Abstract
N-Acetyl-S-(p-chlorophenylcarbamoyl)cysteine (NACC) was identified as a metabolite of sulofenur. Sulofenur was demonstrated to have broad activity against solid tumors in preclinical studies but exhibited disappointing clinical responses due to its high protein binding related adverse effects. NACC exhibited low protein binding and excellent activity against a sulofenur sensitive human colon cancer cell line. In this study, analogs of NACC were synthesized and evaluated with four human cancer cell lines. Two of the NACC analogs showed excellent activity against two human melanoma cell lines, while NACC remains the most potent of the series. All three compounds were more potent than dacarbazine, which is used extensively in treating melanoma. NACC was shown to induce apoptosis without affecting the cell cycle. Further, NACC exhibited low toxicity against monkey kidney cells. The selective anticancer activity, low toxicity, an unknown yet but unique anticancer mechanism and ready obtainability through synthesis make NACC and its analogs promising anticancer agents.
1. Introduction
Following heart disease, cancer is the second leading cause of death in the developed world.1 For the majority of cancers, chemotherapy, either alone or as an adjunct to radiotherapy or surgery, remains the treatment of choice. Most of the anticancer agents used in clinical practice are broadly acting cytotoxic drugs. These agents impact structure and function of the rapidly proliferating cancer cells and will often arrest the cell cycle in a specific phase depending on the mechanism of action of the agent. Unfortunately, they also lack specificity and produce adverse effects related to the impact on rapidly dividing noncancerous cells.2, 3 Discovery of more specific anticancer agents in order to improve efficacy and decrease the adverse effect potential is one of the goals in developing new anticancer drugs. Another major goal for developing new anticancer agents is to overcome cancer resistance to drug treatment that has made many of the currently available chemotherapeutic agents ineffective.4
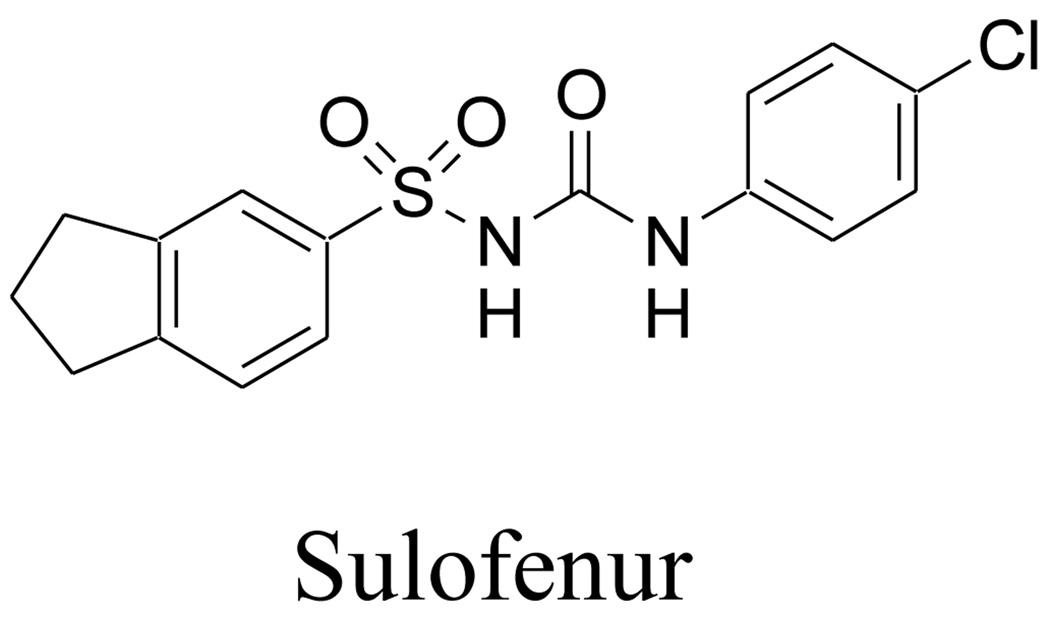
Sulofenur (LY 186641, Fig. 1), a diarylsulfonylurea developed in Lilly Research Laboratories, has been reported to exhibit broad spectrum activity against murine solid tumors and human tumor xenografts.5 Sulofenur is also effective against childhood rhabdomyosarcoma.6 It has undergone clinical evaluation in lung, breast, colon, ovarian, pancreatic, renal and gastric cancers.5 However, due to its high protein binding, high dosages of sulofenur were required for clinical responses. As a consequence, hemolytic anemia and methemoglobinemia were observed with sulofenur in clinical trials.7, 8
Figure 1.
Structure of sulofenur
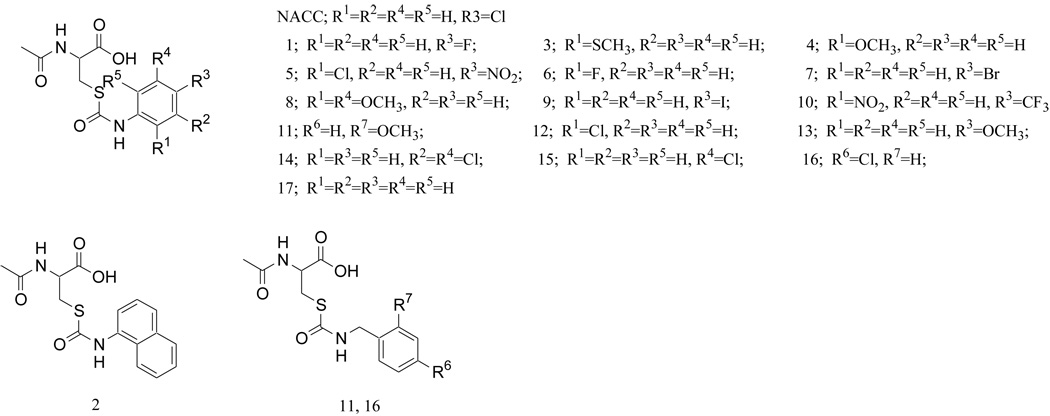
In our earlier study of sulofenur metabolism in rats, it was found that the mercapturic acid conjugate of sulofenur, N-acetyl-S-(p-chlorophenylcarbamoyl)cysteine (NACC, Fig. 2), had much lower protein binding and exhibited anticancer activity that was comparable to sulofenur against one sulofenur sensitive human colon cancer cell line (GC3/c1).9 Encouraged by the low protein binding, significant anticancer activity, and ready obtainability through a one-step synthesis, we designed and prepared a series of analogs of NACC in an effort to search for a novel class of anticancer agents. Seventeen analogs (Fig. 2) of NACC were prepared, and their anticancer activities were evaluated against three different human cancer cell lines: UACC-62, GC3/c1 and OVCAR-3 (human ovarian cancer) cells. The toxicity of NACC against normal cells was investigated using CV-1 cells - a monkey kidney cell line. Our data demonstrate that NACC and two of its analogs exhibit selective anticancer activities and low toxicity against normal cells. Compared with dacarbazine, a commonly used anticancer drug in treating melanoma and various other cancers, NACC, analog 1 and analog 7 were 490, 328, and 37-fold more active respectively than dacarbazine against UACC-62. The selective activity against melanoma was further confirmed later with SK-MEL-2 cells, another human melanoma cell line. NACC, analog 1 and analog 7 were found 16000, 279, and 1000 times more active than dacarbazine respectively against SK-MEL-2 cells. Mechanistic studies of NACC in UACC-62 cells revealed that NACC induced cell apoptosis without affecting the cell cycle. The selective anticancer activities, low toxicity against normal cells, unique anticancer mechanism, and simple chemical structures that can be readily obtained through a one step synthesis from commercially available starting materials make NACC and its analogs promising anticancer agents.
Figure 2.
Structures of NACC and its analogs
2. Chemistry
NACC was synthesized by coupling of N-acetyl-L-cysteine (NAC) and 4-chlorophenylisocyanate.9 Based on the commercial availability of isocyanates, we decided to investigate the effects of the substituents at the different positions of the aromatic ring. Various electron withdrawing groups (EWGs) and electron donating groups (EDGs) were chosen to determine the electronic effects of the substituent on the anticancer activity. We also selected a naphthalene group (analog 2) to check whether an increase in hydrophobicity and aromaticity would increase the anticancer activity. In addition, analogs 11 and 16 were prepared to determine whether the p-π conjugation between the thiocarbamoyl group and the aromatic ring is required for the activity. The compounds designed are presented in Figure 2.
3. Results and Discussion
3.1. Synthesis
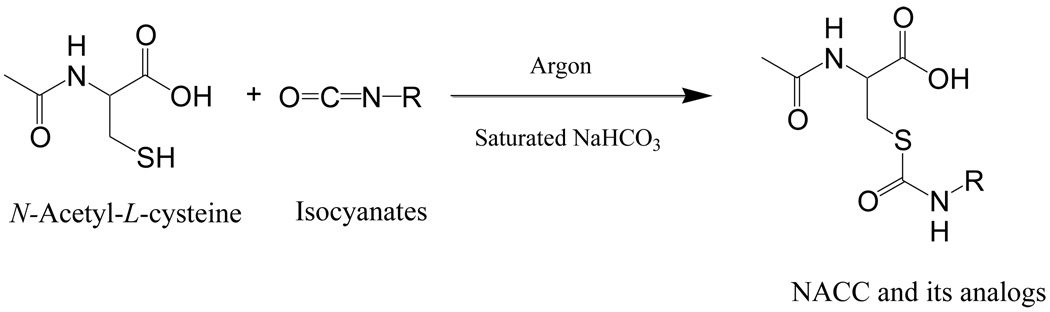
NACC and its analogs were synthesized by a one-step reaction of commercially available NAC and isocyanates (Scheme 1). The reaction yields ranged between 10–93%. The purity of the compounds was determined to be >95% by two different HPLC mobile systems.
Scheme 1.
3.2. In Vitro Cytotoxicity Evaluation
The anticancer activities of NACC and its analogs were initially evaluated with three different human cancer cell lines: GC3/c1, UACC-62, and OVCAR-3. Due to the finding that NACC, analog 1 and analog 7 were effective against human melanoma (UACC-62), the selective activity against melanoma of these three compounds was further evaluated with SK-MEL-2 cells, another human melanoma cell line. Cells were treated with a compound for 6 days before the cell viability was determined by the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). A shorter length (1 to 3 days) treatment resulted in no activity (data not shown). IC50 values, the concentration producing 50% cell growth inhibition, were derived from the corresponding dose-response curves. To place the data in perspective, dacarbazine was chosen as a positive control. It needs to be noted that dacarbazine is a prodrug and requires activation by P-450 or light.10 In this protocol, dacarbazine was handled under natural light and was incubated with cells for 6 days. The protocol ensures dacarbazine to be activated and serve as a positive control.
As demonstrated in Table 1, the cytotoxicity of NACC against GC3/c1 cells was comparable to dacarbazine. Disappointingly, none of the analogs showed significant activity against the colon cancer cells except analog 1 and analog 7 which exhibited moderate activity. NACC and its analogs were then screened against UACC-62 cells. NACC, analog 1 and analog 7 were found to be 490, 328, and 37-fold more active respectively than dacarbazine against UACC-62 cells. The selective activity against melanoma was further confirmed with SK-MEL-2 cells. NACC, analog 1 and analog 7 were found to be 16000, 279, and 1000 times more active than dacarbazine respectively against SK-MEL-2 cells confirming the selective activity against melanoma. When NACC and its analogs were evaluated with OVCAR-3 cells, none of the compounds exhibited significant activity demonstrating the selectivity of NACC and its analogs against specific cancers. The selectivity was further confirmed by screening of NACC against other human cancer cell lines that included PC-3 (human prostate cancer), NCI-H226 (human lung cancer), UO-31 (human renal cancer), and A431(human skin cancer). Poor activities were observed (unpublished data from this laboratory). Since NACC was the most potent anticancer agent of the series, the toxicity of NACC against normal cells was evaluated with CV-1 cells. The IC50 value of NACC against CV-1 cells was determined to be 521.67±7.64 µM demonstrating that NACC exhibited low toxicity toward a normal cell line. Therefore, NACC exhibits not only high selectivity against different cancers but also high selectivity between cancer cells and normal cells.
Table1.
Determination of in vitro cytotoxicity of NACC and its analogs against various human cancer cell lines.
| Compounds | IC50 (µM) a |
|||
|---|---|---|---|---|
| UACC-62 | GC3/c1 | OVCAR-3 | SK-MEL-2 | |
| NACC | 0.024 ± 0.01 | 8.13 ± 4.07 | >200 | 0.008 ± 0.001 |
| 1 | 0.036 ± 0.023 | 114.49 ± 1.21 | >200 | 0.430 ± 0.230 |
| 2 | >200 | >200 | >200 | N.D. |
| 3 | >200 | >200 | >200 | N.D. |
| 4 | >200 | >200 | >200 | N.D. |
| 5 | >200 | >200 | >200 | N.D. |
| 6 | >200 | >200 | >200 | N.D. |
| 7 | 0.32 ± 0.11 | 63.47 ± 1.27 | >200 | 0.120 ± 0.030 |
| 8 | >200 | >200 | >200 | N.D. |
| 9 | 46.16 ± 2.61 | >200 | >200 | N.D. |
| 10 | >200 | >200 | >200 | N.D. |
| 11 | >200 | >200 | >200 | N.D. |
| 12 | >200 | >200 | >200 | N.D. |
| 13 | 75.23 ± 4.80 | >200 | >200 | N.D. |
| 14 | >200 | >200 | >200 | N.D. |
| 15 | >200 | >200 | >200 | N.D. |
| 16 | >200 | >200 | >200 | N.D. |
| 17 | 125.00 ± 5.00 | 178.33 ± 7.64 | 153.33 ± 15.28 | N.D. |
| Dacarbazine | 11.83 ± 1.03 | 7.67 ± 2.39 | 17.67 ± 1.70 | 120.00 ± 75.5 |
Cells were treated with a compound for 6 days. Cell survival rates were determined by the MTT assay. IC50 values were derived from dose-response curves and ar presented as the mean ± SD of three independent experiments. N.D.: Not determined.
The selective activity of NACC and its analogs against human melanoma cells is encouraging. Malignant melanoma is an aggressive, therapy-resistant malignancy of melanocytes.11 The incidence of melanoma has been increasing dramatically worldwide in the last few decades.11–14 Early-stage melanoma is curable, but advanced, metastatic melanoma is almost uniformly fatal.15 In most cases, primary surgery excision remains the mainstay in the treatment of malignant melanoma. Early diagnosis combined with appropriate surgical therapy is the only curative treatment.16, 17 Single-agent or combination chemotherapies have not made a major impact on metastatic melanoma, and complete or durable responses are rare. Dacarbazine remains the only ‘‘standard’’ agent for melanoma chemotherapy18 and is the only small molecule drug approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. However, it has a response rate of only 10% to 20%, and complete or durable responses are much lower. Other chemotherapy agents which have been used in the treatment of melanoma with activity similar to dacarbazine include platinum compounds, vinca alkaloids, nitrosoureas, and taxanes. These drugs have not improved clinical outcome.13 Therefore, compounds with selective activity against melanoma are of therapeutic potential.
3.3. Structure Activity Relationship
The anticancer activity screening in UACC-62 cells (Table 1) revealed that the most potent compounds appear to be from the para-substituted derivatives. Any substitution at meta or ortho position resulted in the loss of activity. Among the para-substituted derivatives, 4-halogenated analogs appear to be the most potent with NACC, analog 1 and analog 7 found to be more potent than dacarbazine respectively. The effect of the substituents does not appear to be caused by the electronic effect of the substituent since moving the chloro or fluoro from the para- position to ortho- position (NACC vs analog 12 or analog 1 vs analog 6), which does not significantly affect the aromatic ring electron density, caused a loss of activity. This notion is further supported by the fact that when compared with NACC, both analog 1 and analog 13, one with a more electron withdrawing group (p-F) and one with a more electron donating group (p-OMe), exhibited lower activity indicating a decrease or increase in aromatic ring electron density is not correlated with the anticancer activity. Further, none of the analogs with EDGs (analogs 3, 4, and 8) or EWGs (analogs 5, 10, 12, 15, 16) at positions other than para are active, again showing no correlation between the aromatic ring electron density and anticancer activity. Therefore, it is concluded that the effect of the substituent at the para position is likely to be related to the size of the substituent, with small substituents (Cl or F) being more active. It is interesting that the unsubstituted compound (analog 17) exhibited no significant activity suggesting that there is an interaction between the para substituent and the target.
When the thiocarbamoyl group is separated from the aromatic ring by a methylene group as shown in analogs 11 and 16, the compounds lost activity indicating that the p-π conjugation between the aromatic ring and the thiocarbamoyl group might be needed for the activity, or the increased distance between the aromatic ring and the thiocarbamoyl group by insertion of a methylene group might be detrimental, or both might be essential for the activity.
3.4. Solubility determination of the active compounds
To assess whether NACC, compounds 1 and 7 exhibit drugable physicochemical properties, their solubility in both organic phase (ethyl acetate) and aqueous phase (0.1 M, pH 7.4 phosphate buffer) was determined. These compounds exhibit good solubility in both organic phase and aqueous phase with the solubility in ethyl acetate 721, 388 and 50 mg/mL and in the buffer 26, 26 and 24 mg/mL respectively for NACC, compounds 1 and 7.
3.5. Determination of Protein Binding by Equilibrium Dialysis
Sulofenur failed in clinical trials due to its high protein binding (greater than 99%) associated hemolytic anemia and methemoglobinemia side effects.7, 8 The protein binding for compounds 1 and 2 was determined to be 67% and 82 % respectively, much lower than sulofenur but similar to NACC (84%) which was reported earlier.9
3.6. Analysis of Cellular DNA Content by Flow Cytometry
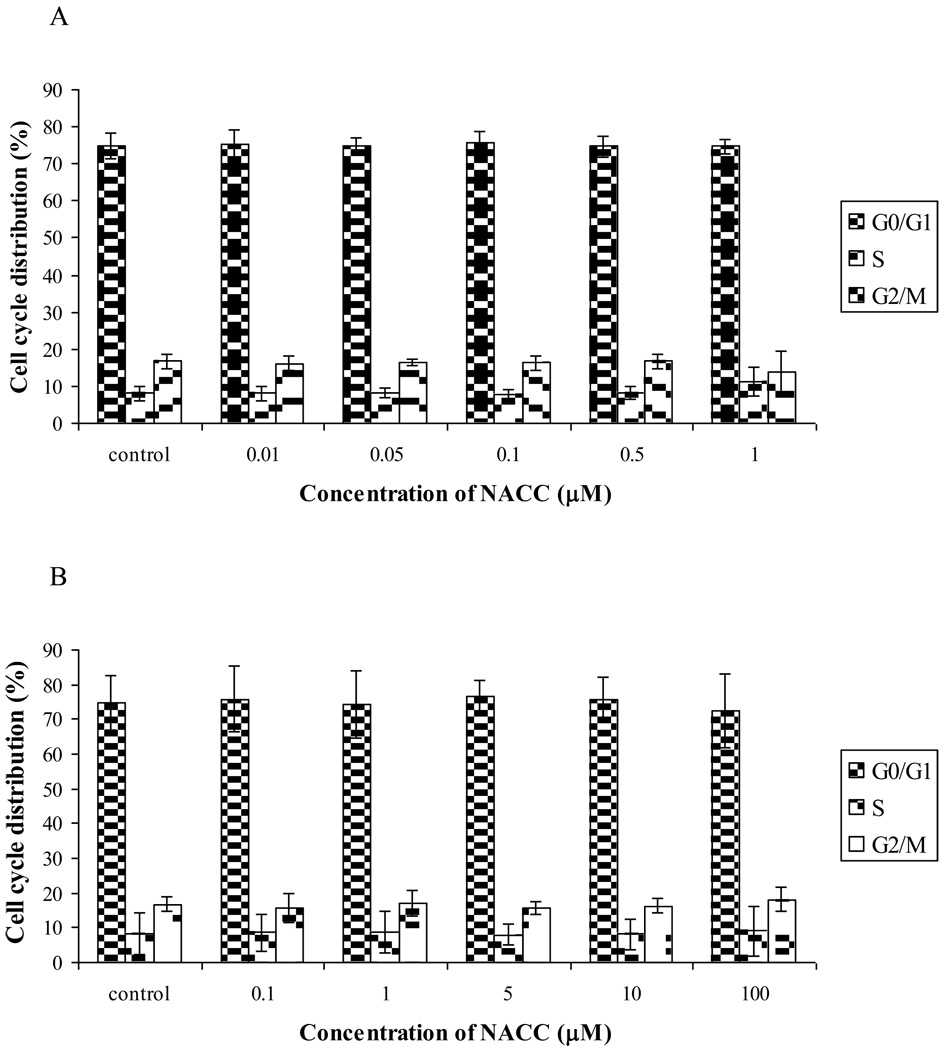
We further investigated the mechanism of the cancer growth inhibition by NACC and its analogs. Since NACC was the most potent of the series, it was selected for the investigation. The effects of NACC on the cell cycle distribution were studied with UACC-62 and GC3/c1 cells by flow cytometric analysis of cellular DNA content using propidium iodide (PI) staining. To be consistent with the cytotoxicity evaluation experiment, UACC-62 and GC3/c1 cells were treated with NACC for 6 days. After the treatment, only attached cells were collected, and intracellular DNA content was determined. As shown in Figure 3A, no change in cell cycle distribution was observed after UACC-62 cells were treated with NACC (0.01 µM to 1 µM). Similar results were obtained with GC3/c1 cells (Fig. 3B). The effect of NACC on the cell cycle is interesting since diarylsulfonylureas were also reported to be non-cell cycle specific. A lack of effects on the cell cycle distinguishes diarylsulfonylureas from other conventional anticancer agents that can arrest cells at a specific phase, mostly at the G1/S or G2/M boundaries.19 It is not clear whether it is this property that makes diarylsulfonylureas lack toxicity to proliferating normal cells, a phenomenon also observed with NACC.
Figure 3.
Effect of NACC on cell cycle distribution in UACC-62 cells (A) and GC3/c1 cells (B). UACC-62 cells and GC3/c1 cells were treated with different concentrations of NACC for 6 days. After staining with PI, cell cycle distribution was analyzed using a flow cytometer. Results are presented as the mean ± SD of three independent experiments.
3.7. Apoptosis Assay by Flow Cytometry
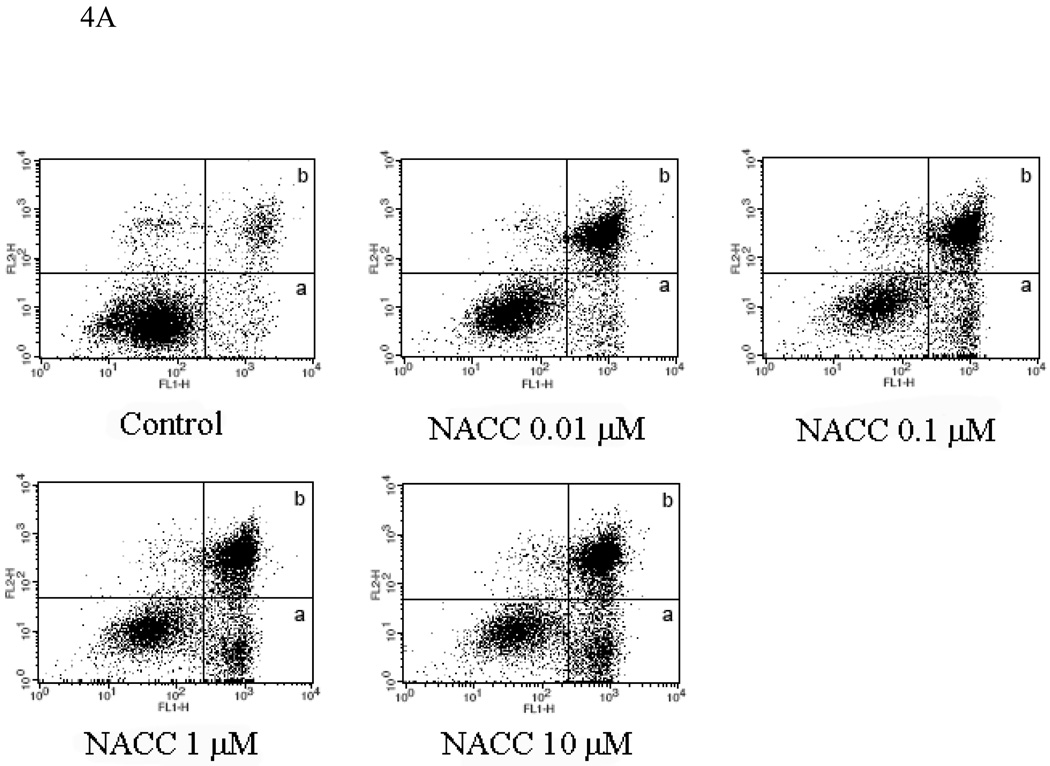
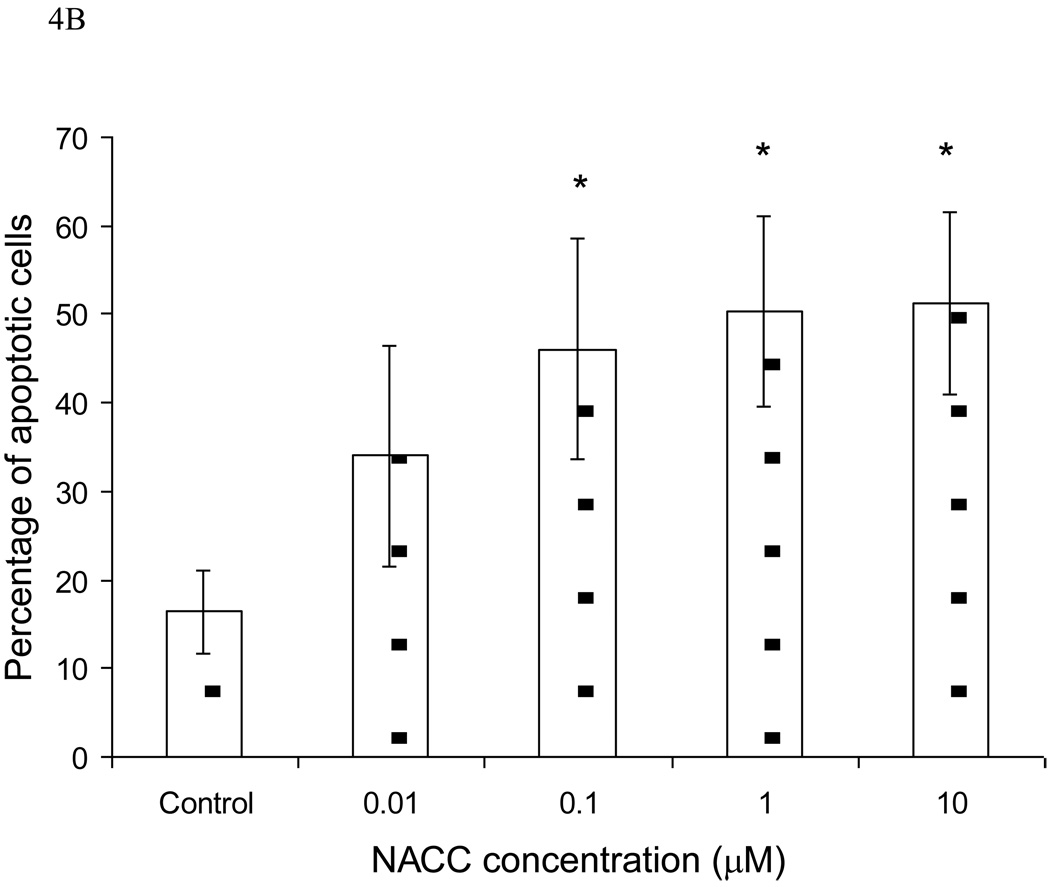
To further investigate the mechanism of action, the effect of NACC on cell apoptosis was conducted with UACC-62 cells. An apoptotic event is marked by the translocation of phosphatidylserine (PS) from the inner layer to the outer layer of the cell membrane. The translocated PS can be detected using fluorescein-labeled annexin V, a Ca2+-dependent phospholipid-binding protein with high affinity to PS. Combined with PI which stains nucleic acids in dead cells, the percentage of the cell population undergoing apoptosis can be determined. After UACC-62 cells were treated with NACC for 6 days, both adherent and suspended cells were collected and analyzed using flow cytometry. As demonstrated in Figure 4A, NACC induced significant apoptosis in UACC-62 cells. It is interesting that more late apoptotic cells (area b) were observed compared to cells in early apoptosis (area a). This might be due to the 6-day treatment in which the induced apoptotic cells were more in the late stage of apoptosis at the end of the 6-day treatment. Figure 4B provides a quantitative presentation of the apoptotic cells. It shows that NACC induced apoptosis in UACC-62 cells in a concentration dependent manner in the concentration range of 0.1 µM to 1 µM. However, when the concentration exceeds 1 µM, no further increase in apoptosis was observed (Fig. 4B). It is likely that at high concentrations, NACC may induce cell death through a mechanism other than apoptosis.
Figure 4.
Effect of NACC on induction of apoptosis in UACC-62 cells. UACC-62 cells were treated with different concentrations of NACC for 6 days. After staining with Annexin V and PI, apoptotic cells was analyzed by flow cytometry. The total percentage of apoptotic cells is the sum of early apoptotic cells (Graph A, area a) and late apoptotic cells (Graph A, area b). Graph A presents apoptotic histograms derived from one of the three independent experiments. Graph B presents the summarized data. Results are presented as the mean ± SD of three independent experiments.
* indicates statistical significance (P < 0.05) in NACC treated groups as compared to the control.
4. Conclusions
In this study, NACC and its seventeen analogs were synthesized through a one-step reaction. Based on the IC50 values, NACC remains the most potent compound of the series. NACC also exhibited low toxicity to noncancerous cells. The low toxicity and its impressive activity against UACC-62 and SK-MEL-2 cells suggest the potential of this class of compounds in the treatment of melanoma. The non-cell cycle specific cell growth inhibition is another unique feature of this class of compounds since most conventional anticancer agents achieve their action through affecting the cell cycle. NACC also induced significant apoptosis in UACC-62 cells. Additionally, NACC and its analogs exhibit excellent physicochemical and pharmaceutical properties with low protein binding and good solubility in both organic and aqueous solutions. Further investigation of NACC and its analogs against melanoma in vivo is needed to evaluate their potential in the treatment of melanoma.
5. Experimental Section
5.1 Chemistry
5.1.1. General
All chemicals and reagents purchased from commercial sources were used as received. Reactions were monitored by thin layer chromatography (TLC) on prepared silica gel plates with UV indicator. Column chromatography was performed with 40 µm average particle diameter silica gel. Yields were calculated based on purified products and not optimized. All final products had a purity of >95% determined by two HPLC methods. Both HPLC methods used Adsorbosil analytical C18 column (3.2 mm×250 mm, 5 µm) with analytes being monitored at 254 nm and a flow rate of 0.5 mL/min. HPLC method A employed a gradient of solvent A [0.1% trifluoroacetic acid (TFA) aqueous solution] and solvent B (acetonitrile) with solvent B being increased from 5% to 80% in 15 min and held for an additional 10 min. HPLC method B employed a gradient of solvent A (0.1% TFA aqueous solution) and solvent B (methanol) with solvent B being increased from 5% to 80% in 10 min and held for an additional 15 min. Melting points were determined on a Büchi melting point apparatus. IR absorptions were recorded on a Nicolet 380 ATR-FTIR instrument and values are reported in cm−1. All 1H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz instrument. Chemical shifts are reported as δ values (ppm) downfield from TMS and all J values are in hertz. The following NMR abbreviations are used: br (broad), s (singlet), d (doublet), dd (doublet of doublets), m (multiplet). Mass spectra were obtained on a Finnigan MAT Navigator mass spectrometer.
5.1.2. General Procedure for the Preparation of NACC and Its Analogs (1–17)
NACC and its seventeen analogs were synthesized through a one-step reaction of an isocyanate with NAC (1.1:1, molar ratio) following a reported procedure with modification (Scheme 1)9. Briefly, an isocyanate (11 mmol) in 10 mL of tetrahydrofuran (THF) was added dropwise through an addition funnel under argon to a solution of NAC (10 mmol) in a saturated NaHCO3 solution (20 mL) at room temperature. After 15 min stirring at room temperature, the mixture was filtered and solid was discarded. THF was removed by a rotary evaporator and the aqueous layer was washed twice with ethyl acetate to remove non-acidic organic by-products. The aqueous solution was then acidified with HCl to pH ~1 over ice followed by extraction with ethyl acetate. The combined ethyl acetate extracts were dried over MgSO4, filtered, concentrated, and purified by flash column chromatography (silica gel, EtOAc/hexane, gradient). The purified product was dissolved in acetonitrile and water (1:1, v/v) and dried by lyophilization. Due to the instabilities of compounds 16 and 17 in ethyl acetate, the reaction solutions of compounds 16 and 17 were lyophilized followed by extraction with methanol. The crude products of 16 and 17 were purified by flash column chromatography (silica gel, methanol/methylene dichloride).
5.1.2.1. N-Acetyl-S-(p-chlorophenylcarbamoyl)cysteine (NACC)
The title compound was obtained from the reaction of NAC and p-chlorophenylisocyanate in 80% yield. The spectral data were identical to that reported earlier.9
5.1.2.2. N-Acetyl-S-(p-fluorophenylcarbamoyl)cysteine (1)
The title compound was obtained from the reaction of NAC with p-fluorophenylisocyanate by following the procedure as described in the method. Yield, 50%; white solid, mp 71–74 °C. 1H NMR (acetone-d6): δ 1.98 (s, 3 H, CH3), 3.29 (dd, J = 7.6, 14.0 Hz, 1 H, CH2), 3.58 (dd, J = 4.8, 14.0 Hz, 1 H, CH2), 4.73 (m, 1 H, NCH), 6.95–7.14 (m, 2 H, phenyl), 7.66–7.45 (m, J = 9.2 Hz, 3H, phenyl, HN), 9.50 (s) 13C NMR (acetone-d6): δ 170.7, 169.8, 164.3, 135.0, 120.7, 115.1, 114.9, 52.3, 30.7, 21.5. MS: 301 (MH+). IR (cm−1): 3277, 1724, 1655, 1618, 1536, 1507.
5.1.2.3. N-Acetyl-S-(naphthalen-1-ylcarbamoyl)cysteine (2)
The title compound was obtained from the reaction of NAC with 1-isocyanatonaphthalene following the procedure as described in the method. Yield, 87%; white solid, mp 128–131 °C. 1H NMR (DMSO-d6): δ 1.87 (s, 3 H, CH3), 3.08 (dd, J = 8.4, 13.6 Hz, 1 H, CH2), 3.42 (dd, J = 4.8, 13.6 Hz, 1 H, CH2), 4.42 (m, 1 H, NCH), 7.60-7.46 (m, 4 H, phenyl), 7.82 (d, J = 8.0 Hz, 1H, phenyl), 8.02-7.92 (m, 2 H, phenyl), 8.29 (d, J = 8.0 Hz, 1H, NH), 10.36 (s, 1 H). 13C NMR (DMSO-d6): δ 170.8, 168.1, 164.8, 132.6, 132.1, 127.3, 126.9, 125.1, 125.0, 124.4, 121.9, 121.7, 116.9, 51.1, 29.7, 21.3. MS: 333 (MH+). IR (cm−1): 3265, 1724, 1650, 1524, 1495.
5.1.2.4. N-Acetyl-S-(o-methylsulfanylphenylcarbamoyl)cysteine (3)
The title compound was obtained from the reaction of NAC with o-methylsulfanylphenylisocyanate. Yield, 69%; white solid, mp 55–60 °C. 1H NMR (acetone-d6): δ 1.96 (s, 3 H, CH3), δ 2.43 (s, 3 H, CH3), 3.33-3.23 (m, 1 H, CH2), 3.59-3.49 (m, 1 H, CH2), 4.71 (m, 1 H, NCH), 7.33-7.15 (m, 2 H, phenyl), 7.55-7.38 (m, 2 H, phenyl), 7.69 (d, J = 6.8 Hz, 1H, NH), 8.75 (s, 1H, NH). 13C NMR (acetone-d6): δ 172.3, 171.2, 167.0, 137.5, 130.6, 128.0, 127.5, 125.9, 125.8, 53.9, 32.4, 23.1, 17.4. MS: 329 (MH+). IR (cm−1): 3291, 1732, 1642, 1499.
5.1.2.5. N-Acetyl-S-(o-methoxyphenylcarbamoyl)cysteine (4)
The title compound was obtained from the reaction of NAC with o-methoxyphenylisocyanate. Yield, 87%; white solid, mp 62–65 °C. 1H NMR (acetone-d6): δ 1.96 (s, 3 H, CH3), 3.25-3.33 (m, 1 H, CH2), 3.60-3.53 (m, 1 H, CH2), δ 3.86 (s, 3 H, OCH3), 4.71 (m, 1 H, NCH), 7.11-6.89 (m, 3 H, phenyl), 7.54 (d, J = 5.2 Hz, 1 H, phenyl), 8.01 (d, J = 8.0 Hz, 1 H, NH), 8.60 (s, 1H, NH). 13C NMR (acetone-d6): δ 170.7, 169.5, 164.3, 148.8, 127.2, 124.2, 120.6, 120.2, 110.5, 55.0, 52.2, 30.8, 21.5. MS: 313 (MH+). IR (cm−1): 3319, 1733, 1652, 1602, 1518.
5.1.2.6. N-Acetyl-S-(o-chloro-p-nitrophenylcarbamoyl)cysteine (5)
The title compound was obtained from the reaction of NAC with o-chloro-p-nitrophenylisocyanate. Yield, 31%; white solid, mp 56–59 °C. 1H NMR (acetone-d6): δ 1.98 (s, 3 H, CH3), 3.33 (dd, J = 7.6, 14.0 Hz, 1 H, CH2), 3.64 (dd, J = 4.8, 14.0 Hz, 1 H, CH2), 4.74 (m, 1 H, NCH), 7.66-7.38 (m, 2 H, phenyl), 8.39-8.15 (m, 3 H, phenyl, 2 NH), 9.33 (s, 1H, OH). 13C NMR (acetone-d6): δ 170.7, 169.5, 166.2, 143.3, 140.4, 124.7, 123.5, 122.9, 122.4, 51.8, 21.2, 21.5. MS: 362 (MH+). IR (cm−1): 3342, 1587, 1537, 1507.
5.1.2.7. N-Acetyl-S-(o-fluorophenylcarbamoyl)cysteine (6)
The title compound was obtained from the reaction of NAC with o-fluorophenylisocyanate. Yield, 61%; white solid, mp 65–70 °C. 1H NMR (acetone-d6): δ 1.97 (s, 3 H, CH3), 3.34-3.23 (m, 1 H, CH2), 3.62-3.51 (m, 1 H, CH2), 4.73 (m, 1 H, NCH), 7.21-7.12 (m, 3 H, phenyl), 7.59-7.46 (m, 1 H, phenyl), 7.98-7.86 (m, 1 H, NH), 9.25 (s, 1 H, NH). 13C NMR (acetone-d6): δ 170.7, 169.6, 165.2, 154.6, 125.9, 125.8, 125.2, 124.1, 115.0, 52.2, 31.8, 21.5. MS: 301 (MH+). IR (cm−1): 3269, 1650, 1614, 1524.
5.1.2.8. N-Acetyl-S-(p-bromophenylcarbamoyl)cysteine (7)
The title compound was obtained from the reaction of NAC with p-bromophenylisocyanate. Yield, 93%; white solid, mp 80–84 °C. 1H NMR (acetone-d6): δ 1.97 (s, 3 H, CH3), 3.33-3.23 (m, 1 H, CH2), 3.62-3.53 (m, 1 H, CH2), 4.73 (m, 1 H, NCH), 7.67-7.39 (m, 5H, phenyl, NH), 9.25 (s, 1 H, NH). 13C NMR (acetone-d6): δ 170.7, 169.7, 164.4, 138.0, 131.5, 120.6, 115.2, 52.2, 30.7, 21.5. MS: 363 (MH+). IR (cm−1): 3281, 1659, 1589, 1524, 1483.
5.1.2.9. N-Acetyl-S-(2,5-dimethoxyphenylcarbamoyl)cysteine (8)
The title compound was obtained from the reaction of NAC with 2,5-dimethoxyphenylisocyanate. Yield, 83%; white solid, mp 144–146 °C. 1H NMR (DMSO-d6): δ 1.86 (s, 3 H, CH3), 3.02 (dd, J = 8.8, 13.8 Hz, 1 H, CH2), 3.37 (dd, J = 4.8, 13.8 Hz, 1 H, CH2), 4.36 (m, 1 H, NCH), 6.68 (dd, J = 3.2, 9.0 Hz, 1 H, phenyl), 6.96 (d, J = 8.8 Hz, 1 H, phenyl), 7.31 (s, 1 H, phenyl), 8.29 (d, J = 8.0 Hz, 1 H, NH), 9.53 (s, 1 H, NH). 13C NMR (DMSO-d6): δ 170.8, 168.2, 163.7, 151.6, 143.5, 126.2, 123.2, 11.2, 108.3, 55.0, 54.2, 51.1, 29.5 21.2. MS: 343 (MH+). IR (cm−1): 3322, 1687, 1605, 1516.
5.1.2.10. N-Acetyl-S-(p-iodophenylcarbamoyl)cysteine (9)
The title compound was obtained from the reaction of NAC with p-iodophenylisocyanate. Yield, 75%; white solid, mp 92–95 °C. 1H NMR (acetone-d6): δ 1.97 (s, 3 H, CH3), 3.29 (dd, J = 7.6, 14.0 Hz, 1 H, CH2), 3.58 (dd, J = 4.4, 14.0 Hz, 1 H, CH2), 4.73 (m, 1 H, NCH), 7.33 (d, J = 8.4 Hz, 2 H, phenyl), 7.55 (d, J = 7.6 Hz, 1 H, NH), 7.66 (d, J = 8.4 Hz, 2H, phenyl), 9.55 (s, 1 H, NH). 13C NMR (acetone-d6): δ 170.7, 169.6, 164.4, 138.6, 137.5, 120.9, 85.8, 52.2, 30.8, 21.5. MS: 409 (MH+). IR (cm−1): 3284, 1659, 1581, 1520.
5.1.2.11. N-Acetyl-S-(o-nitro-p-trifluoromethylphenylcarbamoyl)cysteine (10)
The title compound was obtained from the reaction of NAC with o-nitro-ptrifluoromethylphenylisocyanate. Yield, 52%; light yellow solid, mp 69–72 °C. 1H NMR (DMSO-d6): δ 1.87 (s, 3 H, CH3), 3.11 (dd, J = 8.8, 13.4 Hz, 1 H, CH2), 3.41 (dd, J = 5.2, 13.6 Hz, 1 H, CH2), 4.37 (m, 1 H, NCH), 7.82 (d, J = 8.4 Hz, 1 H, phenyl), 8.09 (dd, J =2.0, 8.8 Hz, 1H, phenyl), 8.34-8.27 (m, 2 H, phenyl, NH), 11.05 (b, 1 H). 13C NMR (DMSO-d6): δ 170.9, 168.2, 165.0, 140.4, 133.0, 129.5, 124.6, 121.6, 120.5, 50.8, 29.8, 21.2, 19.9. MS: 396 (MH+). IR (cm−1): 3309, 1626, 1581, 1512.
5.1.2.12. N-Acetyl-S-(o-methoxybenzylcarbamoyl)cysteine (11)
The title compound was obtained from the reaction of NAC with o-methoxybenzylisocyanate. Yield, 61%; white solid, mp 63–65 °C. 1H NMR (acetone-d6): δ 1.90 (s, 3 H, CH3), 3.23 (dd, J = 8.4, 14.0 Hz, 1 H, CH2), 3.46 (dd, J = 4.4, 14.0 Hz, 1 H, CH2), 3.84 (s, 3 H, OCH3), 4.43(m, 2H, CH2), 4.61 (m, 1 H, NCH), 6.99-6.86 (m, 2 H, phenyl), 7.29-7.21 (m 2H, phenyl), 7.46 (d, J = 7.2 Hz, 1H, NH), 7.67 (s, 1H, NH). 13C NMR (acetone-d6): δ 170.7, 169.6, 165.8, 156.9, 128.2, 126.0, 120.0, 110.0, 54.5, 52.8, 39.7, 30.4, 21.5. MS: 327 (MH+). IR (cm−1): 3311, 1654, 1493.
5.1.2.13. N-Acetyl-S-(o-chlorophenylcarbamoyl)cysteine (12)
The title compound was obtained from the reaction of NAC with o-chlorophenylisocyanate. Yield, 85%; white solid, mp 64–66 °C. 1H NMR (acetone-d6): δ 2.00 (s, 3 H, CH3), 3.30 (dd, J = 7.6, 14.0 Hz, 1 H, CH2), 3.58 (dd, J = 4.4, 14.0 Hz, 1 H, CH2), 4.73 (m, 1 H, NCH), 7.24-7.17 (m, 1 H, phenyl), 7.38-7.31 (m, 1H, phenyl), 7.47 (dd, J = 1.6, 8.0 Hz, 1H, phenyl), 7.56 (d, J = 8.0 Hz, 1H, NH), 7.87 (dd, J = 8.2, 1.6 Hz,1H, NH), 8.95 (s, 1H, COOH). 13C NMR (acetone-d6): δ 170.7, 169.6, 165.5, 134.5, 129.3, 127.3, 126.1, 125.8, 125.1, 52.2, 30.9, 21.5. MS: 317 (MH+). IR (cm−1): 3330, 1646, 1589, 1512.
5.1.2.14. N-Acetyl-S-(p-methoxyphenylcarbamoyl)cysteine (13)
The title compound was obtained from the reaction of NAC with p-methoxyphenylisocyanate. Yield, 85%; white solid, mp 69–72 °C. 1H NMR (acetone-d6): δ 1.93 (s, 3 H, CH3), 3.26 (dd, J = 7.6, 13.7 Hz, 1 H, CH2), 3.54 (dd, J = 4.6, 13.7 Hz, 1 H, CH2), 3.76 (s, 3 H, OCH3), 4.66 (m, 1 H, NCH), 6.88 (d, J = 9.3 Hz, 2 H, phenyl), 7.47 (d, J = 9.3 Hz, 2H, phenyl). 13C NMR (acetone-d6): δ 168.8, 167.9, 167.8, 162.1, 154.1, 129.8, 118.8, 57.4, 50.5, 28.7, 19.5. MS: 313 (MH+). IR (cm−1): 3271 (broad), 1729, 1656, 1509.
5.1.2.15. N-Acetyl-S-(3,5-dichlorophenylcarbamoyl)cysteine (14)
The title compound was obtained from the reaction of NAC with 3,5-dichlorophenylisocyanate. Yield, 85%; white solid, mp 93–95 °C. 1H NMR (acetone-d6): δ 1.93 (s, 3 H, CH3), 3.27 (dd, J = 7.6, 14.0 Hz, 1 H, CH2), 3.59 (dd, J = 4.4, 14.0 Hz, 1 H, CH2), 4.69 (m, 1 H, NCH), 7.16 (t, J = 1.8 Hz, 1 H, phenyl), 7.62 (d, J = 1.8 Hz, 2H, phenyl). 13C NMR (acetone-d6): δ 168.7, 167.5, 163.2, 138.9, 132.6, 120.8, 115.0, 57.4, 49.9, 19.5. MS: 353 (MH+). IR (cm−1): 3257 (broad), 1728, 1695, 1658, 1536.
5.1.2.16. N-Acetyl-S-(3-chlorophenylcarbamoyl)cysteine (15)
The title compound was obtained from the reaction of NAC with 3-chlorophenylisocyanate. Yield, 85%; white solid, mp 96–100 °C. 1H NMR (acetone-d6): δ 1.93 (s, 3 H, CH3), 3.28 (dd, J = 7.8, 14.0 Hz, 1 H, CH2), 3.57 (dd, J = 4.6, 14.0 Hz, 1 H, CH2), 4.71 (m, 1 H, NCH), 7.10 (m, 1 H, phenyl), 7.40 (m, 3H, phenyl), 7.75 (m, 1 H, phenyl). 13C NMR (acetone-d6): δ 168.8, 168.5, 162.8, 138.0, 131.8, 128.1, 121.2, 116.7, 115.1, 50.2, 28.8, 19.5. MS: 317 (MH+). IR (cm−1): 3271 (broad), 1729, 1660, 1534.
5.1.2.17. N-Acetyl-S-(4-chlorobenzylcarbamoyl)cysteine (16)
The title compound was obtained from the reaction of NAC with 4-chlorobenzylisocyanate. Yield, 44%; white solid, mp 115–119 °C. 1H NMR (methyl alcohol-d4): δ 1.90 (s, 3 H, CH3), 3.22 (dd, J = 8.0, 13.6 Hz, 1 H, CH2), 3.50 (dd, J = 4.0, 14.0 Hz, 1 H, CH2), 4.35 (s, 2 H, CH2), 4.42 (m, 1 H, NCH), 7.33-7.23 (m, 4 H, phenyl). 13C NMR (methyl alcohol-d4): δ 176.8, 172.8, 169.8, 132.6, 139.0, 134.0, 130.2, 129.6, 56.6, 45.0, 33.3, 22.9. MS: 331 (MH+). IR (cm−1): 2873, 1650, 1602.
5.1.2.18. N-Acetyl-S-(phenylcarbamoyl)cysteine (17)
The title compound was obtained from the reaction of NAC with phenylisocyanate. Yield, 10%; light yellow solid, mp 95–98 °C. 1H NMR (methyl alcohol-d4): δ 1.97 (s, 3 H, CH3), 3.23 (dd, J = 8.0, 14.0 Hz, 1 H, CH2), 3.57 (dd, J = 4.4, 14.0 Hz, 1 H, CH2), 4.61 (m, 1 H, NCH), 7.10-7.02 (m, 1 H, phenyl), 7.34-7.24 (m, 2H, phenyl), 7.53-7.44 (m, 2H, phenyl). 13C NMR (methyl alcohol-d4): δ 174.4, 173.3, 166.9, 162.5, 140.1, 129.9, 125.0, 120.6, 54.8, 32.3, 22.6. MS: 283 (MH+). IR (cm−1): 2359, 1622.
5.2. Biology
5.2.1 Cell Lines and Culture Conditions
UACC-62, OVCAR-3 and SK-MEL-2 cells were obtained from the National Cancer Institute (NCI). CV-1 cells were obtained from American Type Culture Collection (ATCC). GC3/c1 cells were obtained as a kind gift from Dr. Peter J. Houghton of St. Jude Children’s Research Hospital. Cells were grown in RPMI 1640 growth medium supplemented with 10% FBS, 100 units/mL of penicillin and 100 µg/mL of streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C.
5.2.2. In Vitro Cytotoxicity Evaluation
NACC and its analogs were tested in vitro for cytotoxicity against UACC-62, GC3/c1, OVCAR-3, SK-MEL-2 and CV-1 cells in a 96-well plate using the MTT assay to determine cell survival rates. Dacarbazine was chosen as a positive control. In brief, cells (1500, 5000, 2000, 4000, 500 cells for UACC-62, GC3/c1, OVCAR-3, SK-MEL-2 and CV-1 cells respectively) were plated into a 96-well plate and allowed to attach for 24 hours. After attachment, the medium was replaced with the medium containing variable concentrations of NACC or its analogs. Cells were exposed to the drug for 6 days followed by the MTT assay to determine cell survival rates.
5.2.3. Determination of Protein Binding by Equilibrium Dialysis
The protein binding of analog 1 and analog 7 was determined based on a procedure reported earlier for the protein binding determination of NACC.9
5.2.4. Analysis of Cellular DNA Content by Flow Cytometry
GC3/c1 (0.3 million) or UACC-62 (0.14 million) cells were plated in 75 cm2 flasks and were directly treated with 30 mL of medium containing various concentrations of NACC for 6 days. At the end of treatment, adherent cells were harvested by trypsinization and washed twice with ice-cold PBS. One million cells of each sample were fixed with 70% ethanol in DPBS at 4 °C. Fixed cells were then centrifuged (2500 rpm × 5 min) and washed with staining buffer. After the wash, the samples were centrifuged (2500 rpm × 5 min) and the pellets were treated with 100 µL of RNase A (1 mg/mL) for 30 min at 37 °C. After incubation, 900 µL of staining buffer was added to the samples to make up the volume to 1 mL followed by addition of 20 µL of PI (1 mg/mL). The sample was incubated in the dark at room temperature for 30 min and analyzed with BD FACScan™ flow cytometer using CellQuest Software.
5.2.5. Apoptosis Assay by Flow Cytometry
Vybrant Apoptosis Kit 2 (Molecular Probes, Carlsbad, CA) was used to quantitate apoptosis in UACC-62 cells treated with NACC. The kit contains annexin V labeled with green-fluorescent Alexa Fluor 488 and PI. In brief, UACC-62 cells were plated and treated with NACC in a 96-well plate as described above for cytotoxicity activity evaluation. At the end of the treatment, adherent and suspended cells were harvested through trypsinization from the plate and washed twice with ice-cold PBS and then cell pellets were resuspended with annexin-binding buffer to give a final concentration of 1×106 cells/mL. Approximately 1×105 cells in 100 µL buffer were incubated in the dark with 5 µL of annexin V and 1 µL of the 100 µg/mL PI solutions for 15 minutes at room temperature. After incubation, 200 µL of annexin-binding buffer was added to each sample, and the samples were analyzed with BD FACScan flow cytometer.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA098810-01, CA120062-01) and 2005 Governor Rounds’ Individual Research Seed Grant Awards. The authors would like to thank Dr. Peter J. Houghton of St. Jude Children’s Research Hospital, Memphis, Tennessee for kindly supplying human colon adenocarcinoma GC3/c1 cells.
Abbreviations
- TFA
trifluoroacetic acid
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- DPBS
Dulbecco’s phosphate buffered saline
- EWG
electron withdrawing group
- EDG
electron donating group
- HPLC
high performance liquid chromatography
- IC50
concentration that produces 50% cell growth inhibition
- NAC
N-acetyl-L-cysteine
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- PBS
phosphate buffered saline
- PI
propidium iodide
- PS
phosphatidylserine
- THF
tetrahydrofuran
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang JY. Nat Rev Drug Discov. 2002;1:101. doi: 10.1038/nrd742. [DOI] [PubMed] [Google Scholar]
- 2.Buolamwini JK. Curr Opin Chem Biol. 1999;3:500. doi: 10.1016/S1367-5931(99)80073-8. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald V. Can Vet J. 2009;50:665. [PMC free article] [PubMed] [Google Scholar]
- 4.Borowski E, Bontemps-Gracz MM, Piwkowska A. Acta Biochim Pol. 2005;52:609. [PubMed] [Google Scholar]
- 5.Mohamadi F, Spees MM, Grindey GB. J Med Chem. 1992;35:3012. doi: 10.1021/jm00094a013. [DOI] [PubMed] [Google Scholar]
- 6.Phelps PC, Jain PT, Berezesky IK, Boder GB, Trump BF. Cancer Lett. 1995;88:27. doi: 10.1016/0304-3835(94)03607-k. [DOI] [PubMed] [Google Scholar]
- 7.Ehlhardt WJ. Drug Metab Dispos. 1991;19:370. [PubMed] [Google Scholar]
- 8.Molthrop DC, Jr, Wheeler RH, Hall KM, Prchal J. Invest New Drugs. 1994;12:99. doi: 10.1007/BF00874438. [DOI] [PubMed] [Google Scholar]
- 9.Guan X, Hoffman BN, McFarland DC, Gilkerson KK, Dwivedi C, Erickson AK, Bebensee S, Pellegrini J. Drug Metab Dispos. 2002;30:331. doi: 10.1124/dmd.30.3.331. [DOI] [PubMed] [Google Scholar]
- 10.Metelmann HR, Von Hoff DD. Int J Cell Cloning. 1983;1:24. doi: 10.1002/stem.5530010105. [DOI] [PubMed] [Google Scholar]
- 11.Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand JL, Laman SD, Kottschade LA, Maples WJ, Pittelkow MR, Pulido JS, Cameron JD, Creagan ET. Mayo Clin Proc. 2007;82:364. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- 12.Cornish D, Holterhues C, van de Poll-Franse LV, Coebergh JW, Nijsten T. Ann Oncol. 2009;20 Suppl 6:vi51. doi: 10.1093/annonc/mdp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Day SJ, Kim CJ, Reintgen DS. Cancer Control. 2002;9:31. doi: 10.1177/107327480200900105. [DOI] [PubMed] [Google Scholar]
- 14.Hocker TL, Singh MK, Tsao H. J Invest Dermatol. 2008;128:2575. doi: 10.1038/jid.2008.226. [DOI] [PubMed] [Google Scholar]
- 15.Gogas HJ, Kirkwood JM, Sondak VK. Cancer. 2007;109:455. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 16.Testori A, Rutkowski P, Marsden J, Bastholt L, Chiarion-Sileni V, Hauschild A, Eggermont AM. Ann Oncol. 2009;20 Suppl 6:vi22. doi: 10.1093/annonc/mdp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand JL, Laman SD, Kottschade LA, Maples WJ, Pittelkow MR, Pulido JS, Cameron JD, Creagan ET. Mayo Clin Proc. 2007;82:490. doi: 10.4065/82.4.490. [DOI] [PubMed] [Google Scholar]
- 18.Flaherty KT. Clin Cancer Res. 2006;12:2366s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro GI, Harper JW. J Clin Invest. 1999;104:1645. doi: 10.1172/JCI9054. [DOI] [PMC free article] [PubMed] [Google Scholar]