068. [DOI] [PubMed] [Google Scholar]
- Rimsky LT, Shugars DC, Matthews TJ. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni de Sio FR, Trono D. APOBEC3G-depleted resting CD4+ T cells remain refractory to HIV1 infection. PLoS One. 2009;4:e6571. doi: 10.1371/journal.pone.0006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M, Munch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Muller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Swiggard WJ, O'Doherty U, McGain D, Jeyakumar D, Malim MH. Long HIV type 1 reverse transcripts can accumulate stably within resting CD4+ T cells while short ones are degraded. AIDS Res Hum Retroviruses. 2004;20:285–295. doi: 10.1089/088922204322996527. [DOI] [PubMed] [Google Scholar]
- Thomas C. Roadblocks in HIV research: five questions. Nat Med. 2009;15:855–859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis SR, Algeciras-Schimnich A, Bou G, Heppelmann CJ, Villasis-Keever A, Collman RC, Paya CV. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J Clin Invest. 2001;107:207–215. doi: 10.1172/JCI11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wyma DJ, Jiang J, Shi J, Zhou J, Lineberger JE, Miller MD, Aiken C. Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail. J Virol. 2004;78:3429–3435. doi: 10.1128/JVI.78.7.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.