Abstract
A quandary of evolution is how to measure change over time. A natural inclination is to use morphologic criteria—the greater the differences between two phenotypes, the greater amount of time needed to evolve these differences. However, appearances may be deceiving, and another approach to infer time is with molecular clocks. Here, the greater the differences between two genomes, on average the greater the time since a common ancestor. Recent advances in DNA sequencing shed new light on how human cancers might evolve.
Introduction
Molecular clocks are fundamental evolutionary tools that compare homologous genome regions to reconstruct ancestries of species and populations (1). The goal of this review is to outline how similar approaches can be used to help reconstruct cancer evolution. Because genomes are ‘almost’ perfect copies of copies, differences between genomes can be used to infer ancestry. The greater the number of differences between genomes, on average the greater the time since their common ancestor (a molecular clock hypothesis). A simple way to enumerate differences between genomes is with a pairwise distance (PWD) that counts the number of differences between homologous sites (Figure 1). Although PWDs are not direct measures of time (because of back mutations and other complications), PWDs generally correlate with time. For example, humans and chimpanzees diverged ∼5 million years ago, and PWDs are ∼10 000 per 1 000 000 bases (Table I). PWDs between unrelated individuals are ∼1000 per million bases, corresponding to the more recent emergence of modern humans out-of-Africa ∼50 000 years ago.
Fig. 1.
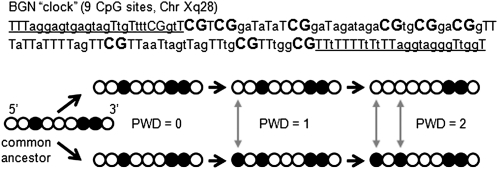
Calculating PWD. (A) PWDs count differences between homologous 5′ to 3′ sites (circles) and can be calculated between allele pairs or groups of alleles (average PWD). (B) Examples of the 5′ to 3′ methylation patterns of eight epialleles sampled from CRC glands from left and right sides of the same tumor (filled circles are methylated CpG sites). Average PWDs measure diversity within and between glands. The glands of Cancer 12 are more diverse than the glands of Cancer 2 (also see Figure 5).
Table I.
PWD comparisons
Although molecular clocks are controversial (7), the underlying data are simply sequences. Interesting patterns emerge when genomes are organized by counting the numbers of differences between them because some genomes are more alike than others. To translate these differences into time, sophisticated algorithms are typically employed to help correct for the possibility that mutations probably do not accumulate in a strictly ‘clock-like’ manner. In this review, a simple ‘fingers and toes’ approach will be used to count differences between genomes. How well do cancer models stand up when genomes are quantitatively compared?
Why somatic cell clocks are needed
Before sequences were widely available, species evolution was largely based on intuition and the comparison of morphologic features. Although evolution could not be directly observed, it was possible to imagine a ladder of progression from more ‘primitive’ to more ‘advanced’ species. This approach was translated to tumor evolution, leading to familiar tumor progression diagrams illustrating a series of morphologic changes (Figure 2A). Such models imply a single stereotypic pathway and a presumption that selection aided by genomic instability will lead to clonal evolution (8) and phenotypic progression whenever and wherever opportunities arise. Sequence data can be incorporated into morphologic progression ladders and a number of specific mutations have been associated with different phases of progression (9). However, this task becomes increasingly awkward because cancer genomes contain thousands of diverse mutations, most of which appear to be neutral passenger mutations (4–6).
Fig. 2.
Cancer evolution. (A) A progression ladder illustrates stepwise changes in phenotypes, thought to be caused by the selection of new driver mutations (arrows). A ladder implies common ‘starts’, intermediate stages and ‘finishes’ and therefore, it should be possible to organize the data from many cancers into a single ladder. However, data from multiple cancers are difficult to incorporate into ladders because starting germ line genomes are very different, most mutations are passenger mutations, and common driver mutations have been difficult to identify. (B) Genealogies follow genomes and not phenotypes. A somatic cell genealogy starts at the zygote and ends at present day genomes, which are almost exact copies of prior copies. Replication errors (primarily passengers) accumulate throughout life, and these errors may record the past. The multiple genomes in a tumor eventually trace back to a common ancestor called the first transformed cell. Clonal cancer mutations accumulate in the interval between the zygote and the first transformed cell. Variant mutations accumulate after transformation. Intervals before or after transformation can be reconstructed by counting the numbers of clonal or variant mutations. The total numbers of clonal somatic mutations in cancer genomes are few enough to be consistent with the numbers of mutations that may accumulate solely due to normal replication errors. Because visible tumors appear later in life, most ‘cancer’ mutations first accumulate in normal appearing tissues.
In contrast, newer molecular clock approaches largely neglect morphology but use sequence data to reconstruct the past. Somatic sequences can reconstruct a genealogy that starts at the zygote and ends with present day genomes (Figure 2B). A cancer genome has had many different cellular phenotypes during its evolution, and it should be possible to infer something about this past by counting the number of alterations accumulated along the way. This approach switches the relationship between the ‘known’ and ‘unknown’. With progression ladders, tumor evolution is known and new genomic data from multiple cancers are combined and filtered to discard the many more uninformative hitchhikers or passengers from the much rarer selective drivers that best explain this evolution. With clocks, tumor evolution is unknown and passengers are the most abundant and informative data. The evolution of an individual cancer is inferred to best fit its own genomic data.
Experimentally, two types of ‘clock’ mutations are present in a tumor. Clonal mutations are present in all cancer cells and accumulate in the interval between the zygote and the first transformed cell (Figure 2B). The number of clonal mutations depends on what happened between conception and transformation. Variant mutations are present in only some cancer cells and accumulate between transformation and tumor removal. A tumor removed later after transformation would be expected to accumulate more variant mutations among its cells compared with a tumor removed sooner after transformation. Clonal mutations are detected when bulk DNA extracted from whole tumors is sequenced and then compared with the germ line sequence of the individual with the cancer. Variant mutations would be difficult to detect by the sequencing of bulk-extracted tumor DNA because their individual frequencies would be low. Detection of variant mutations requires comparisons between single cancer genomes, for example by the sequencing of different cloned polymerase chain reaction products from a single tumor.
Cancer genetic instability?
It is commonly stated that cancers exhibit ‘genetic instability’, which would facilitate clonal evolution because new selective mutations would frequently arise. Recent sequencing studies document the numbers of clonal mutations (point mutations or small insertions/deletions) per cancer genome (4–6). The mutational load of repair proficient cancers (Table I) is typically <10 per million bases and on average about four per million bases compared with their germ line genomes (5). Thousands of somatic point mutations or small insertions/deletions in a cancer genome may seem substantial but are small compared with the numbers of differences between genomes from different individuals (Table I). The differences between two normal human germ line genomes are ∼100-fold greater than the differences between a cancer genome and its germ line sequence. These comparisons illustrate that PWDs reflect both mutation rates and the passage of time.
To see if mutation rates are elevated during progression, the numbers of clonal cancer somatic mutations can be compared with the numbers of mutations expected to accumulate solely from replication errors (Figure 2B). All genomes are almost perfect copies of copies because replication errors are inevitable. For example, genome-wide sequencing of siblings and their parents reveal ∼70 new germ line point mutations per generation (10). The normal replication error rate is uncertain (and depends on sequence context) but has been estimated at ∼10−10 to 10−9 mutations per base per division (11). Calculations illustrate that human colorectal cancer (CRC) mutation frequencies are consistent with normal mutation and division rates (11). For example, a CRC genome removed from a 70 year old requires only ∼57 divisions/year with a mutation rate of 10−9 per base per division to accumulate a PWD of four per million bases compared with its germ line sequence.
These PWD comparisons suggest that some cancers may arise from normal cell division and mutation rates. Cancers are typically more common in the elderly, and more replication errors should accumulate with age in normal mitotic cells, increasing the probability that a single genome will accumulate a proper combination of driver mutations. Although the probability of accumulating multiple driver mutations within a single cell is low (12), the probability is not zero, and chance, many divisions and many cells at risk can mathematically account for the increased incidence of CRC with age (13). A cancer genealogy (Figure 2B) is consistent with relatively few mutations because although mutation rates may increase with progression, most of this genealogy resides before a gatekeeper mutation (9) in normal appearing tissue.
Single progression pathway?
A progression ladder (Figure 2A) implies common ‘starts’ and ‘destinations’, but the start of each human cancer genealogy is quite different because the variation between genomes from different individuals is ∼100-fold greater than the variation between a cancer genome and its germ line sequence. Given the widely different starts, it is perhaps less surprising that the overlap of somatic mutations in different human cancers is minimal. For example, any two CRCs share only about 2–3 common gene mutations per ∼70 mutations (4).
Inbreed mouse models anticipate outbreed human progression heterogeneity. Differences between genomes from different individuals are similar to the differences between inbreed mouse strains [PWDs of 50–4500 per million bases (14)] and phenotypes often vary when the same tumor susceptibility allele is placed in a different mouse genetic background (15,16). A mutation in one individual may not confer the same selective value in another individual.
Progression between stages?
Progression ladders (Figure 2A) also imply that phenotypic differences are caused by new driver mutations. However, without genetic instability, new bona fide driver mutations may rarely arise, especially in the relatively short intervals between transformation and tumor removal. Sequencing has generally failed to identify drivers responsible for metastasis. Comparisons reveal that ∼97% of the clonal mutations in CRC metastases were already present in their primary lesions, and the few additional differences did not appear to be drivers (17). Sequencing of three different samples (primary, xenograft of primary and metastasis) of a basal-like breast cancer revealed 96% mutational similarity also with no obvious drivers responsible for the metastasis (18). One study found 19 new mutations (of 32 mutations) in a lobular breast cancer relapse, but the relapse occurred after 9 years and irradiation (19). The lack of marked mutational differences between different parts of the same cancer (except after many years) is consistent with a lack of genetic instability even after transformation.
Survival of the fittest?
The lack of genomic instability or elevated mutation rates may not impair sequential clonal evolution if selection can discriminate even small differences between cells. Selection optimizes fitness but it is uncertain how much cells must differ before clonal evolution occurs. The puzzling lack of readily identifiable driver mutations between phenotypically different parts of the same cancer despite genome-wide searches may mean that clonal evolution rarely occurs after transformation. Alternatively, selection may be so efficient that even ‘passenger’ mutations may confer subtle phenotypic differences sufficient for clonal evolution. Progression may also be driven by chromosomal structural alterations and non-genetic factors such as epigenetic alterations. Epigenetic changes such as cytosine DNA methylation are widespread in cancer genomes and may also drive progression (20).
Although selection is fundamental to evolution, selection in human cancers is customarily not measured because serial observations are impractical. Instead, differences in phenotype are often presumed to be caused by new mutations. By linking phenotypic changes with clonal evolution, an observer can ‘see’ selection wherever differences are judged to exist. At its extreme, mutation and selection may appear to be relentless because many different phenotypes are present in a cancer.
A weakness with this approach is the potential for cancer cell phenotypic plasticity or the ability of a cancer genome to aberrantly express different phenotypes in different microenvironments without requiring new mutations. Indeed, it is possible that the full malignant potential may already be present in the first transformed cell (21). This ability of a single altered genome to express many different phenotypes is more akin to normal development, albeit without normal checkpoints.
Measuring somatic cell selection
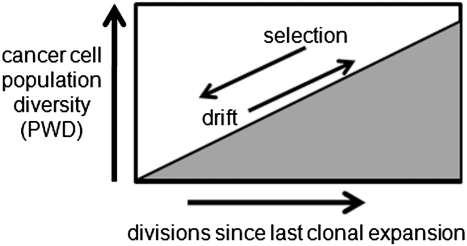
Instead of visual comparisons, genome comparisons can infer selection by measuring the amount of genomic variation between cells. The diversity of a population is a function of time and selection (22). Genomic diversity may accumulate in a cancer cell population due to independent replication errors (Figure 3). Selection reduces this diversity by eliminating less fit variants (background selection) or through hitchhiking with selective sweeps (23). Therefore, PWD is an inverse metric of selection—the greater the variation in a cell population, the greater the time since the last selective sweep or clonal evolutionary bottleneck. The greater the diversity, the weaker the force of selection.
Fig. 3.
Inferring selection. PWDs can infer selection because PWDs are low after selection and clonal expansion, but then progressively increase from the accumulation of random replication errors. A diverse population is an older population, which implies inefficient selection because the last sweep occurred long ago.
Experimentally, it is difficult to compare genomes sampled from a large tumor because it is uncertain if it represents a single population or multiple populations sequentially created by clonal evolution (Figure 5A). Because the immediate battle for survival is between adjacent cells, an easier and more sensitive way to detect selection is to measure the amount of variation among neighboring cells, such as within small gland fragments (2000–10 000 cells) of a colorectal adenocarcinoma (24,25). [More formally, fixation occurs faster in smaller population (26)]. Different parts of a CRC can be sampled by an ethylenediaminetetraacetic acid-washout method (24) that yields purified 2000–10 000 cell gland fragments or more precisely from a microscopic tissue section by laser capture microscopy. Although progeny of a single selected cell may not sweep widely, at a minimum they should be able to homogenize their immediate neighborhood, reverting the mitotic age (divisions since a last common progenitor) and PWD of its population back toward zero. If single cancer glands are diverse populations, then selection is a relatively weak force because it cannot endow even local dominance. By measuring PWDs within and between glands from different parts of the same cancer, it should be possible to infer selection and distinguish newer clonal expansions (low PWDs) from older more diverse regions (high PWDs).
Fig. 5.
Cancer evolution after transformation. (A) Stepwise cancer evolution (Figure 2A) can be ‘reconstructed’ by simple visual inspection, assuming changes in phenotype are caused by the sequential selection of new mutations. It is also possible that the cancer represents a single clonal expansion. The diversity of CRC glands sampled from different parts of the same cancer differs between these models. Glands are small neighboring populations of about 2000–10 000 cells that can be isolated from fresh CRCs by an ethylenediaminetetraacetic acid-washout method. There will be a gradient of diversity with stepwise progression (oldest regions with more diverse glands), whereas all glands of a single clonal expansion will have similar diversities and ages. (B) Data from two human CRCs (24) illustrate that PWDs of individual cancer glands (circles) are low in Cancer 2 and higher in Cancer 12. Consistent with single clonal expansions, glands from the left and right sides of the same cancer have similar PWDs or mitotic ages. Gland data from these cancers are also presented in Figure 1B. (C) The mitotic age of a cancer may be estimated by measuring PWDs between epialleles sampled from the least related cells or cells from opposite cancer sides. The data illustrate that PWDs between glands are similar to PWDs within glands, consistent with a single clonal expansion and infrequent selection and clonal evolution after transformation. (D) Epialleles sampled from laser capture microscopy isolated glands (circles on microscopic section) allow comparisons between physical and PWD. Consistent with a single clonal expansion and a star phylogeny, there was no significant increase in PWD with physical distance, with adjacent glands as related as more distant glands.
Epigenetic molecular clocks
Searching for new variant mutations that arise after transformation is difficult because the intervals between transformation and tumor removal are usually short. The lack of genetic instability makes it expensive to use genetic clocks because millions of bases have to be sequenced to find the few clonal mutations that accumulate between conception and transformation. Many more bases would have to be sequenced to detect variant mutations that arise after transformation (Table I). An alternative to sequence based clocks are epigenetic clocks (27) that use a 5′ to 3′ order of cytosine methylation at CpG sites instead of a 5′ to 3′ base order (Figure 4). Methylation patterns can be read by bisulfite sequencing (28), and differences between homologous CpG sites can be enumerated with a PWD. The epigenetic clocks (27) are short stretches of CpG sites amplified by polymerase chain reaction after bisulfite treatment (Figure 4). Methylation of the clock region is probably to be selectively neutral (‘passenger’) because its gene is not expressed in the colon. The passenger clock region is on the X-chromosome and the tumors are from male patients (potentially one methylated pattern or epiallele per cell).
Fig. 4.
Epigenetic molecular clocks. The 5′ to 3′ order of methylation can be used to calculate PWDs (filled circles are methylated CpG sites). Epigenetic molecular clocks are more suitable for somatic cell evolution reconstruction because patterns are copied almost exactly between generations, but replication error rates are relatively high. Variant methylation patterns can be read after bisulfite sequencing of cloned polymerase chain reaction products. Illustrated is the BGN clock with nine CpG sites after bisulfite treatment (primer sites are underlined, CpG sites in bold).
Like sequences, epigenetic information is inherited between generations because methylation patterns are copied after DNA replication by DNA methyltransferases. The parental pattern is propagated because the old strand is used as a template, but methylation replication error rates are much greater than DNA base replication error rates (∼10 000-fold higher per CpG site) at certain CpG rich regions (28,29). This higher replication error rate means passenger methylation patterns drift faster and should become measurably polymorphic after a few weeks. Methylation patterns are often polymorphic in human cell populations. For example, nearly every possible methylation pattern in several short CpG rich stretches was found in a human breast cancer after deep bisulfite sequencing (30).
Lack of clonal evolution after transformation
If stepwise selection and clonal evolution is assumed, tumor histories can be reconstructed by visual inspection—invasive areas are younger than superficial areas (Figure 5A). However, an alternative possibility is that clonal evolution only occurred at transformation, producing a single clonal expansion. The different phenotypes within the tumor are due to aberrant differentiation rather than the sequential selection of new driver mutations.
It is possible to distinguish between these models by measuring diversity in different parts of the same tumor. Simplistically, the question is whether the glands within a single cancer have similar or different mitotic ages. With stepwise selection, older superficial areas should be more diverse than newer invasive areas, and physically adjacent regions will tend to be more related (smaller PWDs) than distant regions. With a single clonal expansion, the entire tumor and its glands have similar ages and diversity. A single clonal expansion can be represented by a star phylogeny originating from the first transformed cell, which has the interesting property that adjacent and distant regions are similarly related. Mechanistically, tumors tend to exhibit Gompertzian growth (31) with rapid exponential expansions followed by progressive declines in growth rates. An initial rapid expansion would tend to homogenize tumor age because the bulk of the tumor is created very early after transformation.
Methylation pattern data for two human CRCs (24) are illustrated in Figure 5B. One cancer appears to have been removed later after transformation because its glands are more diverse populations with greater intragland PWDs. The methylation patterns appear to represent passenger changes because they are polymorphic and differ between glands (Figure 1B). This drift is more consistent with a single clonal expansion because intragland PWDs are similar whether sampled from the left or the right sides of the same tumor, with the scatter that might be expected of random replication errors. In other words, the age or diversity of a small part of a tumor appears to be representative of the diversity in other parts of the same tumor.
A critical question is whether individual glands are as old as their tumors or are younger because of past remodeling from selection and clonal evolution. As a rough approximation, tumor mitotic ages (divisions since transformation) may be estimated from PWDs between glands from opposite cancer sides because these cells probably last shared a common ancestor around the time of transformation. This strategy uses cells from physically distant glands as ‘out groups’ for the comparisons of neighboring cells within a gland. PWDs between epialleles sampled from opposite cancer sides were similar to intragland PWDs (Figure 5C) and consistent with a model where individual cancer glands are as old as the entire tumor (24).
The diversity in different parts of the same cancer can also be analyzed with laser capture microscopy to compare PWDs with physical distances. This topographical tumor sampling mimics the sampling of individuals from different parts of the world used to reconstruct the human diaspora. Frequent selection and clonal evolution after transformation would create new neighborhoods such that adjacent cells are more related than distant cells. However, more consistent with a single clonal expansion and a star phylogeny, adjacent and distant tumor regions were similarly related with no significant changes in PWDs with physical distances (Figure 5D). Invasive tumor regions were as diverse as superficial regions (24).
The above two tumors appear to be single clonal expansions, suggesting that clonal evolution seldom occurs after transformation even within small glands. Passenger methylation patterns in most human CRCs appear to be consistent with relatively simple single clonal expansions (24,32). It is uncertain whether these inferred histories are correct because the pasts of most human cancers are unknown and are probably to vary even between similar appearing tumors. However, there is consistency between cancer genome sequence data and this passenger methylation data. The inferred lack of selection after transformation manifested by the high diversity of variant passenger methylation in CRC glands is consistent with the lack of genomic instability before transformation inferred from the relatively small numbers of clonal cancer mutations. Without elevated mutation rates, it would be difficult to acquire new driver mutations in the short intervals between transformation and tumor removal, especially within small populations such as single CRC glands. It is possible to obtain additional supporting evidence by comparing multiple genomic regions. The past becomes more certain when variations at multiple independent loci retell the same ancestry.
Practical applications
Inferring that many human cancers are relatively simple and stable single clonal expansions has important clinical implications. If most cancers were complex, rapidly evolving heterogenous mixtures of different clones, many clinical practices would be less justifiable. Small often superficial diagnostic biopsies of large tumors would more probably not be representative of the entire tumor. It would be difficult to define a cancer ‘genome’ if the sequences of individual genomes within a tumor were highly variable. Treatment delays due to bureaucratic inefficiencies would be expected to lead to further progression and poorer outcomes, but it has been difficult to document increased mortality even when surgery for CRC is delayed for months (33,34).
The ability to infer something about the mitotic age (numbers of divisions since transformation) of an individual human cancer would have practical applications. Knowing the age of a cancer may make it easier to understand why certain clinical approaches fail. For example, polyp removal should prevent most CRCs, but inevitably some CRCs appear shortly after colonoscopy (35). The dilemma is whether these interval CRCs arose because a smaller lesion was missed at a prior colonoscopy or if these CRCs progressed faster than usual. Measuring tumor diversity may help distinguish between these possibilities because a missed lesion would be expected to have a greater mitotic age and therefore higher diversity relative to a recently transformed CRC. Similarly, chemotherapeutic resistance is often thought to be secondary to preexisting variants that arise after transformation (36). An older more diverse cancer might be more refractory or prone to relapse because of a greater probability that rare resistant variants are already present in its tumor population.
Summary
Molecular clocks often use sophisticated algorithms and statistics that are not presented in this review. There are many uncertain assumptions and technical problems that can confound the analysis of genetic or epigenetic variation. Technically, it is difficult to isolate pure cancer cell populations and adequately sample their diversity without bias. The stochastic nature of replication errors complicates modeling, especially because error rates may differ in cells of different phenotypes and in different microenvironments. Yet, these potential complications are not unique to somatic cell evolution but are standard problems of molecular clocks. Even for more traditional applications, molecular clock approaches have been criticized as unreliable (7,37).
Despite these potential flaws, genome comparisons provide another approach to test the predictions of different evolutionary models. Models of stepwise selection and clonal evolution (Figure 2A), although logical and widely used, are also based on a number of assumptions that are seldom considered. Recent cancer genome sequence data illustrate that the genomic instability thought to underlie tumor progression appears to be lower than what might be anticipated if selection and clonal evolution frequently occur. Although cancer genomes contain many mutations, the difference between a cancer genome and its germ line sequence is ∼100-fold less that the differences between genomes from different individuals. Normal mutation and cell division rates can account for the relatively small numbers of cancer genome somatic mutations (11), and most of the mutations appear to be neutral passenger mutations (4–6). Therefore, selective driver mutations sufficient to cause clonal evolution and changes in phenotype may only rarely arise during tumor progression. Consistent with the idea that selection rarely occurs, variant passenger methylation patterns within small CRC glands are diverse, suggesting cancer cell populations are relatively stable.
This review has attempted to illustrate how comparisons between somatic cell genomes can be used to experimentally address questions about human cancer evolution. This is an uncertain task because there are many ways to compare genomes and interpret their differences with molecular phylogeny. With refinement, it may be possible to reliably ‘read’ the histories encoded within cancer genomes. Modern evolution is molecular evolution or the reconstruction of the past from genomes. Translation of this basic and often contentious field of biology to epigenetic and genetic somatic variations may bring better insight into how individual human cancers actually evolve.
Funding
National Institutes of Health (CA149990).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CRC
colorectal cancer
- PWD
pairwise distance
References
- 1.Bromham L, et al. The modern molecular clock. Nat. Rev. Genet. 2003;4:216–224. doi: 10.1038/nrg1020. [DOI] [PubMed] [Google Scholar]
- 2.Chimpanzee Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 3.Tishkoff SA, et al. Implications of biogeography of human populations for ‘race’ and medicine. Nat. Genet. 2004;36:S21–S27. doi: 10.1038/ng1438. [DOI] [PubMed] [Google Scholar]
- 4.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 5.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleasance ED, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields R. Pushing the envelope on molecular dating. Trends Genet. 2004;20:221–222. doi: 10.1016/j.tig.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 9.Kinzler KW, et al. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 10.Roach JC, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010;328:636–639. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TL, et al. Prevalence of somatic alterations in the colorectal cancer cell genome. Proc. Natl Acad. Sci. USA. 2002;99:3076–3080. doi: 10.1073/pnas.261714699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 13.Calabrese P, et al. A simple algebraic cancer equation: calculating how cancers may arise with normal mutation rates. BMC Cancer. 2010;10:3. doi: 10.1186/1471-2407-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wade CM, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 15.Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol. Biol. 2009;530:423–433. doi: 10.1007/978-1-59745-471-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halberg RB, et al. The pleiotropic phenotype of Apc mutations in the mouse: allele specificity and effects of the genetic background. Genetics. 2008;180:601–609. doi: 10.1534/genetics.108.091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SP, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, et al. Cancer epigenetics comes of age. Nat. Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 21.Bernards R, et al. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 22.Charlesworth B, et al. The effect of deleterious mutations on neutral molecular variation. Genetics. 1993;134:1289–1303. doi: 10.1093/genetics/134.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlesworth B. Evolution. Variation catches a ride. Science. 2010;330:326–327. doi: 10.1126/science.1197700. [DOI] [PubMed] [Google Scholar]
- 24.Siegmund KD, et al. Inferring clonal expansion and cancer stem cell dynamics from DNA methylation patterns in colorectal cancers. Proc. Natl Acad. Sci. USA. 2009;106:4828–4833. doi: 10.1073/pnas.0810276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegmund KD, et al. Many colorectal cancers are “flat” clonal expansions. Cell Cycle. 2009;8:2187–2193. doi: 10.4161/cc.8.14.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M, et al. The average number of generations until fixation of a mutant gene in a finite population. Genetics. 1969;61:763–771. doi: 10.1093/genetics/61.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata D, et al. Counting divisions in a human somatic cell tree: how, what and why? Cell Cycle. 2006;5:610–614. doi: 10.4161/cc.5.6.2570. [DOI] [PubMed] [Google Scholar]
- 28.Clark SJ, et al. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yatabe Y, et al. Investigating stem cells in human colon by using methylation patterns. Proc. Natl Acad. Sci. USA. 2001;98:10839–10844. doi: 10.1073/pnas.191225998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korshunova Y, et al. Massively parallel bisulphite pyrosequencing reveals the molecular complexity of breast cancer-associated cytosine-methylation patterns obtained from tissue and serum DNA. Genome Res. 2008;18:19–29. doi: 10.1101/gr.6883307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton LA. Gompertzian model of human breast cancer growth. Cancer Res. 1988;48:7067–7071. [PubMed] [Google Scholar]
- 32.Hong YJ, et al. Using DNA methylation patterns to infer tumor ancestry. PLoS One. 2010;5:e12002. doi: 10.1371/journal.pone.0012002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos M, et al. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur. J. Cancer. 2007;43:2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Terhaar sive Droste JS, et al. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC Cancer. 2010;10:332. doi: 10.1186/1471-2407-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh H, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am. J. Gastroenterol. 2010 doi: 10.1038/ajg.2010.390. doi 10.1038ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 36.Goldie JH, et al. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984;44:3643–3653. [PubMed] [Google Scholar]
- 37.Ayala FJ. Molecular clock mirages. Bioessays. 1999;21:71–75. doi: 10.1002/(SICI)1521-1878(199901)21:1<71::AID-BIES9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]