Abstract
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), the most abundant heterocyclic amine produced during the cooking of meats and fish, is suspected to be a human carcinogen. Metabolic activation of PhIP is primarily mediated by the enzyme cytochrome P450 (CYP) 1A2. Metabolism of PhIP by CYP1A2 differs considerably between humans and rodents, with more N2-hydroxylation (activation) and less 4′-hydroxylation (detoxication) in humans. Transgenic CYP1A-humanized mice (hCYP1A-mice), which have the human CYP1A1 and CYP1A2 genes but lack the murine orthologs Cyp1a1 and Cyp1a2, provide an excellent opportunity to develop a relevant model to study dietary-induced colon carcinogenesis. The treatment with 200 mg/kg PhIP by oral gavage, followed by 1.5% dextran sodium sulfate (DSS) in the drinking water for 7 days, was found to be an effective combination to induce colon carcinogenesis in hCYP1A-mice. Tumor multiplicity at week 6 was calculated to be 3.75 ± 0.70 and for week 10 was 3.90 ± 0.61 with 80–95% of the tumors being adenocarcinomas. No tumors were found in the similarly treated wild-type mice. Western blots revealed overexpression of β-catenin, c-Myc, cyclin D1, inducible nitric oxide synthase and cyclooxygenase-2 in colon tumor samples. Strong nuclear localization of β-catenin was observed in tumors. These results illustrate that PhIP and DSS combination produces rapid colon carcinogenesis in hCYP1A-mice and this is an effective model to mimic human colon carcinogenesis.
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in both men and women in the USA (1). Although the causes remain largely unknown, dietary factors have been implicated in the etiology of this disease, with several studies demonstrating associations between consumption of fried or grilled meats with colorectal cancer (2–4).
The procarcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is the most abundant heterocyclic amine produced during the cooking of meats and fish. The estimated daily human dietary intake of PhIP has been reported to be 0.1–13.8 μg (5) and in another study, 0–865 ng/day with a mean of 72 ng/day (6). Initial metabolic activation of PhIP by N-hydroxylation is principally mediated by the hepatic cytochrome P450 (CYP) 1A2 enzyme (7,8). CYP1A1 and CYP1B1 can also metabolize PhIP but are considerably less active toward PhIP than CYP1A2 (9). Following N-hydroxylation of PhIP, it is then further conjugated to a glucuronide in the liver (10) and transported via the bile into the colon or transported as stable N-hydroxy- or N-acetoxy-arylamines via the circulation to peripheral tissues including the colon (11). Once reabsorbed into the colonic mucosa, N-hydroxyarylamines can be further activated by N-acetyltransferases (12) or sulfotransferases (13). These activated esters can react covalently with DNA and other macromolecules forming adducts that may cause mutations and lead to the induction of cancer. A higher CYP1A2 activity in combination with higher N-acetyltransferase activity has been associated with an elevated risk for colon cancer in individuals eating well-cooked meats, which are a rich source of heterocyclic amines (14,15).
PhIP induces aberrant crypt foci (ACF), which are putative precursor lesions for colon adenocarcinomas, in rats (16) and mice (17). Studies have shown that rat colon cancers have been induced with PhIP (18,19), whereas, in mice, administration of PhIP mainly induces non-epithelial malignancies such as malignant lymphomas and leukemia (20–22). No reports of colon carcinogenicity from treatment with PhIP alone exist so far (22). This suggests a weak cancer initiating capability of PhIP in the colon of mice. Recent studies have demonstrated that adenocarcinomas can be rapidly induced in the colon of mice by combined treatment with PhIP and dextran sodium sulfate (DSS) (23–25). DSS is a potent inducer of colitis in experimental animals and DSS-induced colitis has been used as a model for ulcerative colitis in humans (26).
Species differences in the oxidative metabolism of PhIP have been observed between humans and rodents (27,28). In rodents, metabolism by PhIP is predominantly oxidation in the ring system (4′-hydroxylation) followed by Phase II conjugation. However, in humans, N2-hydroxylation to the proximate mutagen N2-hydroxy-PhIP is the major metabolic pathway followed by glucuronidation. Since differences exist in the metabolism of PhIP between humans and rodents, appropriate extrapolation of cancer risk from experimental animals to humans is of concern, particularly in establishing safe thresholds for human exposure to PhIP.
Humanized transgenic mice have been developed in an effort to create more reliable in vivo systems to study and predict human responses to xenobiotics (29). In particular, CYP1A2-humanized mice, which express the human CYP1A2 gene but not the mouse Cyp1a2 gene, were shown to accurately express CYP1A2 protein reflective of their expression in humans (30,31). When compared with wild-type mice, preferential N2-hydroxylation of PhIP was demonstrated in these CYP1A2-humanized mice, a pathway for PhIP metabolism that in vitro studies revealed as predominant with the human ortholog (30). More recently, a transgenic mouse model, CYP1A-humanized mice (hCYP1A-mice), was developed expressing both the human CYP1A1 and CYP1A2 genes and deficient in both the murine Cyp1a1 and Cyp1a2 genes (32).
In studying cancer prevention and carcinogenesis, a model relevant to human colon carcinogenesis that accurately represents the metabolic activation of a dietary carcinogen such as PhIP is urgently needed. Therefore, the primary goal of this study is to develop a model for PhIP-induced colon carcinogenesis using hCYP1A-mice. It is anticipated that the increased metabolic activation of PhIP in hCYP1A-mice (30) will result in greater carcinogenic effect when compared with wild-type mice. Colon cancer is expected to be the predominant cancer in mice given a combination of PhIP and DSS as observed in previous studies (23–25), whereas, other types of cancer such as prostate cancer and mammary cancer may also develop in mice given PhIP alone. The hCYP1A-mice may also be a good model for other cancers. The results described herein, demonstrate that a PhIP and DSS treatment combination to hCYP1A-mice provides a more effective model of colon carcinogenesis when compared with wild-type mice. Colon tumors are shown to be optimally formed at 6–10 weeks after a treatment with 200 mg/kg PhIP followed by 1.5% DSS. The PhIP-induced colon carcinogenesis model using hCYP1A-mice presents an excellent model for colon cancer prevention studies.
Materials and methods
Chemicals
PhIP was obtained from Toronto Research Chemicals (North York, Ontario, Canada) and dissolved in the vehicle [20% (vol/vol) dimethyl sulfoxide (DMSO) in milliQ water], adjusted to approximately pH 3.5 with HCl. DSS (molecular weight 35 000–44 000) was purchased from MP Biomedicals (Solon, OH) and dissolved in milliQ water to 1 or 1.5% (wt/vol). DMSO was bought from Sigma–Aldrich (St Louis, MO).
Animals
All animal procedures and handling was in accordance with the animal study protocol number 02-027 approved by the Rutgers University Institutional Animal Care and Use Committee. Mice were maintained under standard 12 h light/dark cycle with water and diet provided ad libitum unless otherwise specified. Male and female Cyp1a2/Cyp1a1tm2Dwn Tg(CYP1A1,CYP1A2)1Dwn/J and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used as founders to establish homozygous breeding colonies in house. Cyp1a2/Cyp1a1tm2Dwn Tg(CYP1A1,CYP1A2)1Dwn/J mice (on a C57BL/6 background) which were homozygous for the human CYP1A1/2 transgene and homozygous for the mouse Cyp1a1/2 null allele were designated as hCYP1A-mice in this study. C57BL/6J mice were used as wild-type controls. Homozygosity of hCYP1A-mice was confirmed by breeding with wild-type mice and polymerase chain reaction genotyping. Breeding was carried out in a sterile animal room and then the mice were transferred to a non-sterile animal room for at least 1 week prior to treatments.
Animal treatments
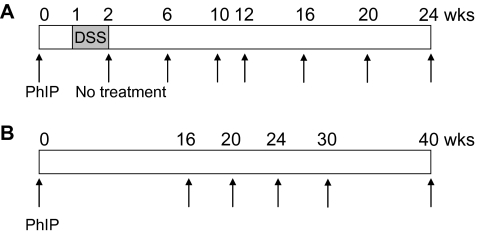
hCYP1A-mice and wild-type mice were treated with a single dose of PhIP (100 or 200 mg/kg body wt) or vehicle (20% DMSO) by oral gavage at 5–8 weeks of age. Both male and female mice were used at a ratio of ∼1 to 1 in each group. After 1 week, mice were divided into groups and some administered 1 or 1.5% (wt/vol) DSS in drinking water for 7 days (Figure 1). Mice were then returned to regular drinking water and continued without any further treatment until sacrificed at the final end points of 24 weeks, for mice given PhIP and DSS combined, and 40 weeks, for mice given PhIP only (Figure 1). Mice were monitored daily during DSS treatment for changes in body weight and clinical signs of colitis such as rectal bleeding. Mice were maintained on an AIN-93M diet (Research Diets, New Brunswick, NJ) throughout the experimental study and body weights were measured weekly. At the termination of the study, mice were sacrificed by CO2 asphyxiation and all organs were carefully inspected for macroscopic pathological lesions. Liver, spleen, lung, mammary and prostate were removed, weighed and fixed in 10% buffered formalin for at least 24 h. The colon was excised, flushed with saline, the length measured, cut open longitudinally, flattened on filter paper and washed with saline. Macroscopic inspection of the colons was carefully carried out, with the number, size and location of visible tumors recorded. The flattened colon tissue was placed between two sheets of filter paper and fixed in 10% buffer formalin for at least 24 h. Colon, liver and tumor (excised from colon) samples from some mice were frozen in liquid nitrogen and stored at −80°C until analysis.
Fig. 1.
Protocols used for investigating colon carcinogenesis. (A) hCYP1A-mice were given a single dose of PhIP (100 or 200 mg/body wt) by oral gavage and 1 week later followed by 1 or 1.5% DSS in their drinking water for 7 days. Mice were sacrificed at various time points from 6 to 24 weeks following PhIP treatment. (B) hCYP1A-mice were given a single dose of 200 mg/kg PhIP by oral gavage and mice sacrificed at various time points from 16 to 40 weeks following PhIP gavage.
Genotyping by polymerase chain reaction
The presence of the human CYP1A1 and human CYP1A2 genes and the mouse Cyp1a1/2 double-knockout allele were verified using polymerase chain reaction primer sequences and conditions provided by Jackson Laboratories. Tail genomic DNA was prepared using the RED Extract-N-Amp Tissue PCR kit (Sigma–Aldrich). When detecting human CYP1A1 or human CYP1A2 genes, mouse epoxide hydrolase 1 gene (Ephx1) primers served as an internal positive control for amplification, yielding an additional fragment of 341 bp (30).
Histopathology
Formalin-fixed colonic tissues were Swiss rolled, processed in paraffin and serially sectioned at 4 μm of thickness. Histopathological analysis of the entire length of the colon was performed on two hematoxylin- and eosin-stained sections (sections 1 and 10) per colon. Hyperplasia and dysplasia were scored as 0 (normal), 1 (mild hyperplasia: epithelial cells lining the colon appear normal, but crypts appear two to four times thicker than normal crypts), 2 (low-grade dysplasia: hyperchromatic cells, –two to four times thicker epithelium, fewer goblet cells and scattered crypts developing an arborizing pattern) or 3 (high-grade dysplasia: hyperchromatic, highly mitotic cells, less than four times thicker epithelium, few or no goblet cells in the crypts with arborizing pattern and crypts extending to muscularis mucosa or submucosa) (33).
Immunohistochemistry
Immunohistochemistry for β-catenin expression was performed on 4 μm thick paraffin-embedded sections from formalin-fixed Swiss-rolled colons. Two different serial sections were examined (sections 2 and 11). In brief, after deparaffinization and rehydration, the sections were treated with antigen unmasking solution (Vector Laboratories, Burlingame, CA) in a microwave for 6 min at full power. The sections were then immersed in 3% hydrogen peroxide. Sections were then incubated with β-catenin antibody (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The sections were then incubated with biotinylated secondary antibody (1:200), followed by streptavidin–biotin peroxidase conjugate (Vector Laboratories). Proteins were visualized with 3,3′-diaminobenzidine and sections were counterstained with hematoxylin and mounted with Permount. On control sections, incubation with primary antibody was omitted. The total number of cells and infiltrated β-catenin-positive cells in mucosa and submucosa was performed by using the Image-Pro Plus Image Processing System (Version 5.0) (Media Cybernetics, Bethesda, MD).
Scoring of colonic aberrant crypts
ACF analysis was carried out on colon tissue using the conventional methylene blue staining method (17). In brief, the formalin-fixed flattened colon tissues were stained with 0.2% methylene blue for 3–5 min and examined under a light microscope. The colon was cut into three sections: proximal, middle and distal and then each section was individually analyzed for the total number of ACF per colon and the multiplicity of crypts per ACF (≥4 and ≥10 aberrant crypts per focus).
Tissue homogenate preparation
For determining β-catenin, c-Myc, cyclin D1, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein expression in the colon by western blots, tissue homogenates were prepared. Colon tumors (∼3) were pooled from each mouse to make each individual tumor sample. Normal untreated colons were used to make the control colon samples. In brief, colon and tumor samples were homogenized in ice-cold T-PER buffer (Thermo Scientific, Rockford, IL) using a glass douncer for 5 min on ice, followed by sonication three times for 15 pulses. The homogenates were centrifuged for 20 min at 3000 r.p.m. and the supernatant was collected. Protein concentrations were measured using the BCA protein assay kit (Thermo Scientific).
Western blotting
Protein (5 μg) was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 10% polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. Membranes were blocked using Odyssey blocking buffer (LI-COR, Lincoln, NB) for 1 h at room temperature followed by incubation with primary antibody overnight at 4°C. The infrared fluorescent-labeled secondary antibody from LI-COR was used at a 1:5000 dilution. Detection of immunoreactive proteins was carried out using the Odyssey Infrared Imaging System (LI-COR). β-Catenin antibody (1:2000) was purchased from BD Bioscience (San Jose, CA). c-Myc (1:200), cyclin D1 (1:200), COX-2 (1:200) and iNOS (1:200) antibodies were purchased from Santa Cruz Biotechnology. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control and this antibody was from Cell Signalling Technology (Danvers, MA).
Statistical analysis
Values are presented as mean ± standard error. Tumor incidence and multiplicity (number of tumors per tumor-bearing mouse) were evaluated with Student’s t-test and one- way analysis of variance for pairwise and group comparisons, respectively (GraphPad Prism version 5).
Results
hCYP1A-mice identified by genotyping as containing the human CYP1A1 and CYP1A2 transgenes and the murine Cyp1a1- and Cyp1a2-knockout allele were used to create homozygous mouse colonies that were used for the carcinogenesis studies described herein.
General observations and survival rates
In this carcinogenesis study, mice were given PhIP or DMSO by oral gavage followed by DSS (0, 1 or 1.5%). Body weights were monitored weekly and the changes are shown in supplementary Figure 1 (available at Carcinogenesis Online). After the initial treatment of PhIP in hCYP1A and wild-type mice, the body weights dropped and continued to do so as DSS was administered. This drop in body weight was more significant in hCYP1A-mice compared with wild-type. Some rectal bleeding was observed after 3 or 4 days of DSS treatment in hCYP1A-mice, which ceased a few days after DSS treatment was stopped, when body weights recovered. These trends have previously been observed in other carcinogenesis models of PhIP and DSS (23,25). Some hCYP1A-mice died approximately 2–3 days after 200 mg/kg PhIP administration, with 90–93% of the treated hCYP1A-mice remaining at week 1 (Table I). This was apparently due to the activation of PhIP to toxic species because no wild-type mice died after PhIP treatment. Administering 1.5% DSS following PhIP treatment resulted in some more deaths with 84% of the original mice remaining at week 2 (Table I). The effect of DSS treatment after DMSO administration also resulted in some deaths to hCYP1A-mice, with 90% remaining at week 2 (Table I). In contrast, no wild-type mice died during treatment with DSS. The greater susceptibility of the hCYP1A-mice to DSS treatment remains to be investigated.
Table I.
Survival rates of mice in the study
| Genotype | Treatment | Duration (weeks) | No. of mice treated | Surviving mice |
|
| 1 week after PhIP or DMSO (%) | 1 week after DSS (%) | ||||
| hCYP1A | 200 mg/kg PhIP + 1.5% DSS | 6–10 | 58 | 52 (90) | 49 (84) |
| hCYP1A | DMSO + 1.5% DSS | 6–10 | 20 | 20 (100) | 18 (90) |
| hCYP1A | DMSO | 24 | 17 | 17 (100) | 17 (100) |
| hCYP1A | 200 mg/kg PhIP | 16–24 | 30 | 28 (93) | 28 (93) |
| Wild-type | 200 mg/kg PhIP + 1.5% DSS | 24 | 11 | 11 (100) | 11 (100) |
| Wild-type | DMSO | 24 | 17 | 17 (100) | 17 (100) |
| Wild-type | 200 mg/kg PhIP | 14–24 | 17 | 17 (100) | 17 (100) |
Mice were given 200 mg/kg PhIP or vehicle (DMSO) by oral gavage and 1 week later were given 0 or 1.5% DSS in the drinking water for 7 days. Duration indicates the number of weeks following PhIP or DMSO administration when the mice were sacrificed.
Colon tumor assessment in hCYP1A-mice
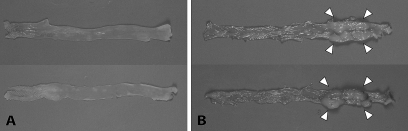
hCYP1A and wild-type mice treated with 200 mg/kg PhIP followed by 1.5% DSS were initially sacrificed at various time points from week 12–24 weeks after PhIP gavage. The next time point for sacrifice was determined based on the pathology of the colon, with the optimal time point established when 80–90% of the mice sacrificed had developed 3–10 colon tumors (adenomas and adenocarcinomas) per mouse. All the hCYP1A-mice which were treated with 200 mg/kg PhIP followed by 1.5% DSS and sacrificed between 12 and 21 weeks, revealed tumors in the colon with 100% incidence and a tumor multiplicity of 4.57 ± 1.39 (Table II). No tumors or other gross abnormalities were observed in the similarly treated wild-type mice sacrificed between 12 and 24 weeks. Since tumors were only observed in hCYP1A-mice treated with 200 mg/kg PhIP followed by 1.5% DSS at weeks 12–21, but not in wild-type mice between weeks 12–24, earlier time points were investigated only in hCYP1A-mice. Tumor multiplicity at week 6 was calculated to be 3.75 ± 0.70 (n = 20) and for week 10 was 3.90 ± 0.61 (n = 29) and tumor incidence 85 and 86%, respectively (Table II). There was no significant difference between tumor multiplicity or tumor incidence at week 6 and week 10 for this treatment. Representative colons from male and female hCYP1A and wild-type mice after 200 mg/kg PhIP and 1.5% treatment are shown in Figure 2. Macroscopic evaluation of the colons revealed no tumors in the colons from wild-type mice at week 24 (Figure 2A) but several tumors in the colons from hCYP1A-mice at week 10 (Figure 2B). The colon tumors were polypoid nodular or flat-type tumors and were located mainly in the middle to distal regions of the colon (Figure 2B). No significant differences in colonic tumor number and size were observed between male and female mice (see supplementary Table I, available at Carcinogenesis Online), so the total tumor multiplicity numbers were combined from both sexes.
Table II.
Incidence of colon tumors in hCYP1A-mice
| Genotype | Treatment | Duration (weeks) | No. of mice examined | Colon tumor total |
Adenoma multiplicity | Adenocarcinoma multiplicity | |
| Incidence (%) | Multiplicity | ||||||
| hCYP1A | 200 mg/kg PhIP + 1.5% DSS | 6 | 20 | 85 | 3.75 ± 0.70* | 0.20 ± 0.13* | 3.5 ± 0.95* |
| 10 | 29 | 86 | 3.90 ± 0.61* | 0.60 ± 0.16* | 2.4 ± 0.45* | ||
| 12–21a | 7 | 100 | 4.57 ± 1.39* | 0 | 4.57 ± 1.39* | ||
| hCYP1A | 100 mg/kg PhIP + 1% DSS | 24–25b | 11 | 9 | 0.09 ± 0.09 | 0 | 0.09 ± 0.09 |
| hCYP1A | 200 mg/kg PhIP | 16–40c | 38 | 0 | 0 | 0 | 0 |
| hCYP1A | DMSO | 24 | 17 | 0 | 0 | 0 | 0 |
| Wild-type | 200 mg/kg PhIP + 1.5% DSS | 12–24d | 9 | 0 | 0 | 0 | 0 |
| Wild-type | 200 mg/kg PhIP | 14–40e | 26 | 0 | 0 | 0 | 0 |
| Wild-type | DMSO | 24 | 17 | 0 | 0 | 0 | 0 |
Mice were given 100 or 200 mg/kg PhIP or vehicle (DMSO) by oral gavage and 1 week later were given 0, 1 or 1.5% DSS in the drinking water for 7 days. Duration indicates the number of weeks following PhIP or DMSO administration, when the mice were sacrificed. Histological analysis was used to determine whether colon tumor was an adenoma or adenocarcinoma. Multiplicity values are mean ± standard error.
Mice examined at weeks 12 (n = 2), 14 (n = 2), 18 (n = 1) and 21 (n = 2).
Mice examined at weeks 24 (n = 7) and 25 (n = 4).
Mice examined at weeks 16 (n = 7), 20 (n = 8), 24 (n = 13), 30 (n = 5) and 40 (n = 5).
Mice examined at weeks 12 (n = 2), 18 (n = 3) and 24 (n = 6).
Mice examined at weeks 14 (n = 4), 20 (n = 6), 24 (n = 7), 30 (n = 4) and 40 (n = 5).
*P < 0.02, statistically different from the value for wild-type mice given 200 mg/kg PhIP + 1.5% DSS (two-tailed Student’s t-test).
Fig. 2.
Macroscopic examination of excised colons. Representative colons from mice treated with PhIP (200 mg/kg) and 1.5% DSS in (A) wild-type mice (upper: female, lower: male), 24 weeks after PhIP treatment and in (B) hCYP1A transgenic mice (upper: female, lower: male), 10 weeks after PhIP treatment. Arrows indicate colonic tumors.
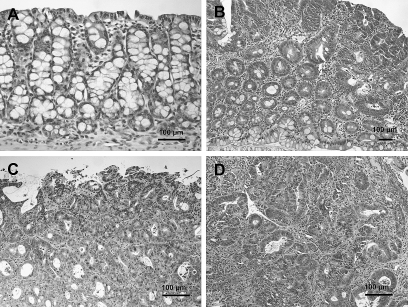
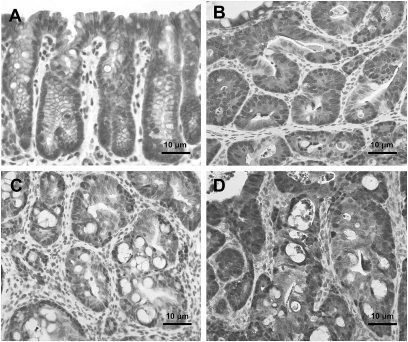
Histopathological diagnosis of colon tumors excised from representative mice treated with 200 mg/kg PhIP and 1.5% DSS revealed that some were tubular adenomas, but the majority at 6 and 10 weeks were tubular adenocarcinomas (Figure 3 and Table II). Severe dysplasia with mucosal ulceration was also observed (Figure 3C). Scoring the colonic tumors after histopathological diagnosis revealed 87% of the tumors observed at weeks 6–10 were adenocarcinomas (Table II).
Fig. 3.
Histopathology of colonic lesions in hCYP1A transgenic mice treated with 200 mg/kg PhIP and 1.5% DSS, sacrificed 6 weeks after PhIP administration. (A) normal colonic mucosa, (B) tubular adenomas, (C) polypoid tubular adenocarcinomas in colon with mucosal ulceration, (D) adenocarcinomas in colon with severe inflammation. Sections were stained with hematoxylin and eosin.
A lower dose of PhIP in combination with DSS was also administered to the hCYP1A and wild-type mice. The 100 mg/kg of PhIP and 1% DSS treatment group revealed 1 colonic adenocarcinoma of the 11 mice sacrificed at week 24–25 after PhIP gavage (9% incidence) (Table II).
No visible colonic tumors or other cancerous malignancies were observed in hCYP1A-mice treated with 200 mg/kg PhIP only (no DSS) in mice examined at week 16 (n = 7), week 20 (n = 8) and week 24–25 (n = 13) (Table II). At week 20 of 200 mg/kg PhIP only treatment to hCYP1A-mice, enlarged lymph nodes and spleens were observed in some mice and at week 24, some rectal bleeding suggesting inflammation. A small number of mice were examined at week 30 (n = 5) and week 40 (n = 5) after PhIP gavage, but again no visible colon tumors were observed in these mice (Table II).
Detection of ACF in hCYP1A-mice
Due to the low tumor incidence in hCYP1A-mice treated with 100 mg/kg PhIP and 1% DSS, the colons of these mice were stained with methylene blue to visualize ACF. In this treatment group, ACF were observed as early as 6 weeks after PhIP gavage, with the total average number of ACF’s observed after 6–16 weeks being 8.3 (n = 11), after 20–22 weeks, the average was 26.0 (n = 9) and after 24–25 weeks, the average was 27.8 (n = 11) (Table III). The incidence of ACF was shown to increase with duration of this treatment with 25 weeks after PhIP gavage presenting the most ACF (Table III). The majority of ACF identified were located in the middle to distal sections of the colon, which corresponds to the regions where tumors were observed. These data suggest that ACF may be considered as precursors for colon polyps in the hCYP1A mouse model.
Table III.
Development of ACF in colons of hCYP1A-mice treated with 100 mg/kg PhIP and DSS
| Duration of treatment | Incidence of ACF (%) | No. of ACF per colon |
| 6–16 weeks | 10/11 (83.3) | 8.3 ± 2.17 |
| 20–22 weeks | 9/9 (100) | 26.0 ± 4.92 |
| 24–25 weeks | 11/11 (100) | 27.8 ± 10.9 |
Mice were treated with 100 mg/kg PhIP by oral gavage and 1 week later given 1% DSS in drinking water for 7 days. Values for no. of ACF per colon are mean ± standard error.
Expression of β-catenin, iNOS, COX-2, cyclin D1 and c-Myc
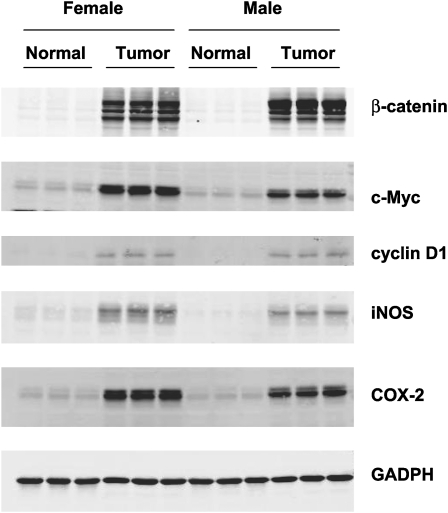
Western blot analysis revealed an overexpression of β-catenin, c-Myc, cyclin D1, iNOS and COX-2 in hCYP1A colonic tumor samples but not in control colon tissue samples from hCYP1A-mice sacrificed 10 weeks post-PhIP gavage (Figure 4). Strong nuclear localization of β-catenin was also shown to be present in colon tumors at week 10 (Figure 5). Minimal staining of β-catenin was observed in the membrane of the crypts of normal colon mucosa (Figure 5A). Strong nuclear localization of β-catenin staining was observed in colons with high-grade dysplasia (Figure 5B). Nuclear staining of β-catenin in a colonic adenoma is more intense compared with normal colonic tissue but weaker compared with a colonic adenocarcinoma, which had very strong staining patterns (Figure 5C–D). These data suggest that proteins that are often overexpressed in human colon cancer are also present in the hCYP1A mouse model for colon cancer.
Fig. 4.
Western blot analysis of β-catenin, c-Myc, cyclin D1, iNOS and COX-2 expression in colonic and tumor tissue of hCYP1A transgenic mice which were treated with PhIP (200 mg/kg) and 1.5% DSS and sacrificed 10 weeks after PhIP administration. Immunodetection of these proteins were carried out using tissue homogenates (5 μg) from untreated normal colon samples and three representative colon tumor samples from male and female hCYP1A-mice. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control.
Fig. 5.
Immunohistochemical analysis of β-catenin in colonic tissue. hCYP1A transgenic mice were treated with 200 mg/kg PhIP and 1.5% DSS and sacrificed 10 weeks after PhIP administration. (A) Normal colon mucosa with brown staining in the membrane and cytoplasm, (B) high-grade dysplasia with brown staining of β-catenin in the nuclei, (C) nuclear staining of β-catenin in colonic adenomas and (D) intense nuclear staining for β-catenin in colonic adenocarcinomas.
Discussion
This study describes the development of a novel model of colon carcinogenesis using PhIP and DSS in mice humanized for CYP1A. Metabolism of PhIP by CYP1A2 differs substantially between humans and rodents with more N2-hydroxylation (activation) and less 4′-hydroxylation (detoxication) in humans (28). Therefore, the human response to PhIP and other heterocyclic amine exposure may not be accurately reflected in the laboratory rodent. There are several research groups, which have aimed to develop efficient experimental models for PhIP-induced colon carcinogenesis using rats (19,34) and mice (23–25). Since it has been shown previously that in CYP1A2-humanized mice there is an increased metabolic activation of PhIP compared with wild-type mice (30), in this study, hCYP1A-mice were used to investigate if there would be a greater carcinogenic effect compared with wild-type mice in the induction of colon carcinogenesis with PhIP. In the model described herein, a single dose of PhIP (200 mg/kg) followed by 1 week administration of DSS (1.5%) to the hCYP1A-mice was found to be the most effective treatment combination to induce colonic adenomas and adenocarcinomas in as early as 6 weeks with a tumor incidence of 85% and multiplicity of 3.70 ± 0.87 (Table II). The amount of PhIP taken up by the gastrointestinal tract is very low, thus high concentrations of PhIP are required to induce formation of preneoplastic and neoplastic lesions in colons of mice as demonstrated in this study and others (27–29). The tumor incidence and multiplicity were much higher than previous studies with wild-type mice receiving the same dose of PhIP (200 mg/kg) and a higher dose of DSS (2%), with tumor incidence of 78% and multiplicity of 1.22 ± 1.20 at week 16 in CD-1 mice (23) and 50% tumor incidence and multiplicity of 1.00 ± 1.30 at week 20 in C57BL/6 mice (25). Thus, clearly this hCYP1A mouse model demonstrates a more effective carcinogenic capability than with wild-type mice alone, with higher tumor incidence and multiplicity after a shorter treatment period.
In this study, no tumors were observed in wild-type (C57BL/6) mice treated with 200 mg/kg PhIP and 1.5% DSS up to week 24, whereas Nakanishi et al. (25) reported a 17% tumor incidence and multiplicity of 0.17 ± 0.38 from 17 C57BL/6 mice treated with 200 mg/kg PhIP and 1.5% DSS at week 20. The reason for this difference is not known. Even if we compare with the data on wild-type mice from Nakanishi et al. (25), the tumor formation in the hCYP1A-mice is clearly much higher. Since doses of 200 mg/kg PhIP without DSS did not induce colonic neoplasms at the time points evaluated in this study, DSS is apparently a powerful tumor-promoting agent. Inflammation caused by DSS exposure in the mouse colon is an important factor in this experimental model. An optimal time point for colonic tumor formation was determined to be 6–10 weeks in the hCYP1A-mice treated with 200 mg/kg PhIP and 1.5% DSS. Since adenomas and adenocarcinomas were observed in mice at 6 weeks (the earliest time point investigated in this study), it can be assumed that tumors can be found even before week 6, and this remains to be investigated.
Following 200 mg/kg PhIP and 1.5% DSS treatment to hCYP1A-mice at week 10, an overexpression of β-catenin, cyclin D1 and c-Myc was observed in colon tumors compared with normal colon tissue (Figure 4). β-Catenin is an intracellular anchoring protein that acts as a transcriptional activator mediating the Wnt signaling pathway (35). Molecular studies have pinpointed activating mutations of the Wnt signaling pathway as the cause of ∼90% of colorectal cancer, with β-catenin and c-Myc being overexpressed or constitutively active in human colon cancers (36). Human colorectal cancers contain mutations that stabilize β-catenin protein causing constitutive activation of β-catenin signaling and overexpression of downstream targets such as c-Myc, c-Jun and cyclin D1 (37). The transcription factor c-Myc is induced by mitogenic signals and regulates downstream cellular responses. If overexpressed, c-Myc promotes malignant transformation (36). Cyclin D1 is an important regulator of the G1 to S phase progression of the cell cycle and increased expression has been observed in human colon cancer (38). Mutations of β-catenin are an early event of colorectal carcinogenesis (39) and mutations in the β-catenin gene have been detected in colon adenocarcinomas induced by PhIP in mice (23) and in rats (34). Analysis of β-catenin mutations in this hCYP1A model in the future would be of value. In human colonic adenomas and adenocarcinomas, β-catenin is universally localized to the cytoplasm and/or nucleus (40) and in this hCYP1A model strong nuclear localization of β-catenin was observed in colon tumor tissue following 200 mg/kg PhIP and 1.5% DSS treatment (Figure 5). Overexpression of iNOS and COX-2 was also observed in colon tumors from mice treated with 200 mg/kg PhIP and 1.5% DSS (Figure 4). Sustained induction of the iNOS in chronic inflammation may be mutagenic through nitric oxide-mediated DNA damage or hindrance to DNA repair, and thus, potentially carcinogenic (41). iNOS may regulate COX-2 production of pro-inflammatory prostaglandins, which are known to play a key role in colon tumor formation (41). Activation of prostaglandin signaling is an early step in tumor formation, and inflammation is thought to upregulate COX-2 (36). Taken together, these data indicate that proteins, which are often overexpressed in human colon cancer are also overexpressed in the hCYP1A mouse model for colon cancer.
When hCYP1A-mice were treated with a single dose of 200 mg/kg of PhIP without the addition of DSS, after week 24 post PhIP gavage, no cancer or other malignancies were observed in the colon. Additionally, after 30 and 40 weeks of 200 mg/kg PhIP treatment, no colonic tumors were observed in the mice examined. However, at week 30 after 200 mg/kg PhIP gavage, preliminary pathological results have revealed severe high-grade prostatic intraepithelial neoplasia in the prostate gland of hCYP1A male mice and at week 40 after PhIP gavage, prostate tumors were observed in four of five hCYP1A-mice but not in wild-type mice (results to be reported elsewhere). High-grade prostatic intraepithelial neoplasia is considered a precursor to the development of prostate adenocarcinomas in humans (42) and these lesions have been detected in PhIP-treated rats (43). Further pathological analysis is currently under way to characterize and quantify the development of prostate cancer as well as other potential malignancies (lymphoma, leukemia, lung or mammary cancers) in the hCYP1A-mice. Therefore, the hCYP1A-mice may be a good model for other cancers.
Through epidemiological studies, it has been suggested that a ‘Western diet’, which consists of high fat, low calcium, low fiber and low vitamin D are some of the major dietary factors involved in the formation of various forms of cancer, specifically colon. A recent study showed that mice fed this Western diet had increased oxidative stress in the colon, leading to promotion of severe inflammation in the colon mucosa (44). In rats, high-fat feeding has been shown to accelerate the induction of carcinogenesis with PhIP (16) and that a high fat low calcium-containing diet results in more ACF in the colon when compared with those on a low-calcium diet (45). Future experiments will be carried out to investigate the promotion of carcinogenesis in hCYP1A-mice treated with 200 mg/kg PhIP (without DSS) by feeding these mice with a high-fat Western diet (40% of calories). The hCYP1A-mice should also be a useful model to study dietary effects on the activation of arylamines. For example, caffeine is an inducer of CYP1A2 and it could enhance the activation of arylamines and therefore enhance carcinogenesis (46).
The significance of this study is the establishment of a novel robust mouse model for colon carcinogenesis using the dietary carcinogen PhIP and with hCYP1A as the activating enzyme. This model is highly relevant to human colon carcinogenesis and therefore makes future chemoprevention studies using this carcinogenesis model extremely pertinent.
Supplementary material
Supplementary Table I and Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health Grants (CA120915, CA120915-03S1) as well as shared core facilities supported by National Cancer Institute Cancer Center Support Grant (CA72720) and National Institute of Environmental Health Sciences Center Grant (ES055022).
Acknowledgments
We thank Yu Hai Sun for the preparation and processing of formalin-fixed tissue for histological analyses.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ACF
aberrant crypt foci
- COX-2
cyclooxygenase-2
- CYP
cytochrome P450
- DMSO
dimethyl sulfoxide
- DSS
dextran sodium sulfate
- iNOS
inducible nitric oxide synthase
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
References
- 1.American Cancer Society. Cancer Facts and Figures, 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2.Gerhardsson de Verdier M, et al. Meat, cooking methods and colorectal cancer: a case-referent study in Stockholm. Int. J. Cancer. 1991;49:520–525. doi: 10.1002/ijc.2910490408. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman MH, et al. Re: “Fried foods and the risk of colon cancer”. Am. J. Epidemiol. 1990;131:376–378. doi: 10.1093/oxfordjournals.aje.a115508. [DOI] [PubMed] [Google Scholar]
- 4.Cross AJ, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakabayashi K, et al. Exposure to heterocyclic amines. Environ. Health Perspect. 1993;99:129–134. doi: 10.1289/ehp.9399129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustsson K, et al. Assessment of the human exposure to heterocyclic amines. Carcinogenesis. 1997;18:1931–1935. doi: 10.1093/carcin/18.10.1931. [DOI] [PubMed] [Google Scholar]
- 7.Butler MA, et al. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc. Natl Acad. Sci. USA. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammons GJ, et al. Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis. 1997;18:851–854. doi: 10.1093/carcin/18.4.851. [DOI] [PubMed] [Google Scholar]
- 9.Crofts FG, et al. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998;19:1969–1973. doi: 10.1093/carcin/19.11.1969. [DOI] [PubMed] [Google Scholar]
- 10.Kaderlik KR, et al. Glucuronidation of N-hydroxy heterocyclic amines by human and rat liver microsomes. Carcinogenesis. 1994;15:1695–1701. doi: 10.1093/carcin/15.8.1695. [DOI] [PubMed] [Google Scholar]
- 11.Kaderlik KR, et al. Metabolic activation pathway for the formation of DNA adducts of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat extrahepatic tissues. Carcinogenesis. 1994;15:1703–1709. doi: 10.1093/carcin/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 12.Turesky RJ, et al. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis. 1991;12:1839–1845. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- 13.Chou HC, et al. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s) Cancer Res. 1995;55:525–529. [PubMed] [Google Scholar]
- 14.Lang NP, et al. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol. Biomarkers Prev. 1994;3:675–682. [PubMed] [Google Scholar]
- 15.Le Marchand L, et al. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:1259–1266. [PubMed] [Google Scholar]
- 16.Ochiai M, et al. Efficient method for rapid induction of aberrant crypt foci in rats with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn. J. Cancer Res. 1996;87:1029–1033. doi: 10.1111/j.1349-7006.1996.tb03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 18.Ubagai T, et al. Efficient induction of rat large intestinal tumors with a new spectrum of mutations by intermittent administration of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in combination with a high fat diet. Carcinogenesis. 2002;23:197–200. doi: 10.1093/carcin/23.1.197. [DOI] [PubMed] [Google Scholar]
- 19.Nakagama H, et al. A rat colon cancer model induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP. Mutat. Res. 2002;506-507:137–144. doi: 10.1016/s0027-5107(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 20.Esumi H, et al. Induction of lymphoma in CDF1 mice by the food mutagen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn. J. Cancer Res. 1989;80:1176–1178. doi: 10.1111/j.1349-7006.1989.tb01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristiansen E, et al. Effects of long-term feeding with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in C57BL/ByA and E mu-pim-1 mice. Cancer Lett. 1998;122:215–220. doi: 10.1016/s0304-3835(97)00388-1. [DOI] [PubMed] [Google Scholar]
- 22.Ochiai M, et al. Induction of intestinal tumors and lymphomas in C57BL/6N mice by a food-borne carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn. J. Cancer Res. 2002;93:478–483. doi: 10.1111/j.1349-7006.2002.tb01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T, et al. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26:229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa A, et al. Induction of colon tumors in C57BL/6J mice fed MeIQx, IQ, or PhIP followed by dextran sulfate sodium treatment. Toxicol. Sci. 2005;84:243–248. doi: 10.1093/toxsci/kfi079. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi M, et al. Mouse strain differences in inflammatory responses of colonic mucosa induced by dextran sulfate sodium cause differential susceptibility to PhIP-induced large bowel carcinogenesis. Cancer Sci. 2007;98:1157–1163. doi: 10.1111/j.1349-7006.2007.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 27.Lin DX, et al. Species differences in the biotransformation of the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by hepatic microsomes and cytosols from humans, rats, and mice. Drug Metab. Dispos. 1995;23:518–524. [PubMed] [Google Scholar]
- 28.Turteltaub KW, et al. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999;143:149–155. doi: 10.1016/s0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 29.Cheung C, et al. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J. Pharmacol. Exp. Ther. 2008;327:288–299. doi: 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung C, et al. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem. Res. Toxicol. 2005;18:1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z, et al. Toward the evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC-transgenic mice carrying the human CYP1A1_CYP1A2 locus. Hum. Mutat. 2005;25:196–206. doi: 10.1002/humu.20134. [DOI] [PubMed] [Google Scholar]
- 32.Dragin N, et al. Generation of 'humanized' hCYP1A1_1A2_Cyp1a1/1a2(-/-) mouse line. Biochem. Biophys. Res. Commun. 2007;359:635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao XP, et al. Beta-catenin expression is altered in human colonic aberrant crypt foci. Cancer Res. 2001;61:8085–8088. [PubMed] [Google Scholar]
- 34.Tsukamoto T, et al. More frequent beta-catenin gene mutations in adenomas than in aberrant crypt foci or adenocarcinomas in the large intestines of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-treated rats. Jpn. J. Cancer Res. 2000;91:792–796. doi: 10.1111/j.1349-7006.2000.tb01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JR, et al. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz SD, et al. Molecular origins of cancer: molecular basis of colorectal cancer. N. Engl. J. Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneikert J, et al. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arber N, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 39.Sparks AB, et al. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 40.Iwamoto M, et al. Expression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis. 2000;21:1935–1940. doi: 10.1093/carcin/21.11.1935. [DOI] [PubMed] [Google Scholar]
- 41.Lala PK, et al. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 42.Bostwick DG, et al. High-grade prostatic intraepithelial neoplasia. Mod. Pathol. 2004;17:360–379. doi: 10.1038/modpathol.3800053. [DOI] [PubMed] [Google Scholar]
- 43.Borowsky AD, et al. Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia. 2006;8:708–715. doi: 10.1593/neo.06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdelyi I, et al. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J. Nutr. 2009;139:2072–2078. doi: 10.3945/jn.108.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisburger JH, et al. The role of fat and calcium in the production of foci of aberrant crypts in the colon of rats fed 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine. Environ. Health Perspect. 1994;102(suppl. 6):53–55. doi: 10.1289/ehp.94102s653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, et al. Protective versus promotional effects of white tea and caffeine on PhIP-induced tumorigenesis and beta-catenin expression in the rat. Carcinogenesis. 2008;29:834–839. doi: 10.1093/carcin/bgn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.