Abstract
It is established that a high-frequency chromosomal deletion of ca. 100 kb accounts for the loss of properties making up the pigmented phenotype (Pgm+) of wild-type Yersinia pestis. These determinants are known to include virulence by peripheral routes of injection, sensitivity to the bacteriocin pesticin, adsorption of exogenous hemin or Congo red at 26 degrees C, and growth in iron-sequestered medium at 37 degrees C. We have now identified the outer membrane as the primary site of exogenous hemin storage in Pgm+ cells grown at 26 degrees C. Significant outer membrane storage of hemin did not occur in Pgm- mutants or in Pgm+ cells cultivated at 37 degrees C. However, both Pgm+ and Pgm- organisms grown at 37 degrees C contained a periplasmic reservoir of hemin, which may be associated with a temperature-dependent ca. 70-kDa peptide recently equated with antigen 5. At 37 degrees C, Pgm+ and Pgm- yersiniae also utilized a cytoplasmic ca. 19-kDa bacterioferritin-like peptide for deposition of inorganic iron. Incorporation of [55Fe]hemin into pools at 37 degrees C was not significantly inhibited by competition with excess unlabeled Fe3+. However, excess unlabeled hemin modestly competed with incorporation of label from 55FeCl3. This relative independence of storage pools observed at 37 degrees C is consistent with physiological linkage to in vivo acquisition and transport of Fe3+ from ferritin and of hemin from hemoglobin, myoglobin, or hemopexin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Harrison P. M., Guest J. R. Cloning, sequencing, and mapping of the bacterioferritin gene (bfr) of Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3940–3947. doi: 10.1128/jb.171.7.3940-3947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Bartsch R. G., Kakuno T., Horio T., Kamen M. D. Preparation and properties of Rhodospirillum rubrum cytochromes c 2, cc', and b 557.5, and flavin mononucleotide protein. J Biol Chem. 1971 Jul 25;246(14):4489–4496. [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhoff H. A., Vinal A. C. Congo red medium to distinguish between invasive and non-invasive Escherichia coli pathogenic for poultry. Avian Dis. 1986 Jan-Mar;30(1):117–121. [PubMed] [Google Scholar]
- Brubaker R. R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991 Jul;4(3):309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R., Lough S. A hemoprotein from azotobacter containing non-heme iron: isolation and crystallization. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1274–1281. doi: 10.1016/0006-291x(73)91125-x. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- CRUMPTON M. J., DAVIES D. A. An antigenic analysis of Pasteurella pestis by diffusion of antigens and antibodies in agar. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):109–134. doi: 10.1098/rspb.1956.0021. [DOI] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Congo red binding phenotype is associated with hemin binding and increased infectivity of Shigella flexneri in the HeLa cell model. Infect Immun. 1987 Jun;55(6):1393–1398. doi: 10.1128/iai.55.6.1393-1398.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Stoebner J. A., Payne S. M. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991 Aug;59(8):2706–2711. doi: 10.1128/iai.59.8.2706-2711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., West E. P., Sparling P. F. Effects of serum carrier proteins on the growth of pathogenic neisseriae with heme-bound iron. Infect Immun. 1987 Sep;55(9):2171–2175. doi: 10.1128/iai.55.9.2171-2175.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms S. D., Oliver J. D., Travis J. C. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984 Aug;45(2):345–349. doi: 10.1128/iai.45.2.345-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Kubo H. The metabolism of Streptomyces griseus. X. A study of Streptomyces griseus cytochrome b. Biochim Biophys Acta. 1965 Oct 25;110(1):57–65. doi: 10.1016/s0926-6593(65)80094-7. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutyrev V. V., Filippov A. A., Oparina O. S., Protsenko O. A. Analysis of Yersinia pestis chromosomal determinants Pgm+ and Psts associated with virulence. Microb Pathog. 1992 Mar;12(3):177–186. doi: 10.1016/0882-4010(92)90051-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucier T. S., Brubaker R. R. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J Bacteriol. 1992 Apr;174(7):2078–2086. doi: 10.1128/jb.174.7.2078-2086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Williams J. M., Treffry A., Harrison P. M. Reconstituted and native iron-cores of bacterioferritin and ferritin. J Mol Biol. 1987 Dec 5;198(3):405–416. doi: 10.1016/0022-2836(87)90290-7. [DOI] [PubMed] [Google Scholar]
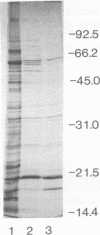
- Mehigh R. J., Braubaker R. R. Major stable peptides of Yersinia pestis synthesized during the low-calcium response. Infect Immun. 1993 Jan;61(1):13–22. doi: 10.1128/iai.61.1.13-22.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehigh R. J., Sample A. K., Brubaker R. R. Expression of the low calcium response in Yersinia pestis. Microb Pathog. 1989 Mar;6(3):203–217. doi: 10.1016/0882-4010(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A. Soluble cytochrome composition of the purple phototrophic bacterium, Rhodopseudomonas sphaeroides ATCC 17023. Biochim Biophys Acta. 1985 May 31;807(3):308–319. doi: 10.1016/0005-2728(85)90263-4. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Mann S., Bannister J. V. Isolation and properties of the complex nonheme-iron-containing cytochrome b557 (bacterioferritin) from Pseudomonas aeruginosa. J Inorg Biochem. 1986 Oct-Nov;28(2-3):329–336. doi: 10.1016/0162-0134(86)80097-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in Shigella. Mol Microbiol. 1989 Sep;3(9):1301–1306. doi: 10.1111/j.1365-2958.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Pendrak M. L., Perry R. D. Characterization of a hemin-storage locus of Yersinia pestis. Biol Met. 1991;4(1):41–47. doi: 10.1007/BF01135556. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Pendrak M. L., Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990 Oct;172(10):5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpic J. K., Robins-Browne R. M., Davey R. B. Differentiation between virulent and avirulent Yersinia enterocolitica isolates by using Congo red agar. J Clin Microbiol. 1983 Sep;18(3):486–490. doi: 10.1128/jcm.18.3.486-490.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample A. K., Brubaker R. R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987 Oct;3(4):239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- Sikkema D. J., Brubaker R. R. Outer membrane peptides of Yersinia pestis mediating siderophore-independent assimilation of iron. Biol Met. 1989;2(3):174–184. doi: 10.1007/BF01142557. [DOI] [PubMed] [Google Scholar]
- Sikkema D. J., Brubaker R. R. Resistance to pesticin, storage of iron, and invasion of HeLa cells by Yersiniae. Infect Immun. 1987 Mar;55(3):572–578. doi: 10.1128/iai.55.3.572-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982 Apr;36(1):129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stugard C. E., Daskaleros P. A., Payne S. M. A 101-kilodalton heme-binding protein associated with congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun. 1989 Nov;57(11):3534–3539. doi: 10.1128/iai.57.11.3534-3539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Une T., Brubaker R. R. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984 Mar;43(3):895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Brubaker R. R. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect Immun. 1982 Dec;38(3):953–959. doi: 10.1128/iai.38.3.953-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck B. S., Verhoef J. Iron and host defence. Eur J Clin Microbiol. 1983 Feb;2(1):6–10. doi: 10.1007/BF02019915. [DOI] [PubMed] [Google Scholar]