Abstract
To find biomarkers for risk prediction of pancreatic cancer (PC), we evaluated the frequency of micronuclei (MN) in peripheral lymphocytes of 346 patients with PC and 449 healthy controls. The levels of baseline MN (mean ± standard error of micronucleated cells per 1000 binucleated cells) were significantly higher in patients (15.3 ± 0.3) than those in controls [9.7 ± 0.5; adjusted for body mass index (BMI), P < 0.001]. Using the median levels found in controls as the cut point, 78.9% of patients and 43.7% of controls had a higher frequency of MN. Logistic regression analysis with adjustment for known risk factors for PC showed that having a higher level of MN was significantly associated with increased risk of PC [odds ratio (OR): 8.32, 95% confidence interval (CI): 5.06–13.67, P < 0.001]; and the risk was much higher in men than in women [OR (95% CI): 14.19 (7.09–28.40) versus 4.19 (1.90–9.27)]. The level of MN was not associated with disease stage or resection status but was related to smoking status in men and to BMI in women among patients. The level of MN was higher in smokers (14.5 ± 0.6) than in nonsmokers (12.1 ± 0.6; P = 0.023) and in obese (25.3 ± 2.8) versus normal weight individuals (17.7 ± 0.8; P = 0.024). These data showed that elevated level of MN in peripheral lymphocytes was associated with increased risk of PC.
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer death in the USA. It is a rapidly fatal disease with a 5 years survival rate of <5% (1). Known risk factors for PC include cigarette smoking, obesity and type II diabetes, a family history of PC, and several inherited familial syndromes. Some of these factors may share a common mechanism involving oxidative stress and inflammation (2,3). Novel approaches are needed to identify and validate biomarkers for risk prediction and early detection of PC.
PC shows extensive genomic instability and aneuploidy. Telomere attrition and mutation in TP53 and BRCA2 genes, as well as defects in the mitotic spindle apparatus conferred by centrosome abnormalities, may contribute to the aneuploidy and genomic instability seen in this type cancer (4). Centrosome abnormalities are detected in 85% of PC, and there is a correlation between levels of such abnormalities and the degree of chromosomal aberrations present (5). It is well known that chromosomal aberrations, such as the background micronuclei (MN), reflect accumulative DNA damage as a result of exposure to genotoxic agents and individual variations in response to DNA damage (6). On the other hand, the level of chromosomal/DNA damage in cultured peripheral lymphocytes that are in vitro treated with genotoxic agents, such as tobacco carcinogens, mutagens and H2O2 under the same exposure conditions, has been used extensively in molecular epidemiology as a phenotypic marker for individual variations in DNA repair (7).
MN originate from chromosomal fragments or whole chromosomes that are not included in the main daughter nuclei during nuclear division (8). Compared with other cytogenetic assays, the MN assay is highly reliable, easier to score and less costly. The Human MicroNucleus (HUMN) project involving >30 laboratories worldwide has provided technical guidelines, identified sources of variations and conducted prospective validation studies on this biomarker (9–11).
MN levels have been extensively used as biomarkers of genetic damage in humans with known exposure to genotoxic agents. The value of MN as a cancer susceptibility marker has been supported by numerous observations. For example, a number of studies have found elevated levels of MN formation in the peripheral lymphocytes of cancer patients prior to chemotherapy or radiotherapy and in patients affected by cancer-prone congenital diseases such as Bloom syndrome or ataxia telangiectasia (9,12,13). Levels of MN in the oral mucosa have been used as surrogate end points in clinical chemoprevention trials for oral malignancies (14). Many MN-inducing genotoxic agents are known to be carcinogenic and the HUMN project has confirmed that an elevated MN frequency in peripheral blood lymphocytes predicts an increased cancer risk (15). A nested case–control study based on a longitudinal cohort study also provided supporting evidence for the efficacy of MN levels as a biomarker for cancer risk in the general population (16).
To demonstrate the associations between the risk of PC and the level of intrinsic chromosome instability and sensitivity to oxidative stress-induced chromosomal damage, we conducted the cytokinesis-block MN assay (8,17) in a large group of patients with pancreatic adenocarcinoma and healthy controls. To our knowledge, this is the largest group of patients in whom this has been studied. We report that the background and hydrogen peroxide (H2O2)-induced MN levels in peripheral lymphocytes are predictive of the risk of PC.
Materials and methods
Study population
Our study design and data collection methods have been described previously in detail (18). Briefly, in this hospital-based case–control study, 346 patients with newly diagnosed and pathologically confirmed primary pancreatic ductal adenocarcinoma and 449 healthy controls were consecutively recruited from The University of Texas M. D. Anderson Cancer Center between January 2002 and June 2006. The response rate (number of individuals enrolled per number of individuals approached) was 83.9% for cases and 83.6% for controls (19). Control individuals were cancer-free and selected from friends and genetically unrelated family members (usually spouses or in-laws) of patients who were diagnosed with cancers other than gastrointestinal cancer, lung cancer or head and neck cancer. Patients and control individuals were frequency matched by age (±5 years), gender and race. In personal interviews, we collected information on cigarette smoking, alcohol consumption, diabetic status, medical history, family history of cancer among first-degree relatives and body mass indices (BMIs) at different age period (19). Because we did not include BMI in the questionnaire until 2004, this information was missing for 106 patients and 287 controls. We created a variable for missing BMI information (yes or no) and this variable was adjusted for in all data analysis. A blood sample was collected from each study participant in heparinized vacutainers. The median time interval between the initial diagnosis and blood drawn was 7 days [95% confidence interval (CI): 1–9]. Patients with PC who had received cytotoxic chemotherapy before the blood collection were excluded from the current study. For cancer patients, we also conducted medical record review to collect information on disease stage, tumor resection status and overall survival time. Each participant gave written informed consent in a protocol approved by the M. D. Anderson Institutional Review Board.
Smokers were defined as individuals who had smoked ≥100 cigarettes during their lifetimes. Cumulative smoking was measured by pack-years smoked, with heavy smokers defined as those having smoked >20 pack-years. Ever-alcohol drinkers were defined as individuals who had consumed at least four alcoholic drinks of beer, wine or hard liquor each month for 6 months in their lifetime. Individuals who had consumed ≥60 g/day alcohol were defined as heavy drinkers. BMI at age 30s was considered in the analysis based on our previous observation that BMI at this age period had the strongest association with risk of PC (19). BMI was categorized according to World Health Organization criteria so that a BMI (kilogram per square meter) of <25 was defined as normal weight, 25–30 as overweight and >30 as obese.
Cytokinesis-block MN assay
The cytokinesis-block MN assay was performed using the protocol of Fenech (8,17,20) and following the HUMN project recommendations (11). Lymphocytes were isolated from fresh blood samples by Ficoll–Paque (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation and were cultured at a concentration of 1 × 106 cells/ml in RPMI 1640 medium (Hyclone; Thermo Scientific, Logan, UT) without fetal bovine serum. All cultures were prepared in duplicate. After 30 min of incubation at 37°C, one of the duplicates was challenged with 75 μM H2O2 (CAS number: 7722-84-1; Sigma, St Louis, MO) that had been freshly prepared in Hanks Buffered Salt Solution (Hyclone; Thermo Scientific). After 30 min of incubation, the cell suspensions were centrifuged at 180g for 2 min and cells were washed in 3 ml of RPMI 1640 without fetal bovine serum. After another centrifugation, cells were resuspended in 750 μl RPMI 1640 with 10% (vol/vol) fetal bovine serum (Hyclone; Thermo Scientific) and 45 μg/ml of phytohemagglutinin (Remel, Lenexa, KS). Forty-four hours post-phytohemagglutinin, cytochalasin B (Sigma) was added to the cultures to a final concentration of 4.5 μg/ml. The cultures for the measurement of background MN without H2O2 treatment were handled in the same manner.
Cells were harvested 72 h after phytohemagglutinin stimulation by transferring directly to a glass slide using a Cytospin 3 (Shandon Southern Products Ltd, Astmoor, UK). The slides were air-dried for 10 min, fixed in absolute methanol for 10 min and stained with Giemsa (Sigma). We used standard scoring criteria for identifying binucleated cells and MN (11).
All slides were coded and scored by an experienced scorer blinded to sample status. MN frequency was reported as the number of micronucleated cells per 1000 binucleated lymphocytes. Some H2O2-exposed samples could not be scored because there were few binucleated cells, which were caused by the toxicity.
Statistical analysis
We used PASW statistical software version 17.0 for data analyses (SPSS, Chicago, IL). Demographic characteristics and risk factors were compared between patients and controls using Pearson’s χ2 test. MN frequencies were transformed by natural log to normalize the variance. The differences in mean values between patients and controls and between the categories of selected variables were analyzed with multivariate analysis of covariance with adjustment for age, sex, race, smoking, alcohol consumption, diabetes, family history of cancer in first-degree relatives and BMI at age 30s. Because BMI were missing for 106 patients and 287 controls, we reported our results from analyses with or without BMI adjustment. For convenience, the means and standard errors of the non-transformed MN frequencies are presented.
The median MN values from the control group were used as the cut point for dichotomizing the MN frequencies. Odds ratios (ORs) and 95% CIs were calculated to provide an estimate of the risk of PC in association with the levels of background and H2O2-induced MN. Unconditional multivariate logistic regression analysis was used to control for confounding factors such as age (continuous), gender (female or male), race/ethnicity (non-Hispanic white, Hispanic, African American and Other), smoking status (yes or no), alcohol consumption (yes or no), history of diabetes (yes or no), family history of cancer among first-degree relatives (yes or no) and BMI (continuous). The receiver operating characteristic curves were constructed and the area under the curve was calculated to evaluate the specificity and sensitivity of the ability to predict case–control status by levels of background and H2O2-induced MN. Analysis of Covariance was performed to compare the mean levels of MN in subgroups at interest with adjustment for demographic and risk factors. A two-tailed P value of <0.05 was indicative of statistical significance.
Results
Characteristics of the study participants
The characteristics of the 346 patients with PC and 449 cancer-free control participants are summarized in Table I. Patients and controls did not differ significantly by sex (P = 0.244) or age distribution (P = 0.139). There were fewer minorities among controls than among patients (8.9% versus 15.3%, P = 0.033). Smoking was associated with a 70% higher risk of PC (95% CI: 1.2–2.4). About 36.7% of the patients and 23.8% of the controls had smoked for >20 pack-years (P < 0.001). Other factors that were significantly associated with increased risk of PC included history of diabetes and family history of cancer in first-degree relatives (Table I). Ever drinkers and heavy drinkers comprised 56.4 and 11.8% of patients and 53.9 and 7.9% of controls, respectively, and the differences between patients and controls were not statistically significant. There were no significant differences in BMI between patients and controls (P = 0.110). The 346 patients include 32 with localized, 89 with locally advanced, 155 with metastatic and 51 with unstaged disease.
Table I.
Distribution of selected variables among patients and control group
| Variables | Patients n = 346 (%) | Controls n = 449 (%) | P (χ2) |
| Age | 0.139 | ||
| ≤50 | 50 (14.4) | 80 (17.8) | |
| 51–60 | 95 (27.5) | 144 (32.1) | |
| 61–70 | 127 (36.7) | 149 (33.2) | |
| >70 | 74 (21.4) | 76 (16.9) | |
| Sex | 0.244 | ||
| Female | 149 (43.1) | 212 (47.2) | |
| Male | 197 (56.9) | 237 (52.8) | |
| Race | 0.033 | ||
| Non-Hispanic white | 293 (84.7) | 409 (91.1) | |
| Hispanic | 28 (8.1) | 22 (4.9) | |
| African–American | 16 (4.6) | 14 (3.1) | |
| Other | 9 (2.6) | 4 (0.9) | |
| Family history of cancer | 0.001 | ||
| No | 117 (33.8) | 202 (45.0) | |
| Yes | 229 (66.2) | 247 (55.0) | |
| Diabetes | <0.001 | ||
| No | 254 (73.4) | 406 (90.4) | |
| Yes | 92 (26.6) | 43 (9.6) | |
| Smoking | 0.003 | ||
| Never | 132 (38.2) | 226 (50.3) | |
| Former | 160 (46.2) | 169 (37.6) | |
| Current | 54 (15.6) | 54 (12.1) | |
| ≤20 pack-years | 87 (25.1) | 116 (25.8) | <0.001 |
| >20 pack-years | 127 (36.7) | 107 (23.9) | |
| Alcohol | 0.489 | ||
| No | 151 (43.6) | 207 (46.1) | |
| Yes | 195 (56.4) | 242 (53.9) | |
| Alcohola | 0.163 | ||
| None | 151 (43.6) | 207 (46.5) | |
| <60 g/day | 154 (44.5) | 203 (45.6) | |
| ≥60 g/day | 41 (11.8) | 35 (7.9) | |
| BMI (kg/m2)b | 0.110 | ||
| <25 | 128 (53.3) | 95 (58.6) | |
| 25–30 | 80 (33.3) | 56 (34.6) | |
| >30 | 32 (13.3) | 11 (6.8) |
Information was missing for four controls.
Information was missing for 106 patients and 287 controls.
Reproducibility of the MN assay
We have conducted repeated assays on the same samples from eight individuals. The background MN frequencies (micronucleated cells per 1000 binucleated cells) (mean ± standard error) were 11.50 ± 1.65 versus 10.75 ± 1.44 [coefficient of variation (CV) = 37.8%] (P = 0.111), and the H2O2-induced MN frequencies were 20.00 ± 1.53 (CV = 20.2%) versus 20.43 ± 1.62 (CV = 20.9%) (P = 0.555) in the two assays. The interindividual variation in background MN was greater than that in H2O2-induced MN. The CV was 40.5 and 37.8% for the repeated assays of the background MN and was 20.2 and 20.9% for the H2O2-induced MN. We have also analyzed blood samples collected at two different time points from three individuals. The CV for the intraindividual variation was 9.4, 12.9 and 0% for background MN and 0, 0 and 6.2% for H2O2-induced MN, respectively, for the three individuals.
MN frequency and risk of PC
The background and H2O2-induced (mean ± standard error) were significantly higher in patients (15.3 ± 0.3 and 19.2 ± 0.4) than in controls (10.7 ± 0.3 and 14.5 ± 0.4) (Ps < 0.001). The level of H2O2-induced MN paralleled the level of background MN (correlation coefficient = 0.846, P < 0.001). Because of the well-known sex difference in MN level, we selected the median value of the controls in each sex group (12/1000 cells in women and 9/1000 cells in men) as the cut point for MN high and low categorization (Table II). In total, 78.9% of the patients and 43.7% of the controls had a higher than median level of background MN. Logistic regression analysis with adjustment for demographic factors and known risk factors (including BMI) for PC found that having a higher frequency of background MN was significantly associated with an increased risk of PC [OR and 95% CI: 8.32 (5.06–13.67), P < 0.001]. The MN-associated risk of PC was much higher in men than in women [OR (95% CI): 14.19 (7.09–28.40) versus 4.19 (1.90–9.27)] (Table II). A higher level of H2O2-induced MN (>14/1000 cells for both men and women) was also significantly associated with increased risk of PC [OR (95% CI): 3.38 (2.05–5.57), P < 0.001].
Table II.
Background MN frequency and risk of PC
| MN | Patient |
Control |
OR (95% CI)a | OR (95% CI)b | ||
| N | % | N | % | |||
| Overallc | ||||||
| Low | 73 | 21.1 | 253 | 56.3 | 1.0 | 1.0 |
| High | 273 | 78.9 | 196 | 45.7 | 4.75 (3.40–6.63) | 8.32 (5.06–13.67) |
| ≤10 | 74 | 21.4 | 247 | 55.0 | 1.0 | 1.0 |
| >10 | 272 | 78.6 | 202 | 45.0 | 5.96 (4.14–8.57) | 9.0 (5.16–15.68) |
| Women | ||||||
| ≤12 | 42 | 28.2 | 113 | 53.3 | 1.0 | 1.0 |
| >12 | 107 | 71.8 | 99 | 46.7 | 2.92 (1.80–4.74) | 4.19 (1.90–9.27) |
| Men | ||||||
| ≤9 | 31 | 15.7 | 140 | 59.1 | 1.0 | 1.0 |
| >9 | 166 | 84.3 | 97 | 40.9 | 7.25 (4.50–11.69) | 14.19 (7.09–28.40) |
OR was adjusted for age, sex, race, smoking, alcohol consumption, history of diabetes and family history of cancer.
OR was adjusted for age, sex, race, smoking, alcohol consumption, history of diabetes, family history of cancer and BMI.
Overall: low and high used the median control value in women (≤12 or >12) and men (≤9 or >9) separately. The median control value of the control group including both women and men was 10.
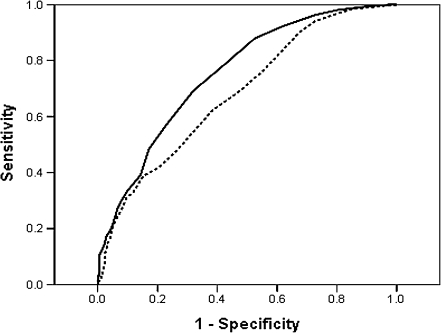
To determine if the levels of MN in the lymphocytes could significantly differentiate between PC patients and healthy controls, receiver operating characteristic curves were constructed. The area under the curve (95% CI) was 0.73 (0.69–0.76, P < 0.001) for the background MN and 0.68 (0.64–0.72, P < 0.001) for the H2O2-induced MN (Figure 1). Using the optimal cut point of 11.5 for background MN and 15.5 for H2O2-induced MN, the sensitivity and specificity was 70.2 and 63.5% and 62.1 and 61.8%, respectively.
Fig. 1.
Receiver operating characteristic curve for MN and PC case–control status. The area under the curve (95% CI) was 0.73 (0.69–0.76, P < 0.001) for the background MN (solid line) and 0.68 (0.64–0.72, P < 0.001) for the H2O2-induced MN (dashed line). Using the optimal cut-point of 11.5 for background MN and 15.5 for H2O2-induced MN, the sensitivity and specificity was 70.2 and 63.5% and 62.1 and 61.8%, respectively.
Factors influencing the level of MN
Because of the significant differences in the distribution of the MN frequencies between patients and controls, we analyzed the impact of age, race, sex, smoking, alcohol, diabetes, BMI and family history of cancer on MN frequency by case–control strata (Table III) A significantly higher frequency of both baseline and H2O2-induced MN level was detected in women than in men for both patients and control groups. We also found that the frequency of background MN in patients with PC was higher in those of >60 years of age (15.9 ± 0.5, n = 201) compared with those of ≤60 years of age (14.3 ± 0.4, n = 145; P = 0.049, Table III). The difference became nonsignificant after adjusting for BMI (P = 0.059). Among controls, MN was negatively associated with BMI (Person’s correlation coefficient = −0.190 and P = 0.015). We also noticed a significantly higher level of MN in controls without the BMI information than those with the BMI information and this difference could not be explained by the age, sex and smoking distribution between the two groups (data not shown). Thus, we created a variable of BMI information (yes or no) and included this variable in the data analysis.
Table III.
Factors influencing the level of MN in patients and controls
| Variables | Background |
H2O2 induced |
||||||||||
| Patients |
Controls |
Patients |
Controls |
|||||||||
| n | Mean ± SE | Pa | n | Mean ± SE | Pa | n | Mean ± SE | Pa | n | Mean ± SE | Pa | |
| Overall | 346 | 15.3 ± 0.3 | 449 | 10.7 ± 0.3 | 322 | 19.2 ± 0.4 | 322 | 14.5 ± 0.4 | ||||
| Age | ||||||||||||
| ≤60 | 145 | 14.3 ± 0.4 | Ref. | 224 | 10.7 ± 0.3 | Ref. | 134 | 18.6 ± 0.6 | Ref. | 157 | 14.9 ± 0.5 | Ref. |
| >60 | 201 | 15.9 ± 0.5 | 0.049 | 225 | 10.7 ± 0.4 | 0.999 | 188 | 19.7 ± 0.6 | 0.982 | 165 | 14.0 ± 0.6 | 0.152 |
| Sex | ||||||||||||
| Women | 149 | 17.1 ± 0.6 | Ref. | 212 | 13.0 ± 0.5 | Ref. | 136 | 22.0 ± 0.8 | Ref. | 147 | 17.7 ± 0.7 | Ref. |
| Men | 197 | 13.4 ± 0.3 | <0.001 | 137 | 8.9 ± 0.3 | <0.001 | 186 | 17.0 ± 0.4 | <0.001 | 175 | 12.1 ± 0.4 | <0.001 |
| Race/ethnicity | ||||||||||||
| White | 293 | 15.3 ± 0.3 | Ref. | 409 | 10.7 ± 0.3 | Ref. | 271 | 19.2 ± 0.4 | Ref. | 296 | 14.5 ± 0.4 | Ref. |
| Non-white | 28 | 15.5 ± 0.9 | 0.418 | 22 | 10.8 ± 1.1 | 0.539 | 28 | 19.6 ± 1.0 | 0.278 | 14 | 14.3 ± 1.4 | 0.629 |
| Smoking | ||||||||||||
| Never-smoker | 132 | 15.0 ± 0.4 | Ref. | 226 | 11.6 ± 0.3 | Ref. | 121 | 19.2 ± 0.6 | Ref. | 169 | 15.3 ± 0.5 | Ref. |
| Former smoker | 160 | 15.3 ± 0.5 | 0.303 | 169 | 9.7 ± 0.5 | 0.077 | 150 | 19.1 ± 0.7 | 0.225 | 114 | 13.4 ± 0.8 | 0.505 |
| Current smoker | 54 | 15.8 ± 0.7 | 0.256 | 54 | 9.9 ± 0.7 | 0.216 | 51 | 20.0 ± 1.0 | 0.266 | 39 | 14.3 ± 1.1 | 0.951 |
| ≤20 pack-years | 87 | 15.5 ± 0.5 | 0.171 | 116 | 10.3 ± 0.4 | 0.319 | 83 | 18.7 ± 0.7 | 0.379 | 76 | 14.3 ± 0.7 | 0.809 |
| >20 pack-years | 127 | 15.3 ± 0.7 | 0.402 | 107 | 9.4 ± 0.7 | 0.023 | 118 | 19.7 ± 0.8 | 0.159 | 77 | 13.0 ± 1.0 | 0.560 |
| Alcohol | ||||||||||||
| Never | 151 | 15.6 ± 0.5 | Ref. | 207 | 11.8 ± 0.5 | Ref. | 138 | 20.1 ± 0.7 | Ref. | 154 | 15.8 ± 0.6 | Ref. |
| <60 g/day | 154 | 15.3 ± 0.4 | 0.422 | 203 | 10.0 ± 0.4 | 0.447 | 148 | 18.5 ± 0.6 | 0.702 | 141 | 13.8 ± 0.6 | 0.554 |
| ≥60 g/day | 41 | 13.2 ± 0.6 | 0.928 | 35 | 9.6 ± 0.7 | 0.969 | 36 | 17.7 ± 0.9 | 0.862 | 24 | 11.5 ± 1.1 | 0.257 |
| Diabetes | ||||||||||||
| No | 254 | 15.5 ± 0.4 | Ref. | 406 | 10.7 ± 0.3 | Ref. | 237 | 19.6 ± 0.5 | Ref. | 289 | 14.5 ± 0.4 | Ref. |
| Yes | 92 | 14.2 ± 0.6 | 0.186 | 43 | 11.5 ± 0.9 | 0.333 | 85 | 17.9 ± 0.7 | 0.167 | 33 | 15.3 ± 1.2 | 0.243 |
| Family history of cancer | ||||||||||||
| No | 117 | 14.9 ± 0.5 | Ref. | 202 | 10.2 ± 0.3 | Ref. | 107 | 18.9 ± 0.6 | Ref. | 156 | 14.0 ± 0.5 | Ref. |
| Yes | 229 | 15.5 ± 0.4 | 0.536 | 247 | 11.0 ± 0.4 | 0.101 | 215 | 19.4 ± 0.5 | 0.574 | 166 | 14.8 ± 0.6 | 0.428 |
| BMI (kg/m2) | ||||||||||||
| <25 | 128 | 16.3 ± 0.5 | Ref. | 95 | 10.6 ± 0.6 | Ref. | 127 | 19.8 ± 0.7 | Ref. | 95 | 14.5 ± 0.8 | Ref. |
| 25–30 | 80 | 13.5 ± 0.5 | 0.197 | 56 | 8.3 ± 0.6 | 0.412 | 79 | 16.3 ± 0.6 | 0.083 | 54 | 11.3 ± 0.8 | 0.648 |
| >30 | 32 | 16.6 ± 1.3 | 0.178 | 11 | 7.4 ± 2.3 | 0.226 | 32 | 20.7 ± 1.7 | 0.193 | 11 | 10.4 ± 3.0 | 0.292 |
| Disease stage | ||||||||||||
| Localized | 32 | 14.4 ± 1.3 | Ref. | NA | 30 | 18.8 ± 1.5 | Ref. | NA | ||||
| Locally Advanced | 89 | 15.6 ± 0.8 | 0.375 | 80 | 19.7 ± 0.9 | 0.608 | ||||||
| Metastatic | 155 | 14.7 ± 0.6 | 0.933 | 146 | 18.3 ± 0.7 | 0.620 | ||||||
| Unstaged | 51 | 16.7 ± 1.0 | 0.433 | 49 | 21.0 ± 1.2 | 0.358 | ||||||
Analysis of covariance test for differences in MN frequency between subgroups with adjustment for age, race/ethnicity, smoking, alcohol consumption, diabetes and family history of cancer.
Because of the significant sex difference in MN level, we further analyzed the data by sex strata. Among female patients, those who were obese had a higher frequency of background MN (25.3 ± 2.8, n = 6) compared with normal weight patients (17.7 ± 0.8, n = 73; P = 0.024). Among men, cigarette smoking had a differential effect on MN formation for both patients and controls. In male patients, a higher frequency of background MN was observed in ever smokers (14.5 ± 0.6, n = 92) than in nonsmokers (12.1 ± 0.6, n = 49) (P = 0.023). In contrast, among men serving as controls, a lower frequency of background MN was detected in heavy smokers (8.3 ± 0.6, n = 82) than in nonsmokers (9.0 ± 0.4, n = 89; P = 0.014). Because BMI is a major risk factor for PC, we also examined the impact of smoking on MN with adjustment for BMI among men (Table IV). We found a significantly higher frequency of baseline MN in ever smokers than nonsmokers among patients but not among controls. We did not observe any substantial differences in MN frequencies by other factors examined.
Table IV.
Background MN frequency between nonsmokers and smokers in men
| Variables | Patients |
Controls |
||||
| n | Mean ± SE | Pa | n | Mean ± SE | Pa | |
| Without BMI adjustment | ||||||
| Nonsmoker | 49 | 12.12 ± 0.56 | Reference | 36 | 8.14 ± 0.66 | Reference |
| Smoker | 92 | 14.48 ± 0.62 | 0.023 | 59 | 7.25 ± 0.79 | 0.593 |
| ≤20 pack-years | 33 | 13.87 ± 0.81 | 0.106 | 27 | 7.61 ± 0.90 | 0.909 |
| >20 pack-years | 59 | 14.59 ± 0.88 | 0.027 | 32 | 7.38 ± 1.23 | 0.323 |
| With BMI adjustmentb | ||||||
| Nonsmoker | 49 | 12.12 ± 0.56 | Reference | 36 | 8.14 ± 0.66 | Reference |
| Smoker | 92 | 14.50 ± 0.62 | 0.023 | 59 | 7.22 ± 0.79 | 0.593 |
| ≤20 pack-years | 33 | 13.79 ± 0.82 | 0.104 | 27 | 7.71 ± 0.91 | 0.913 |
| >20 pack-years | 59 | 14.65 ± 0.88 | 0.027 | 32 | 7.27 ± 1.24 | 0.326 |
Analysis of covariance test for differences in MN frequency between subgroups with adjustment for age, race/ethnicity, alcohol consumption, diabetes and family history of cancer.
BMI was adjusted as a continuous variable.
Discussion
Our observations add to existing evidence supporting the value of MN as a cancer susceptibility biomarker (13). In the present hospital-based case–control study, a significant increase in baseline and H2O2-induced MN frequency in peripheral lymphocytes was observed in patients with PC over healthy controls. This higher level of MN was associated with an 8-fold increase in risk of PC. Furthermore, we have for the first time demonstrated a differential association of smoking and levels of background MN between male patients and controls and a lack of association between levels of background MN and disease stage.
MN has been successfully used as a marker for DNA damage in populations with known exposure to genotoxic agents. Our study has demonstrated an association between levels of MN formation and the risk of PC. It is possible that the higher frequency of MN in patients with PC reflects the accumulated genetic alterations caused by known and suspected genotoxic factors, such as cigarette smoking, obesity and heavy alcohol consumption as well as individual variations in susceptibility to these factors. However, it is difficult to establish a causal relationship between increased MN levels and cancer risk because a high MN level in cancer patients could be a consequence of the disease (known as reverse causality) or reflect an individual’s susceptibility to events causing genomic instability (9). The predictive value of background levels of MN as a biomarker for cancer risk in the general population has been preliminarily validated by two ongoing cohort studies, which involve plans to extend the length of follow-up for those study cohorts (15,16). The lack of association between the level of MN and disease stage argue against the possibility that the observed higher level of MN was mainly due to the disease.
There is a growing awareness that oxidative stress and/or inflammation plays a contributory role in numerous pathologies, including pancreatic carcinogenesis (3). To find out whether individual susceptibility to oxidative stress-induced DNA damage was associated with risk of PC, we measured the MN frequency in peripheral lymphocytes treated in vitro with H2O2. We found that the background and H2O2-induced MN frequencies were parallel, i.e. those with a higher level of background MN also had a higher level of H2O2-induced MN, suggesting that the individual did indeed have an increased susceptibility to oxidative stress. These observations suggest an association between elevated level of background MN and increased risk of PC.
The current study confirmed that MN formation differs according to age and sex as these were the demographic variables that most consistently predicted the levels of MN found in peripheral lymphocytes (21–25). The effect of age seems to reflect the accumulated genetic damage over a lifetime. The sex difference could be ascribed to an aneuploidy phenomenon, i.e. the X chromosome tends to lag behind in female lymphocyte anaphase, being micronucleated more efficiently than autosomes (26).
Previously, reports on the association of smoking with background MN frequency are inconsistent. Despite a few reports showing a positive association (27–29), the majority of studies did not find any association between levels of MN frequency and smoking (15,16,30–32). In men, we observed different associations between smoking and MN levels between patients and controls. Among patients, smokers, especially heavy smokers, had higher levels of MN than nonsmokers; however, among men in the control group, heavy smokers had a lower frequency of MN than light or never-smokers. Our observation in the control group was in agreement with the results reported by the HUMN project that current and former smokers experienced a small decrease of MN frequencies compared with never-smokers (32). These observations suggest that the higher level of MN in patients who had smoked is largely determined by genetic susceptibility to such exposure because smokers in the control group did not have increased level of MN. The lower levels of MN among men in the control group who had smoked heavily may have induced some protective mechanisms, e.g. detoxification enzymes and DNA repair enzymes that actually led to a lower level of MN. In addition, smoking may induce apoptosis and the micronucleated cells may be preferentially eliminated by apoptosis or the damaged cells may not divide and fail to form binucleated cells (32). On the other hand, patients may have more cells with DNA damage because of their deficiency in apoptosis signaling. It is not known whether female hormones have a protective effect against DNA damage that could have negated the effects of smoking among women, but the question needs further investigation.
Interestingly, we observed a significant negative association between BMI and MN level in controls but a positive association between obesity, an established risk factor for PC, and MN levels among female patients (19). Increased oxidative stress and inflammatory conditions could be underlying mechanisms contributing to the elevated levels of MN in obese women; but the negative association between BMI and MN in controls did not support this hypothesis. Whether there is a difference in host response to oxidative DNA damage between patients and controls that lead to the differential effect of BMI on MN needs further investigation. The small sample size for the obese group also suggests these observations need to be confirmed in a larger study.
The strengths of the current study include a large overall sample size, the collection of detailed epidemiologic information and having a single researcher scoring all the samples to control for observer variations. The limitations of the study include the case–control design and its inherent recall bias. However, the risk estimates for major risk factors such as smoking, obesity, diabetes and family history of PC in our study were quite comparable with that reported in cohort studies (18,19), which do not suggest a significant recall bias. Other limitations include the unbalanced match on race/ethnicity, incompleteness of the data on BMI and the small sample size in some of the subgroup analyses. Additionally, only actual chromosome breakage (in the form of micronucleated cells) was measured in our study, so other chromosomal damage endpoints, such as chromosome rearrangements as indicated by nucleoplasmic bridges and gene amplification as indicated by nuclear buds, were not evaluated. The impact of dietary nutrients (33) and genetic variations in DNA damage response also need further study.
In summary, data from this study provide convincing evidence that elevated levels of MN in peripheral lymphocytes are associated with risk of PC. Other large-scale epidemiological studies in different populations, preferably in a prospective design, are warranted to confirm the causal role of MN formation in the occurrence of genetic instability and PC.
Funding
National Institutes of Health (NIH) (CA98380); National Institute of Environmental Health Sciences center (P30 ES07784); NIH core (CA16672); Lockton Pancreatic Cancer Research Funds.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- HUMN
Human MicroNucleus
- MN
micronuclei
- OR
odds ratio
- PC
pancreatic cancer
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Garcea G, et al. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 3.Greer JB, et al. Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy N, et al. Pancreatic cancer biology and genetics. Nat. Rev. Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, et al. Correlation between centrosome abnormalities and chromosomal instability in human pancreatic cancer cells. Cancer Genet. Cytogenet. 2001;126:13–19. doi: 10.1016/s0165-4608(00)00384-8. [DOI] [PubMed] [Google Scholar]
- 6.Solomon E, et al. Chromosome aberrations and cancer. Science. 1991;254:1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- 7.Norppa H. Cytogenetic biomarkers and genetic polymorphisms. Toxicol. Lett. 2004;149:309–334. doi: 10.1016/j.toxlet.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M. The in vitro micronucleus technique. Mutat. Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Fenech M, et al. The HUman MicroNucleus Project—An international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat. Res. 1999;428:271–283. doi: 10.1016/s1383-5742(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 10.Fenech M, et al. Intra- and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes. Results of an international slide-scoring exercise by the HUMN project. Mutat. Res. 2003;534:45–64. doi: 10.1016/s1383-5718(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 11.Fenech M, et al. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 12.Fenech M. Chromosomal biomarkers of genomic instability relevant to cancer. Drug Discov. Today. 2002;7:1128–1137. doi: 10.1016/s1359-6446(02)02502-3. [DOI] [PubMed] [Google Scholar]
- 13.Iarmarcovai G, et al. Micronuclei frequency in peripheral blood lymphocytes of cancer patients: a meta-analysis. Mutat. Res. 2008;659:274–283. doi: 10.1016/j.mrrev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Van Schooten FJ, et al. Effects of oral administration of N-acetyl-L-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol. Biomarkers Prev. 2002;11:167–175. [PubMed] [Google Scholar]
- 15.Bonassi S, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 16.Murgia E, et al. Validation of micronuclei frequency in peripheral blood lymphocytes as early cancer risk biomarker in a nested case-control study. Mutat. Res. 2008;639:27–34. doi: 10.1016/j.mrfmmm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat. Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 18.Hassan MM, et al. Risk factors for pancreatic cancer: case-control study. Am. J. Gastroenterol. 2007;102:2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenech M, et al. Necrosis, apoptosis, cytostasis and DNA damage in human lymphocytes measured simultaneously within the cytokinesis-block micronucleus assay: description of the method and results for hydrogen peroxide. Mutagenesis. 1999;14:605–612. doi: 10.1093/mutage/14.6.605. [DOI] [PubMed] [Google Scholar]
- 21.Barale R, et al. Sister chromatid exchange and micronucleus frequency in human lymphocytes of 1,650 subjects in an Italian population: II. Contribution of sex, age, and lifestyle. Environ. Mol. Mutagen. 1998;31:228–242. doi: 10.1002/(sici)1098-2280(1998)31:3<228::aid-em4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Fenech M. Important variables that influence base-line micronucleus frequency in cytokinesis-blocked lymphocytes-a biomarker for DNA damage in human populations. Mutat. Res. 1998;404:155–165. doi: 10.1016/s0027-5107(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 23.Bonassi S, et al. HUman MicroNucleus project: international database comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ. Mol. Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 24.Bonassi S, et al. Influence of sex on cytogenetic end points: evidence from a large human sample and review of the literature. Cancer Epidemiol. Biomarkers Prev. 1995;4:671–679. [PubMed] [Google Scholar]
- 25.Bolognesi C, et al. Age-related increase of baseline frequencies of sister chromatid exchanges, chromosome aberrations, and micronuclei in human lymphocytes. Cancer Epidemiol. Biomarkers Prev. 1997;6:249–256. [PubMed] [Google Scholar]
- 26.Norppa H, et al. What do human micronuclei contain? Mutagenesis. 2003;18:221–233. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 27.Cheng TJ, et al. Increased micronucleus frequency in lymphocytes from smokers with lung cancer. Mutat. Res. 1996;349:43–50. doi: 10.1016/0027-5107(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 28.Duffaud F, et al. Comparison between micronucleated lymphocyte rates observed in healthy subjects and cancer patients. Mutagenesis. 1997;12:227–231. doi: 10.1093/mutage/12.4.227. [DOI] [PubMed] [Google Scholar]
- 29.Duffaud F, et al. Micronucleated lymphocyte rates from head-and-neck cancer patients. Mutat. Res. 1999;439:259–266. doi: 10.1016/s1383-5718(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 30.Bolognesi C, et al. A molecular epidemiology case control study on pleural malignant mesothelioma. Cancer Epidemiol. Biomarkers Prev. 2005;14:1741–1746. doi: 10.1158/1055-9965.EPI-04-0903. [DOI] [PubMed] [Google Scholar]
- 31.El-Zein RA, et al. Cytokinesis-blocked micronucleus assay as a novel biomarker for lung cancer risk. Cancer Res. 2006;66:6449–6456. doi: 10.1158/0008-5472.CAN-06-0326. [DOI] [PubMed] [Google Scholar]
- 32.Bonassi S, et al. Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat. Res. 2003;543:155–166. doi: 10.1016/s1383-5742(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 33.Fenech M, et al. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability—results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis. 2005;26:991–999. doi: 10.1093/carcin/bgi042. [DOI] [PubMed] [Google Scholar]