Abstract
The presence of day-night variations in cardiovascular and metabolic functioning is well known. However, only recently it has been shown that cardiovascular and metabolic processes are not only affected by the behavioral sleep/wake cycle but are partly under direct control of the master circadian pacemaker located in the suprachiasmatic nucleus (SCN). Heart rate, cardiac autonomic activity, glucose metabolism and leptin —involved in appetite control—all show circadian variation (i.e., under constant behavioral and environmental conditions). This knowledge of behavioral vs. circadian modulation of cardiometabolic function is of clinical relevance given the morning peak in adverse cardiovascular incidents observed in epidemiological studies and given the increased risk for the development of diabetes, obesity, and cardiovascular disease in shift workers. We will review the evidence for circadian control of cardiometabolic functioning, as well its sensitivity to light and melatonin, and discuss potential implication for therapy.
Keywords: cardiovascular system, circadian misalignment, light, metabolic function, shift work
1. Introduction
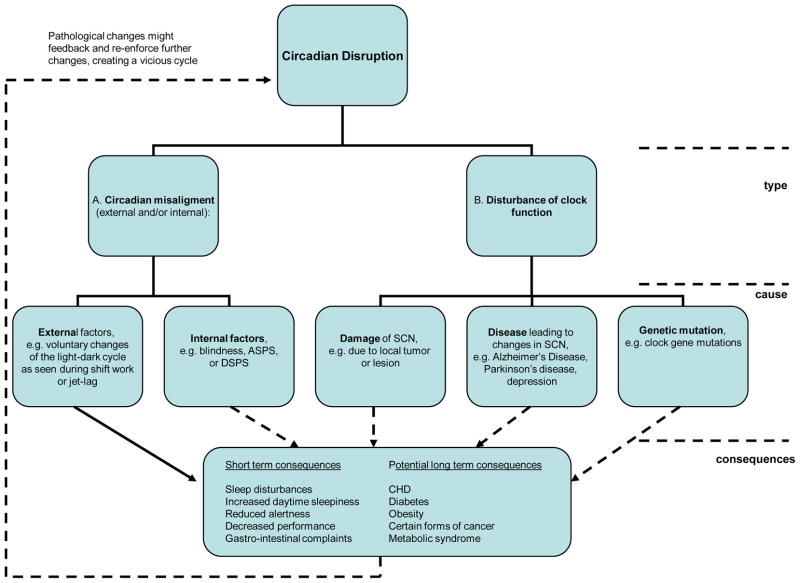
In this review, we will examine the effects of the circadian system and circadian disruption on the cardiometabolic system and their potential health implications. The term circadian rhythm was coined by Hallberg and is defined as a rhythm that a) displays a length of approximately 24-hrs and—most importantly—persists in the absence of external stimuli and b) can be entrained to external stimuli such as light, food or activity [1]. Furthermore we use the term biological night to refer to the habitual dark episode, i.e., the time normally characterized by behavioral inactivity in diurnal species and behavioral activity in nocturnal species. We will begin with a brief introduction of the circadian system, followed by a review of the literature on circadian regulation of the cardiometabolic system, the effects of light (the major Zeitgeber of the circadian system) on cardiometabolic function, the effects of melatonin (as nighttime signal of the circadian system) on cardiometabolic function, and finally, the adverse health consequences of circadian disruption (Figure 1), including its relevance to shift work, jet lag and potential involvement in the increased risk for the development of hypertension, obesity and diabetes.
Fig. 1.
Schematic overview of the different types of circadian disruption, their causes and potential short-term and long-term health consequences.
2. The circadian system
Many aspects of human physiology, metabolism and behavior vary over the 24-hour day and can have a major impact on our health and well-being. For example, the risk for adverse cardiovascular incidents shows a peak in the morning, temporal lobe epileptic seizures are more frequent in the late afternoon and asthma is generally worse at night [2–4]. This variation over 24-hrs can either be caused by changes in behavioral activity, posture, the sleep/wake cycle, the fast/feeding cycle, the light-dark cycle, or be centrally orchestrated by an endogenous circadian (near 24-hour) oscillator [5–11]. In mammals, the master circadian oscillator is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus [12,13]. The cells in the SCN orchestrate rhythms in endocrine, physiological, and behavioral parameters with a period close to, but not exactly 24 hours [14]. In addition, more recent work shows that the heart and many other peripheral tissues including the lungs and kidneys can act as semi-independent circadian oscillators, termed peripheral oscillators. The cells in these organs, including the heart, contain the same core circadian clock genes that are required to generate spontaneous circadian rhythms in the SCN. These peripheral organs continue to generate circadian rhythms for several cycles in vitro, demonstrating their potential for controlling circadian rhythms to some degree independently of the SCN (for review [14]). The SCN optimally regulates the internal alignment of circadian rhythms in different peripheral tissues.
Furthermore, in order for the circadian pacemaker to ensure that physiology and behavior are appropriately timed to anticipate events in the outside world, environmental time cues are required to reset this internal clock. The primary environmental circadian time cue in mammals is the 24-hour light-dark cycle generated by the earth‘s axial rotation. Light information to the circadian system is captured exclusively by the eyes using specialized intrinsic photosensitive retinal ganglion cells (ipRGCs), and transduced directly to the SCN via a dedicated neural pathway, the retinohypothalamic tract (RHT). Each day the light-dark cycle resets the internal clock which in turn synchronizes the physiology and behavior controlled by the clock. Circadian misalignment occurs when the internal timing system runs out of sync with the behavioral cycle (including e.g., the sleep/wake and fasting/feeding cycle), occurring for example during transmeridian travel or night shift work. In the case of shift workers optimal physiology and metabolism are compromised by them staying awake and eating during a circadian phase that is suited for sleeping and fasting, whereas they try to sleep at a circadian phase suited for activity and food intake. In addition, due to the changes in the behavioral cycle shift workers or transmeridian travelers are exposed to abnormal light-dark cycles, which can result in suppression of melatonin and effects on other physiological variables such as heart rate, cortisol and temperature during the biological night which may have adverse downstream effects on cardiometabolic regulation. But, light being the major Zeitgeber of the circadian system, can also facilitate re-entrainment between the circadian system and the environmental light-dark cycle when appropriately timed. Jet lag and experimental short-term circadian misalignment in the laboratory, results in sleep problems, fatigue, poor cognitive performance and memory, and gastrointestinal problems. Based on epidemiologic data in shift workers and recent laboratory studies isolating circadian misalignment from socioeconomic and lifestyle factors, circadian misalignment may increase the risk of developing cardiovascular disease, obesity,diabetes and some types of cancer.
3. Circadian control of the cardiometabolic system
Proper cardiovascular function ensures that oxygen and nutrients are provided to all organs of the body based on their needs. Over a period of 24-hours, the demands on the heart and the local blood flow change dramatically based on our activities, with concomitant cycles in rest/activity, fasting/feeding, postural changes, and thermoregulatory demands. A good example for such a transition period is the end of the nightly sleep episode in humans: from lying down and resting, we get up and become physically active. This requires the heart to change from a state of relative inactivity: low heart rate and sympathetic tone and high vagal tone, to a state of high activity: increased heart rate and sympathetic tone and decreased vagal tone. In order to prepare for the demands of such transitions between behavioral states, it would be beneficial for the cardiovascular system to be able to anticipate those transitions. One way of —anticipating those changes in demands on the cardiovascular system would be through modulation by the circadian timing system.
3.1. Day-night changes of the cardiometabolic system and its involvement in health and disease
The presence of a daily rhythm in heart rate, blood pressure, platelet function and endothelial function has been known for some time and there are many epidemiological reports, noting a peak in the occurrence of cardiovascular incidents, including ischemic strokes, myocardial infarction (MI), sudden cardiac death, and ventricular arrhythmias in the morning hours [2,15,16]. However, initially it was unclear if the reported morning peak in adverse cardiovascular events was due to a reporting bias of family and physicians or represented a real phenomenon. Subsequent analysis, using creatine kinase MB (CK-MB), a marker of myocardial damage that is used to confirm the timing of the MI, confirmed that there was indeed a morning peak in MIs. The peak based on CK-MB occurred between early morning to noon, coincident with the peak in reported chest pain [17]. Using data from defibrillators and pacemakers, two other studies confirmed a morning peak in the occurrence of ischemic strokes [18] and cardiac arrhythmias [19]. Besides the morning peak in CK-MB and reported pain, Muller et al also observed a secondary peak in CK-MB in the evening, that was unmatched by a peak in reported pain. In a recent review on ischemic and hemorrhagic stroke, Manfredini [20] also reports a double peak pattern with a secondary peak in the evening that interestingly is also seen in the occurrence of MIs in patients with sleep apnea [21]. One trigger for ischemic strokes and MI is blood clots caused by increased platelet aggregability. Two studies showed that platelet aggregability is higher in the morning hours and sensitive to postural changes, i.e. transitioning from a supine to an upright position [22,23]. This indicates that change of posture/behavior might play a role in the morning peak in adverse cardiovascular incidents. However, whether there is a circadian rhythm in platelet function, thus independent of behavioral changes, is unknown.
Despite the epidemiological on a day-night rhythm in adverse cardiac events, one can not distinguish whether this morning peak in cardiovascular incidents is solely due to behavioral and environmental factors versus an endogenous circadian rhythm. Furthermore, these epidemiological studies can not easily address the underlying neuroendocrine mechanisms that lead to the adverse cardiovascular outcomes.
3.2. Circadian regulation of the cardiac system
To determine the potential role of the circadian system on physiology, it is necessary to distinguish the influence of the circadian timing system from the influence of the daily rhythm in behavioral activity, including sleep/wake, fast/feeding, posture and physical activity cycles, and environmental conditions, including light and temperature, which greatly influences physiology. One way to do accomplish this is to measure physiology under constant routine‘ (CR) conditions, i.e. during constant wakefulness, (low) activity, posture, temperature, dim light, and with evenly spread isocaloric meals. The CR protocol hereby permits the study of the underlying circadian variation in a given parameter [24–26]. The second method to study underlying circadian rhythms is the —forced desynchrony (FD) protocol. During a FD protocol, the sleep-wake cycle is scheduled to a cycle length under dim light conditions to which the circadian system can not entrain (i.e. a 28-h day‘), thereby uncoupling the sleep-wake cycle and the circadian pacemaker.
One of the first studies to exploit the CR protocol to investigate the presence of circadian variation in cardiovascular variables was Kräuchi et al. (17), finding a circadian rhythm in heart rate with a peak-to-trough difference of ~7 bpm, a peak between 11:00 to 12:00, and a trough during the biological night, i.e. the habitual resting or sleeping period. These findings were confirmed by Kerkhof et al. [25] and Van Dongen et al.[27]. These findings of a circadian rhythm in heart rate obtained using CR protocols were further confirmed by data from FD protocols. Hu and colleagues, using an 11-day, 28-h cycle, FD protocol found the longest R-R interval, i.e. lowest HR, during the biological night [28]. A similar timing and amplitude in heart rate of ~7 bpm was also observed using repeated resting conditions across the day and night, taking into account dim light, supine posture, inactivity and prior fasting state [29].
CR protocols also revealed circadian rhythms in non-invasive markers for cardiac autonomic regulation. In an elegant study, Burgess et al. investigated the relative influence of the circadian cycle and the sleep/wake cycle by scheduling two CR protocols, where one was modified to permit for sleep at the time of each individual‘s habitual sleep episode [30]. This study showed that a measure for parasympathetic modulation of heart rate (respiratory sinus arrhythmia) was mostly driven by the circadian system, while a marker for sympathetic cardiac modulation (pre-ejection period) was mostly driven by the sleep/wake cycle. A significant circadian rhythm in the same parasympathetic marker and the absence of that of the sympathetic markers was confirmed in a separate CR protocol [31]. More recently, Ivanov et al. confirmed a circadian variation in average heart rate (R-R interval) and average standard deviation of inter-beat interval (STDRR) using a 38-hour CR protocol, with the longest R-R intervals (thus slowest HR) and largest STDRR (measure of parasympathetic cardiac modulation) occurring during the biological night [32]. Similar findings with regards to the presence of a clear circadian rhythm in autonomic cardiac modulation have been further supported by FD studies. Hilton et al., using a 27-day FD protocol, showed a circadian rhythm in pNN50 (a parasympathetic cardiac marker; the percentage of consecutive inter-beat intervals differing by more than 50 msec; for technical details see [33]), with a peak during the biological night (equivalent to ~5 AM) and the trough during the middle of the biological day (~1 PM to 5 PM) [34]. The authors further showed a marked drop in R-R (i.e., increase in HR) from ~5 AM to 9 AM. The authors propose that the increase in HR preceding the drop in parasympathetic cardiac modulation may suggest an increase in cardiac sympathetic activity at that time. These observations were also consistent with a stronger daily rhythm in parasympathetic (root mean square of successive differences of the inter-beat interval) as compared to sympathetic (pre-ejection period) cardiac modulation, assessed using a simple and practical protocol designed to minimize masking effects of behavior and environment by measuring cardiac autonomic modulation repeatedly across day and night while minimizing influences of behavioral activity, posture, environmental light, and meal intake [29].
Only few studies investigated the presence of a circadian rhythm in blood pressure. Kerkhof et al. [25] and Van Dongen et al. [27] were not able to show a circadian rhythm in blood pressure under CR conditions. However, using three different circadian protocols: a 40-h CR protocol, a 7-cycle 28-h FD protocol and a 12-cycle, 20-h FD protocol, one of us with colleagues was able to consistently demonstrate a significant circadian rhythm in systolic blood pressure with a peak-to-trough difference of ~3–6 mmHg with a peak at a circadian phase equivalent to ~9 PM and a trough equivalent to ~9 AM (Scheer FA et al., unpublished data). Importantly, the timing of the peak suggests that the timing of the circadian rhythm in blood pressure does not contribute to the morning peak in adverse cardiovascular incidents.
In addition to the classic cardiac risk markers of heart rate, cardiac autonomic modulation and blood pressure, recent studies have investigated the presence of a circadian rhythm in scale-invariant properties of inter-beat intervals. Specifically, Hu and colleagues studied the scaling exponent α of heartbeat fluctuations under FD conditions (11-day, 28-h cycle) [28]. This scaling exponent α describes the temporal correlation between heartbeat fluctuations over different time scales, displaying a rather stable pattern that only seems to be compromised under pathological conditions, making it a sensitive marker for cardiac vulnerability and mortality [35]. The scaling exponent displays a circadian pattern, with a peak, i.e. a value closer to a random occurrence of behavior, during the biological morning (equivalent to ~10 AM), the time window during which epidemiological studies report the peak in adverse cardiac events and strokes [28,32].
Taken together, the results from the CR and FD protocols strongly imply that cardiovascular function is at least partly under the control of the circadian system and that changes in behavior and/or sleep wake cycle might be able to worsen pre-existing conditions if they happen at a circadian phase when the cardiovascular system is the most susceptible to behavioral impact, e.g. during the transitions period from rest to waking in the morning when heart rate increases to sustain the bigger demand of blood flow needed to change from a supine to an upright position.
3..3. Circadian regulation of the metabolic system
Recent work showed that metabolic processes and regulatory hormones, e.g., leptin and glucose, also exhibit circadian rhythms, as demonstrated under controlled conditions, i.e. during CR [7] and FD protocols, which might be effected by circadian misalignment and lead to adverse short-and long term consequences [36]. In humans, Van Cauter and colleagues showed circadian rhythmicity in glucose and insulin levels by using a protocol with extended wakefulness for 28-h, with glucose and insulin levels increasing during the biological night (habitual sleep episode) [37]. Simon and colleagues measured leptin levels in 7 subjects under constant enteral nutrition conditions and bed rest either during a nocturnal sleep period or after delaying the sleep period by 8 hrs. The leptin rhythms rose to a maximum during the nocturnal sleep episode. During daytime sleep protocol, leptin levels rose slightly during nocturnal sleep deprivation but rose to a maximum during daytime sleep, suggesting both the sleep/wake cycle and the circadian system affect leptin [38]. Shea and colleagues were able to show circadian variation in leptin and glucose in humans [7] by using constant routine conditions, with a circadian peak in leptin around the end of the habitual sleep episode. During normal entrained conditions, the influence of the circadian system may not be obvious to us in every day life. The potential clinical relevance of these data becomes apparent when looking at the increased risk of diabetes, obesity, and cardiovascular disease amongst shift workers, who eat and fast at abnormal circadian phases. However, the question remains whether these adverse health consequences are due to circadian misalignment or due to differences in lifestyle and socioeconomic status.
In order to reveal the neuroanatomical substrate and pathways responsible for circadian rhythms in cardiovascular and metabolic parameters, animal experiments are required. Animal lesion and tracing studies offer the tool to demonstrate this. Scheer and colleagues [39] showed a clear and robust circadian rhythm in resting heart rate in SCN-intact rats under constant dim light conditions with a higher heart rate during the biological night (their habitual active period) and a lower resting heart rate during the biological day. These data showed that the circadian rhythm in heart rate was independent of the circadian rhythm in locomotor activity, a dominant masking factor for heart rate. Furthermore, lesioning the SCN abolished the circadian rhythmicity in heart rate. The level of resting heart rate in SCNx animals was found to be in-between the biological day and biological night level recorded in intact animals, suggesting that the SCN has an inhibitory as well as an excitatory influence on the cardiovascular system in rats. These findings are similar to the demonstration that SCN lesioning leads to intermediate melatonin levels, which has subsequently been shown to be under both inhibitory (i.e., GABA) and stimulatory (i.e., glutamate) control by the SCN (see below). Future studies are required to identify the SCN neurotransmitters and neural pathways by which this cardiac influence is mediated. Regarding metabolism, circadian rhythms—even independent of locomotor activity—have been demonstrated in body temperature (a balance of heat production and dissipation). Lesioning of the SCN has been demonstrated to abolish this rhythm [40]. Furthermore, circadian rhythms—even independent of meal timing—have been demonstrated in glucose, insulin and leptin [41,42].La Fleur and colleagues subjected SCN intact and SCN-lesioned (SCNx) animals to either a fasting regimen or a 6-meals per day feeding regimen (to uniformly distribute meal intake across the circadian cycle). SCN intact animals subjected to fasting or 6-meal per day feeding showed a 24-h variation of glucose, whereas in SCNx animals, the glucose rhythm was abolished[41]. The same authors were able to show a circadian variation in glucose tolerance, which was also abolished by lesioning the SCN [43]. Ruiter and co-workers showed that the SCN and feeding behavior orchestrate the circadian variation in glucagon, and that this rhythm is absent in SCNx animals [44]. Another crucial player in the regulation of metabolism and food intake is leptin, which is under circadian control as well, as SCN lesions abolish rhythmicity in rats [42]. The pathway by which the SCN regulates these rhythms involves direct projections to the liver, pancreas and white adipose tissues [45–47].
3.4.. Neural pathways underlying SCN control on the cardiometabolic system
In order to test the presence of neural pathways involved in the circadian control of the cardiovascular system in the rat, Scheer et al. [11,39] used pseudorabis virus injections into the heart muscle of rats. They found a multisynaptic pathway from the SCN to the heart that involved the autonomic nervous system and most likely includes direct projections from the SCN to the paraventricular nucleus of the hypothalamus (PVN), and subsequently to dorsal motor nucleus of the vagus, regulating parasympathetic tone to the heart and to the intermediolateral column of the spinal cord (IML), regulating sympathetic tone to the heart [11]. Of the several hypothalamic nuclei receiving input both from the SCN and projecting to the cardiovascular system, the PVN showed the most PRV labeling [39]. By demonstrating neural projections between the SCN and the heart muscle, these studies provide evidence for the potential neuroanatomic substrate for the physiological effects of the SCN on heart rate. Using the same technique, multi-synaptic projections have also been shown from the SCN to the vasculature, kidney and adrenal gland, together with the heart, important in the regulation of hemodynamics and cardiovascular function. In the control of metabolism, similar projections have been shown from the SCN to the liver, pancreas, white adipose tissue, and brown adipose tissue [48–50].
Summary of circadian control of cardiometabolic system
In summary, numerous markers of cardiac function and metabolism exhibit an endogenous circadian rhythm in humans, independent of changes in behavior and environment, with lowest HR during the middle of the biological night (~4–5 AM), a strong circadian rhythm in cardiac vagal markers peaking during the middle of the biological night, a less strong circadian rhythm in sympathetic markers showing higher levels during the biological day, and scaling exponent α—considered predictive of cardiac risk—peaking during the biological morning (~10 AM). Animal experimental work has shown the requirement of the presence of a functional SCN for the circadian rhythms in cardiometabolic function and the possible neural substrate of these influences. Although the cardiovascular system is partly under the control of the circadian system, the question remains if rhythms in cardiovascular parameters, such as BP or HR, can be either be shifted or acutely influenced by light, the signal of the day and major Zeitgeber, or exogenous melatonin, the signal of the night.
4. Effects of light on cardiometabolic functioning
4.1. Circadian, non-image forming, light perception
As mentioned in the Introduction, light is the major Zeitgeber to entrain the circadian system, i.e. to keep it synchronized to the environmental light-dark cycle [51–54]. Light information is perceived via the retina and then transmitted to the SCN directly via the retinohypothalamic tract (RHT) [55,56].
Until recently, it was believed that the mammalian retina consists only of two classes of photoreceptors: rods and cones, which are responsible for the image-forming functions of the retina. Recent findings from animal studies have proven that the retina hosts a third set of photoreceptors located in intrinsically-photosensitive retinal ganglion cells [57–60], that project directly to the SCN. These receptors do not primarily transmit visual information, but serve as relatively insensitive irradiance detectors. In humans, a post-mortem approach was used to establish these direct projections from the retina to the SCN [61–63]. Animal studies have shown that these intrinsically photosensitive retinal ganglion cells contain the vitamin A1-based novel photopigment melanopsin, the photopigment primarily mediating the non-visual‘ effects of light such as circadian phase resetting, melatonin suppression, and pupillary light reflex [60,64–69]. Support for melanopsin functioning as the primary circadian photopigment came from further animal studies showing that the spectral sensitivity of circadian phase resetting by light (λmax ~480 nm) closely matches the action spectrum for light-induced electrical responses of the melanopsin cells themselves (λmax ~484 nm)[70,71]. In melanopsin-deficient mice, circadian responses to light are attenuated but not absent, indicating that rods and cones play an additional role in circadian photoreception. Indeed, light resetting of circadian rhythms and light-induced suppression of melatonin are abolished only after loss of melanopsin and visual photoreceptor function. Hence melanopsin, rods, and cones function as circadian photoreceptors in lower mammals [64,72]. In humans, the spectral sensitivity of light-induced melatonin suppression (λmax = 460 nm) is also blue-shifted relative to the three-cone photopic visual system (λmax = 555 nm) suggesting that a novel photoreceptor (i.e., a non-rod, non-cone) system mediates circadian responses to light [54,73–75].
Light can elicit different types of effects: longer-lasting, i.e. phase shifting, versus acute effects of light. The effect magnitude of both, phase-shifts and acute effects of light depends on the following factors: Timing, wavelength, intensity, and duration of the light pulse. For example light pulses given in the evening, i.e. before the minimum of the core body temperature will create delays in physiological and behavioral rhythms whereas light given in the morning, i.e. after the minimum of core body temperature, will create phase advances in a multitude of rhythms [76–78] [79]
4.2 Effects of light on the cardiometabolic system
Light can affect cardiac function in two ways. First, given that cardiac circadian rhythms are controlled primarily by the central circadian pacemaker in the SCN, light-induced phase shifts of the circadian system, would be expected to induce phase shifts in the cardiac system. Campos et al. [80]showed that inverting the light dark cycles of transgenic mice from LD to DL caused the acrophase of BP, HR, and locomotor activity to shift to the new dark period. To date there are no direct studies examining light-induced circadian resetting of cardiac rhythms in humans, however, phase-shifts in heart rate variability have been observed following treatment with exogenous melatonin, which mirrored those of temperature and sleep EEG [81,82].
Light is also able to acutely elevate heart rate in humans. Most studies report acute effects of light using bright white light exposures of different intensities and durations during the nighttime in an extended wakefulness paradigm. Despite the different intensities (100–10,000 lux) and different durations (10–240 mins) these studies showed uniformly that bright light increased HR, HRV, and low/high frequency ratio of HRV[11,29,83,83–86] [87]. Scheer et al. found a time-of-day dependent light-induced elevation of HR, with an increase at night and in the morning and no effect during the daytime, in a within-subject design [29]. This study furthermore showed a dose-dependent stimulatory effect of white light, with 800 lux causing a larger increase in HR than 100 lux. This finding was consistent with a subsequent study by Rueger et al [83], who showed that a 4-hrs exposure to 5000 lux of full spectrum white light during the nighttime (midnight to 4 am) acutely elevates HR, increase core body temperature, suppresses melatonin, and increases alertness. Using the same experimental set-up, the authors found in an another group of subjects that the same light exposure given during the daytime did not elevate heart rate, suppresses melatonin, increase core body temperature, but yet still increased alertness. The light-induced increase in HR may be dependent primarily to a light-induced increase in sympathetic cardiac activity [86]. In addition, it has been shown that these effects depend on the wavelength of light [88] as do other measures of arousal ([88,89], with short-wavelength blue light being most effective. Cajochen and colleagues [88] showed an increase in heart rate by a 2-hour exposure to bright monochromatic blue, but not green light, in the late evening (21:00–23:00 h). This short-wavelength sensitivity supports the notion that light-induced elevation of heart-rate, and other non-visual‘ effects of light such melatonin suppression, circadian phase shifting and improvements in alertness and performance [54,71,73,74,88–90] are mediated by ipRGCs with an important role for melanopsin as a photoreceptor.
Likewise, cortisol shows a clear circadian rhythm with a peak around awakening [91] and therefore could be susceptible to the phase shifting and acute effects of light. Scheer and Buijs [92] showed that the cortisol peak after awakening is present in total darkness and can be further enhanced by 1 hour of 800 lux applied at habitual time of waking. They furthermore showed that the same exposure in the hour prior to the habitual bedtime had no effect at all on cortisol levels. Similarly, Leproult et al. [93] showed that in sleep deprived subjects 3 hours of bright light exposure (4500 lux) in the early morning (0500–0800) induced an increase in cortisol levels whereas afternoon (1300–1600) bright light exposure had no effect on cortisol. This was also consistent with the findings by Rueger et al. [83] who also found no effect of afternoon light exposure (12:00–4:00 PM) on cortisol levels. Thorn et al. (2004) showed that gradually increasing luminance levels (250 lux over 30 minutes) during awakening (dawn simulation) increased cortisol levels as compared to the control conditions,. This increase in cortisol was accompanied by a higher level of reported arousal but not of reported stress. In addition to heart rate and cortisol, light is able to acutely elevate and/or phase shift core body temperature [94–96,96] [77,97], as well as suppress and/or phase shift the melatonin rhythm [77,98,99].
5. The cardiovascular system and melatonin
Mounting evidence for the involvement of the circadian pacemaker in the regulation of the cardiovascular system opened the discussion for the use of exogenous melatonin as a chronotherapeutic. However, the existing literature on the effects and use of exogenous melatonin as a potential chronotherapeutic for the adjunct treatment of cardiovascular disorders is complex, because of differences in effects dependent on timing of administration (day vs. night), acute vs. chronic effect, dose (physiological vs. pharmacological), species (e.g., diurnal humans vs. nocturnal rodents). We therefore will rather focus on general concepts and selected studies to highlight them (for detailed review see [100]or [101]).
5.1. Daytime melatonin administration
Based on the rational that low BP and HR during the nighttime coincide with high endogenous melatonin levels, several studies investigated whether exogenous dose of melatonin during the daytime lowers BP and HR. One of the first studies to report blood pressure lowering effects of exogenous melatonin administration during the daytime was by Birau et al. [102]. Arangino and co-workers [24] showed that a single administration of 1 mg melatonin administration in healthy male subjects between 2:30 and 5:30 PM reduced BP, norepinephrine levels, and pulsatility index in the internal carotid artery (PI; as measure of vascular reactivity) compared to placebo administration. Cagnacci and co-workers [103] showed a reduction of systolic and diastolic BP and PI by a single daytime melatonin dose also in menopausal women with hormonal replacement therapy. However, a potential concern with daytime melatonin administration is that melatonin induces sleepiness, which most likely is an unwanted side-effect in the treatment of high BP or HR. Furthermore, daytime administration will disrupt the normal profile of melatonin with potential additional adverse side effects. Thus, for therapeutic use, nighttime administration would be most relevant. Therefore several studies have investigated whether exogenous melatonin will elicit similar blood lowering effect during nighttime administration, when endogenous melatonin is high.
5.2. Nighttime melatonin administration
Lusardi and colleagues showed that 4 weeks of nighttime melatonin administration in hypertensive patients treated with the calcium channel blocker nefedipine increased blood pressure and heart rate, suggesting that melatonin counteracted the effects of nefedipine [104]. Scheer et al. [105] studied the effect of (i) a single dose and (ii) a daily dose for three weeks of oral 2,5 mg melatonin one hour prior to scheduled bedtime in a double-blind, randomized, placebo-controlled cross-over study in 16 patients with essential hypertension without any anti-hypertensive medication. They showed that 3-week melatonin administration reduced systolic and diastolic blood pressure during the nighttime and increased the day/night amplitude of systolic and diastolic blood pressure rhythms. There was no effect on HR. Of interest, in these same subjects, the single dose of melatonin had no effect on systolic or diastolic blood pressure or HR during the day or night. This suggests that the mechanism was different from that of a direct vasodilator drug like α-adrenergic receptor antagonist and that the mechanism of nighttime administration (when endogenous melatonin plasma levels are normally already high) is distinct from that of daytime melatonin administration (when endogenous melatonin plasma levels are very low). The 3-week melatonin administration, but not a single dose, furthermore increased sleep efficiency and total sleep time as assessed by actigraphy. There was no correlation between the changes in sleep and nighttime blood pressure as measured on the same nights, suggesting increased sleep quantity was not the main mechanism for the reduced blood pressure. However future studies using polysomnography are required to verify whether improved sleep is part of the underlying mechanism by which melatonin may lower nighttime blood pressure. Cagnacci et al. [106] confirmed the results of Scheer et al. in a mixed group of normontensive and hypertensive female subjects in a double-blind, randomized, cross-over study. Melatonin (3mg; slow release) 1 hour before bedtime for three weeks, lowered nocturnal systolic, diastolic, and mean BP but had no effect on diurnal BP or HR for the group as a whole. Grossman et al.[107] showed that nighttime exogenous melatonin administration lowered systolic blood pressure in patients with nocturnal hypertension and that the effect was greatest between 2 and 5 am. Potential mechanisms by which melatonin reduces BP include a direct effect via high-affinity melatonin receptors Mel1a (MT1) and/or Mel1b (MT2) on: (1) the SCN, changing its autonomic and/or humoral signaling to the cardiovascular system; (2) other hypothalamic or brainstem targets such as the paraventricular nucleus, a core autonomic and humoral relay station [108]; and/or (3) heart, kidney and vasculature [109], possibly affecting peripheral oscillators therein. Alternative possibilities include antioxidative effects [110].
Amongst the first to show that the endogenous heart rhythm could be shifted by using exogenous melatonin, were Krauchi et al., showing a phase advance after administering 5 mg melatonin or 5mg or 100 mg of a melatonin agonist compared to a placebo condition under constant routine conditions[111]. Vandewalle et al. [81] also showed that the endogenous rhythm in heart rate and heart rate variability could also be shifted by administering exogenous melatonin. In a 13-day double-blind, crossover protocol in 8 young adult male subjects they assessed initial phase during a 29-h constant routine, administering either 1.5 mg melatonin or placebo at 4 PM for 8 consecutive days, and re-assessed circadian phase during a second 29-h constant routine. The authors showed robust circadian rhythms for HR, HRV, SDNN, and rMSSD (during CR1) as well phase advances in these cardiac rhythms after melatonin treatment (during CR2). The parallel shift of HR and vagal markers together with other circadian markers, such as melatonin rhythm, in this study suggests these effects are due to a shift of the central circadian pacemaker and not only due to a shift in peripheral circadian oscillators in the heart. If melatonin is able to shift the rhythm of HR and vagal output measures, it might also elicit immediate effects on other aspects of cardiovascular functioning and may be useful as a chronobiotic in treating cardiovascular diseases such as hypertension.
Based on the previous findings, we can summarize that a) there is an endogenous circadian rhythm in cardiac function, cardiac autonomic function, and metabolic function in humans and animals and b) that these rhythms in humans and animals can be altered either by changes in the light-dark cycle or the administration of exogenous melatonin. We will therefore now review the potential negative health outcomes that circadian misalignment can have on cardiometabolic functioning.
6. Health consequences of circadian disruption/misaligment
One can think of circadian disruption/misalignment as a continuum; ranging from the most extreme forms seen in blind people who are not entrained or entrained at an adverse phase angle [112], during shift work, or (although shorted-lived) during jet-lag to more moderate examples like Advanced Sleep Phase Syndrome to mild ones occurring in early or late Chronotypes or after purposely staying up late while studying for an exam. Symptoms of circadian misalignment include increased daytime sleepiness, sleep disturbances, reduced cognitive performance, reduced alertness, gastrointestinal complaints, and an overall feeling of being somatically unwell [113]. Exposure to bright light can re-entrain the circadian pacemaker to the new time zone or to the shift work schedule when appropriately timed [114–116]. Similar approached can be taken with patients suffering from Advanced- and Delayed- Sleep Phase Syndrome (ASPS and DSPS, respectively) in order to regain and maintain appropriate alignment of their sleep-wake rhythm to the environmental light-dark cycle. In some cases, like jet-lag or purposely staying up late, symptoms and complaints are short-lived, i.e. they resolved in a matter of days, but in others like shift work, they persists over a longer period of time, potentially leading to pathological changes leading to chronic diseases such as obesity, diabetes, and cardiovascular disease. We will review the evidence for negative cardiometabolic health outcomes caused by circadian misalignment based on the evidence from epidemiological and laboratory studies.
6.1. Epidemiological and field studies
There is a vast body of evidence from epidemiological studies linking shift work to an increased risk for cardiovascular disease, diabetes, obesity, and cancer [117–121]. The central characteristic of shift work, especially night shift work, is exposure to an unusual light/dark and fasting/feeding cycle that causes misalignment between the internal timing system and the behavioral and light/dark cycle, referred to as external desynchrony, and possibly between different internal organ systems, referred to as internal desynchrony. This misalignment results in physiology, metabolism and behavior occurring out-of-synch‘ with the desired sleep/wake schedule and, in the short-term, leads to increased daytime sleepiness, reduced cognitive performance, disturbed sleep, and gastro-intestinal complaints, which are similar to the symptoms of jet-lag. However, jet-lag symptoms typically resolve over a number of days after adjusting to the new time zone, whereas shift works experience the before mentioned symptoms over extended periods of time. Exposure to sun light during their commute will prevent their circadian system to adjust fully or at all to the night work schedule. Furthermore, the shift worker is compelled to shift his sleep/wake rhythm to meet the needs of his work hours, however, most shift workers revert back to a normal daytime activity schedule during days off in order to share time with family and friends. This constant back and forth shifting of their sleep/wake rhythm is too fast for the internal circadian system to adapt to and is likely to result in mal-adaptation and pathological changes.
One of the first epidemiological studies to address the relationship between shift work and cardiovascular changes was Knutsson et al.[122] who found a dose-relationship between years of shift work and risk for coronary heart disease in paper mill workers, i.e. more years of shift work the greater the risk for coronary heart disease after controlling for age and smoking. Two other studies, one by Kawachi et al. [123] in American nurses and one by Tenkanen and colleagues [124] in Swedish industrial workers, both also showed increased risk for MI or CHD with increased years of rotating shifts worked which persisted after controlling for age, smoking status, body mass index, diabetes mellitus, use of contraception, and alcohol intake. Similarly, in a more recent analysis of the Nurse Health study data, Brown et al. (2009) found a linear association between the duration of shift work and the risk for ischemic stroke, with a 4 % increased risk of ischemic stroke for each five years of shift work. However, only in the group of nurse reporting 15 years or more of rotating shift work the increase in risk was significantly greater compared to those nurse never having worked shift worked.
Coronary Heart Disease (CHD) is part of the metabolic syndrome, besides obesity, dyslipideamia, and high cholesterol, which has been linked to shift work. Karlsson and co-workers [125] found that high tryglycerides, obesity, and low HDL cholesterol clustered together in shift workers as compared to day workers, indicating a link between shift work and the metabolic syndrome. A potential mechanism involved in the development of metabolic syndrome and/or CHD in shift workers might be an inappropriate hormonal response to meals. Lund and colleagues [126] studied 12 night shift workers on a British Artic Station and their response to standardized meals at different times of day. All meals were either consumed a) during a day time working shift, b) at the beginning of a night shift, or c) during the day after finishing a night shift. Postprandial insulin, glucose, and triacyglycerol (TAG) levels were significantly elevated during night shifts compared to the day time shift. Two days after returning to regular day time working hours, glucose and insulin levels returned to pre-shift work levels whereas TAG levels remained elevated.
Chronic changes in glucose and insulin metabolism can lead to diabetes, which also shows an increased incident rate in amongst shift workers. Morikawa and co-workers found an increased, age-adjusted, incident rate for diabetes mellitus amongst Japanese male blue collar shift workers workers compared to the white collar workers in an eight year prospective study [127]. Kroenke et al. [128] found similar result in female nurses that took part in the Nurses Health Study II, with the lowest diabetes risk amongst those nurses working part time and the highest risk in those working over time when compared to nurses fulltime but regular hours. In addition, the authors found a positive association between duration of shift work and risk for diabetes, that was entirely mediated by body weight, stressing the association between shift work, BMI and obesity, and risk for diabetes. In a retrospective longitudinal study in Italian municipal enterprise employees, Biggi and colleagues [129]found that night shift workers had higher BMI, smoked more, had higher cholesterol values and a higher risk for developing CHD than day time workers while controlling for age, cardiovascular disease at baseline, BMI, cholesterol, and lifestyle variable such as smoking and alcohol intake.
Besides CHD, cancer has been associated with shift work for some time and has recently been announced —probably carcinogenic by the cancer related subcommittee of the World Health Organization. In a study of women in the Nurses Health Study, Schernhammer et al. (2001), found an increased risk for breast cancer in women doing shift work compared to those who did not and further reported that, similar to the risk of ischemic stroke, women who had the longest duration of shift work, 30 year or longer, had the greatest risk for developing breast cancer. Data from mice studies showed that circadian disruption due to lesioning the SCN leads to an increased tumor growth rate compared to sham operated animals with intact SCN [130].
As mentioned earlier, shift work and jet-lag share some symptoms; however, there is very little data on long term health consequences with respect to repeated jet-lag. One of the few studies is an Icelandic study showing an overall increased risk for cancer, especially breast cancer and malignant melanoma amongst the female flight attendances [131]. It is important to note that the separate contributions of radiation, circadian disruption, and life style habits of the flight attendance can not be determined due to the nature of the study.
In summary, data from epidemiological and field studies show that shift work and possibly repeated jet-lag is associated with an increased risk for CHD, diabetes, cancer, and ischemic stroke. Nonetheless, the physiological mechanisms cannot be determined from epidemiological data: the disruption of hormonal rhythms, sleep curtailment/deprivation caused by the circadian disruption, or behavioral changes based on a changed sleep/wake and fast/feeding cycle due to the circadian disruption. Highly standardized laboratory studies offer the possibilities to control for some of the confounding aspects in order to unveil the underlying mechanisms.
6.2. Laboratory studies
One of the first laboratory studies to investigate potential metabolic changes due to circadian disruption/misalignment was by Hampton and co-workers [132] in a simulated shift work study. Nine healthy subjects were subjected to eating a standardized meal at the same clock time (13:30), either while being entrained to their normal rhythm or after a 9 hour phase advance of their sleep/wake cycle. All subjects received a high-fat pre-meal at 08:00. Significant difference in postprandial glucose and insulin levels were observed after the 9 hour phase shift compared to before and were most apparent in the last 4 hours of the 6-hour post-meal sampling period. The TAG peak was delayed after the phase shift and kept on rising throughout the study. In a follow-up study, Ribeiro et al [133] extended the previous design by lengthening the post-meal sampling period from 9 hours and varying the caloric content of the test meal. In this study, the twelve subjects received a non-fat pre-test meal at 08:00 before the actual test meal at 13:00, being consumed either under circadian entrainment or after a 9 hour phase advance of the sleep/wake cycle. In contrast to the results of Hampton (11861}, postprandial plasma glucose and insulin levels were not elevated after the test meal. Immediately after the phase shift, fatty acids levels were significantly lower whereas TAG levels were significantly higher. This suggests that that the nutritional content of meals consumed before or during a shift might influence the type and size of metabolic changes occurring. This is important when thinking of interventions to improve shift work conditions, e.g. the food available to shift workers while being on shift. Interpretation of the pure effect of circadian misalignment from these studies is complicated due to differences in pre-meal conditions between the night and day shifts such as in the duration of wakefulness before test meals.
To address these limitations and further investigate the impact of circadian misalignment on cardiac, metabolic, and endocrine variables, Scheer and colleagues used a FD protocol [36]. Ten subjects were subjected to an 11-day FD protocol consisting of repeated 28-h ‘days‘, including 4 scheduled isocaloric meals per 28-h day. The authors demonstrated that misalignment between the circadian pacemaker and the sleep/wake and fasting/feeding cycle, common in shift workers, lead to increased glucose, insulin levels, and arterial pressure, and reduced leptin levels and sleep efficiency of the subjects. The effects of circadian misalignment on the cardiometabolic system appeared to be beyond any effect of sleep efficiency per se. In addition, the authors found that the circadian misalignment pushed the postprandial glucose response of three of their subjects into the pre-diabetic range. This study revealed some of the direct physiological consequences of circadian misalignment and thereby potential mechanisms to explain the increased risk for the development of obesity, diabetes and cardiovascular disease in shift workers.
Due to the circadian modulation of sleep propensity [134], one of the effects of circadian misalignment is decreased sleep quality, that in itself can induce metabolic changes. Spiegel and colleagues [135]showed that restricting sleep to 4 hours per night for six consecutive nights in healthy, non-diabetic men resulted in reduced glucose clearance, impaired acute insulin response, and lower insulin sensitivity. In the same study, the authors also showed that on day 5 of the sleep deprivation the otherwise healthy subjects had a disposition index (DI), i.e. the product of acute insulin response and insulin sensitivity, similar to the DI values of a group of elderly subjects with a known impaired glucose tolerance and increased diabetes risk [136]. Sleep deprivation furthermore led to a significant reduction of leptin and increase of ghrelin [137] despite constant caloric intake, and significantly increased the subjective feeling of hunger [138]. Nedeltcheva and co-workers [139] allowing ad libitum snacks showed that sleep restriction to 5.5 hrs vs. 8.5 hrs per night for 14 nights, resulted in increased snack consumption but no changes in ghrelin or leptin levels, nor surprising since ghrelin and leptin are counter-regulatory hormones. Indeed, the majority of studies shows a change in hormonal levels as well as in reported feelings of hunger or caloric intake when food intake is restricted and increased caloric intake (but no hormonal changes) with ad libitum food access. However, further studies are required to establish whether or not increased caloric intake during circadian misalignment or following sleep deprivation would lead to weight gain, and is not compensating either increased energy expenditure or impaired absorption. Regardless, the decreased glucose tolerance and insulin sensitivity during circadian misalignment is a precursor of diabetes mellitus [140]. Diabetes itself can lead to retinal impairment and blindness, reduced wound healing, impaired cognition and memory in the long run, secondary negative outcomes that might not be immediately apparent[141,142].
In summary, we can conclude that circadian misalignment (acute and chronic) is able to influence hormones critically involved in the control of metabolic processes, e.g. insulin, leptin, and ghrelin, which may provide the physiological mechanisms underlying the increased risk of shift work for the development of CHD, diabetes and obesity.
7. Summary
The role of the circadian system in the control of cardiometabolic functioning gained clinical relevance based on epidemiological data reporting a day/night pattern in adverse cardiovascular events with a peak in the morning as well as a higher incident of CHD, diabetes and obesity amongst shift workers. This lead to a series of laboratory studies in humans showing that (a) HR and BP show a circadian rhythm with low HR and BP during the biological night, i.e. habitual sleep episode, and an increase during the biological day, i.e. habitual wake episode, (b) that the peak in vagal activity occurs during the biological night, whereas the peak of sympathetic activity occurs during the biological day, and (c) the scaling exponent α of heartbeat fluctuations, a potential marker for the risk of an adverse cardiovascular event, exhibits a circadian rhythm with a peak in the morning. Results from animal studies established that (a) the SCN innervates the heart and other organs involved in hemodynamic control, such as kidney, vasculature and adrenal, via a multisynaptiv pathway, probably including direct projections form the SCN to the paraventricular nucleus of the hypothalamus (PVN), (b) heart rate shows a circadian rhythm that is not the secondary result of a circadian rhythm in locomotor activity, and that (c) ablation of the SCN leads to abolishment of the circadian rhythm in HR and BP. Light, the major Zeitgeber of the circadian system and melatonin, the body‘s internal dark signal, both impact cardiometabolic functioning in humans and animals. Light in humans has been shown to (a) acutely elevate HR, BP, core body temperature and cortisol and suppress melatonin during the biological night but not during the biological day, and (b) phase shift the rhythms melatonin and cortisol. Correctly timed, light can help to facilitate re-entrainment of circadian rhythms after shift work or jet-lag, and potentially in treating ASPS and DSPS. Exogenous daytime and repeated nighttime melatonin administration has proven to be able to lower BP in normotensive and hypertensive subjects, with nighttime administration most clinically useful. Circadian misalignment, i.e. the mismatch of the circadian system with the desired sleep/wake cycle, is a hallmark of shift work and jet-lag and causes a multitude of negative symptoms and chronic exposure has been associated with several negative health consequences such as increased risk for cardiovascular disease, diabetes, obesity, and certain forms of cancer. However, from the epidemiological data can not distinguish if these negative health effects of shift work are directly mediated by (a) differences in socioeconomic status and life style of shift workers, (b) the mismatch of the circadian system and behavioral rhythms such as sleep/wake, rest-activity, or fast/feeding, or (c) chronic sleep deprivation as a direct result of circadian misalignment, which in itself can lead to a variety of adverse cardiac, metabolic, and endocrine changes. However, in recent laboratory studies, it has been demonstrated that circadian misalignment itself can lead to cardiometabolic changes that in the long run may lead to increased risk for the development of obesity, diabetes and cardiovascular disease [36]. This is important in determining the potential therapeutic application of the above summarized findings to counteract the negative health effects of circadian misalignment seen in shift work. In addition, recent findings indicate that circadian misalignment may also leads to the internal desynchronization between the SCN, central internal clock, and the clock cells in peripheral organs such as the kidney or the liver. Our understanding of the relationship between central and peripheral clocks is at the very beginning and limited, but is seems internal desynchronization might impact cardiometabolic functioning and overall health in the end as well. Further studies identifying the exact mechanism involved in these processes is warranted and might lead to (a) behavioral therapies to change the timing of behaviors contributing to adverse cardiometabolic effects, or (b) to identify useful biomarkers in order to help to identify individuals with a greater risk for adverse cardiovascular and metabolic consequences due to circadian misalignment.
Acknowledgments
The authors would like to thank Dr. Steven W. Lockley for initial discussion. M.R. was supported by National Institute of Neurological disorders and Stroke Grant 5R01NS54277-3 and National Institute of Mental Health Grant 5R01MH45130-19. F.A.J.L.S. was supported by National Center for Complementary and Alternative Medicine Grant R21-AT002713, and Biomedical Research Institute Fund to Sustain Research Excellence from Brigham and Women's Hospital.
Reference List
- 1.Halberg F. Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle. Z Vitamin Horm Formentforsch. 1959;10:225–296. [PubMed] [Google Scholar]
- 2.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 3.Dethlefsen U, Repges R. Ein neues therapieprinzip bei nachtlichem asthma. Asthma Klin. 1985;80:44–47. [Google Scholar]
- 4.Pavlova MK, Shea SA, Bromfield EB. Day/night patterns of focal seizures. Epilepsy Behav. 2004;5:44–49. doi: 10.1016/j.yebeh.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms. 2002;17:364–376. doi: 10.1177/074873040201700409. [DOI] [PubMed] [Google Scholar]
- 6.Underwood H, Siopes T, Barret RK. Does a biological clock reside in the eye of quail? J Biol Rhythms. 1988;3:323–331. doi: 10.1177/074873048800300402. [DOI] [PubMed] [Google Scholar]
- 7.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray MS, Young ME. Diurnal variations in myocardial metabolism. Cardiovasc Res. 2008;79:228–237. doi: 10.1093/cvr/cvn054. [DOI] [PubMed] [Google Scholar]
- 9.Young ME, Bray MS. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007;8:656–667. doi: 10.1016/j.sleep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 11.Scheer FAJL, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 12.Stephan FK, Zucker I. Rat drinking rhythms: central visual pathways and endocrine factors mediating responsiveness to environmental illumination. Physiol Behav. 1972;8:315–326. doi: 10.1016/0031-9384(72)90379-4. [DOI] [PubMed] [Google Scholar]
- 13.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: The mind's clock. New York: Oxford University Press; 1991. [Google Scholar]
- 14.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 15.Marsh EE, III, Biller J, Adams HP, Jr, Marler JR, Hulbert JR, Love BB, Gordon DL. Circadian variation in onset of acute ischemic stroke. Arch Neurol. 1990;47:1178–1180. doi: 10.1001/archneur.1990.00530110032012. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Shetty H. Circadian variation in stroke - a prospective hospital-based study. Int J Clin Pract. 2005;59:1272–1275. doi: 10.1111/j.1368-5031.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 17.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Hartwell TD, Scheiner E, Gold HK, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 18.Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, Hier DB, Wolf PA, Caplan LR, Foulkes MA. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476. doi: 10.1161/01.str.20.4.473. [DOI] [PubMed] [Google Scholar]
- 19.Tofler GH, Gebara OC, Mittleman MA, Taylor P, Siegel W, Venditti FJ, Jr, Rasmussen CA, Muller JE. Morning peak in ventricular tachyarrhythmias detected by time of implantable cardioverter/defibrillator therapy. The CPI Investigators Circulation. 1995;92:1203–1208. doi: 10.1161/01.cir.92.5.1203. [DOI] [PubMed] [Google Scholar]
- 20.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria MA, Haus E, Manfredini F. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22:417–453. doi: 10.1081/CBI-200062927. [DOI] [PubMed] [Google Scholar]
- 21.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der WC, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 23.Brezinski DA, Muller JE, Tofler GH, Pohjola-Sintonen S. Postural change and increases in platelet aggregability: associations with myocardial infarction, sudden cardiac death and myocardial ischemia. <None Specified> 1992 [Google Scholar]
- 24.Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 25.Kerkhof GA, Van Dongen HPA, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am. J Hypertens. 1998;11:373–377. doi: 10.1016/s0895-7061(97)00461-5. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 27.Van Dongen HPA, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int. 2001;18:85–98. doi: 10.1081/cbi-100001178. [DOI] [PubMed] [Google Scholar]
- 28.Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheer FAJL, van Doornen LJP, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms. 1999;14:202–212. doi: 10.1177/074873099129000614. [DOI] [PubMed] [Google Scholar]
- 30.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and Circadian Influences on Cardiac Autonomic Nervous System Activity. Am J Physiol Heart Circ Physiol. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 31.van Eekelen AP, Houtveen JH, Kerkhof GA. Circadian variation in base rate measures of cardiac autonomic activity. Eur J Appl Physiol. 2004;93:39–46. doi: 10.1007/s00421-004-1158-6. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov PC, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci U S A. 2007;104:20702–20707. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 34.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Computers in Cardiology. 2000;27:197–200. [PubMed] [Google Scholar]
- 35.Huikuri HV, Makikallio TH. Heart rate variability in ischemic heart disease. Auton Neurosci. 2001;90:95–101. doi: 10.1016/S1566-0702(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 36.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 39.Scheer FAJL, Ter Horst GJ, van der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 40.Scheer FA, Pirovano C, VanSomeren EJW, Buijs RM. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 41.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 42.Kalsbeek A, Fliers E, Romijn JA, la Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 43.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 44.Ruiter M, la Fleur SE, van Heijningen C, van d V, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. doi: 10.2337/diabetes.52.7.1709. [DOI] [PubMed] [Google Scholar]
- 45.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 46.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 49.Shibata S. Neural regulation of the hepatic circadian rhythm. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:901–909. doi: 10.1002/ar.a.20095. [DOI] [PubMed] [Google Scholar]
- 50.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 51.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 52.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 53.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Sensitivity of the human circadian pacemaker to moderately bright light. J Biol Rhythms. 1994;9:315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- 54.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 55.Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- 56.Moore RY. Organization of the mammalian circadian system. In: Waterhouse JM, editor. Circadian clocks and their adjustment; Chichester. Ciba Foundation Symp 183; John Wiley and Sons, Inc; 1995. pp. 88–99. [PubMed] [Google Scholar]
- 57.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 58.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 59.Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai J, van der Vliet J, Swaab DF, Buijs RM. Human retinohypothalamic tract as revealed by in vitro postmortem tracing. J Comp Neurol. 1998;397:357–370. [PubMed] [Google Scholar]
- 62.Sadun AA, Schaechter JD, Smith LEH. A retinohypothalamic pathway in man: Light mediation of circadian rhythms. Brain Res. 1984;302:371–377. doi: 10.1016/0006-8993(84)90252-x. [DOI] [PubMed] [Google Scholar]
- 63.Friedman DI, Johnson JK, Chorsky RL, Stopa EG. Labeling of human retinohypothalamic tract with the carbocyanine dye, DiI. Brain Res. 1991;560:297–302. doi: 10.1016/0006-8993(91)91246-w. [DOI] [PubMed] [Google Scholar]
- 64.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140:1520–1524. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- 66.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 67.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau K-W. Diminished pupillary light reflex at high irradiance in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 68.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 69.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 71.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Selby CP, Thompson C, Schmitz TM, Van Gelder RN, Sancar A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc Natl Acad Sci USA. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brainard GC, Hanifin JP, Rollag MD, Greeson J, Byrne B, Glickman G, Gerner E, Sanford B. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- 74.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalmic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- 77.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 79.Beersma DGM, Daan S. Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8:340–347. doi: 10.1177/074873049300800407. [DOI] [PubMed] [Google Scholar]
- 80.Campos LA, Plehm R, Cipolla-Neto J, Bader M, Baltatu OC. Altered circadian rhythm reentrainment to light phase shifts in rats with low levels of brain angiotensinogen. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1122–R1127. doi: 10.1152/ajpregu.00703.2005. [DOI] [PubMed] [Google Scholar]
- 81.Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res. 2007;16:148–155. doi: 10.1111/j.1365-2869.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 82.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561:339–351. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 84.Tsunoda M, Endo T, Hashimoto S, Honma S, Honma K-I. Effects of light and sleep stages on heart rate variability in humans. Psychiatr Clin Neurosci. 2001;55:286. doi: 10.1046/j.1440-1819.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- 85.Burgess HJ, Sletten T, Savic N, Gilbert SS, Dawson D. Effects of bright light and melatonin on sleep propensity, temperature, and cardiac activity at night. J Appl Physiol. 2001;91:1214–1222. doi: 10.1152/jappl.2001.91.3.1214. [DOI] [PubMed] [Google Scholar]
- 86.Scheer FA, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton Neurosci. 2004;110:44–48. doi: 10.1016/j.autneu.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Yokoi M, Aoki K, Shimomura Y, Iwanaga K, Katsuura T. Exposure to bright light modifies HRV responses to mental tasks during nocturnal sleep deprivation. J Physiol Anthropol. 2006;25:153–161. doi: 10.2114/jpa2.25.153. [DOI] [PubMed] [Google Scholar]
- 88.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 89.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 90.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 91.Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 92.Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- 93.Box GE. Robustness in the Strategy of Scientific Model Building. In: Launer RL, Wilkinson GN, editors. Robustness in Statistics. New York: Academic Press; 1979. pp. 201–236. [Google Scholar]
- 94.Badia P, Culpepper J, Myers B, Boecker M, Harsh J. Psychophysiological and behavioral effects of bright and dim light. In: Chase MH, Lydic R, O'Connor C, editors. Sleep Research. Los Angeles: Brain Information Service; 1990. p. 387. [Google Scholar]
- 95.Badia P, Myers B, Boecker M, Culpepper J, Harsch JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 96.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J Biol Rhythms. 2003;18:409–419. doi: 10.1177/0748730403256650. [DOI] [PubMed] [Google Scholar]
- 97.Kubota T, Uchiyama M, Suzuki H, Shibui K, Kim K, Tan X, Tagaya H, Okawa M, Inoue S. Effects of nocturnal bright light on saliva melatonin, core body temperature and sleep propensity rhythms in human subjects. Neurosci Res. 2002;42:115–122. doi: 10.1016/s0168-0102(01)00310-8. [DOI] [PubMed] [Google Scholar]
- 98.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 99.Shanahan TL, Czeisler CA. Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab. 1991;73:227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- 100.Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9:5–9. doi: 10.1016/j.smrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 101.Scheer FA, Cajochen C, Turek FW, Czeisler CA. Melatonin in the regulation of sleep and circadian rhythms. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2005. pp. 395–404. [Google Scholar]
- 102.Birau N, Peterssen U, Meyer C, Gottschalk J. Hypotensive effect of melatonin in essential hypertension. IRCS Med Sci. 1981;9:906. [Google Scholar]
- 103.Cagnacci A, Zanni AL, Venerit MG, Menozzi R, Volpe A, Del Riot G. Influence of exogenous melatonin on catecholamine levels inpostmenopausal woman prior and during oestradiol replacement. Clin Endocrinol. 2000;53:367–372. doi: 10.1046/j.1365-2265.2000.01099.x. [DOI] [PubMed] [Google Scholar]
- 104.Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br J Clin Pharmacol. 2000;49:423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scheer FA, Van Montfrans GA, Van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertens. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 106.Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am J Hypertens. 2005;18:1614–1618. doi: 10.1016/j.amjhyper.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 107.Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119:898–902. doi: 10.1016/j.amjmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Wu YH, Zhou JN, Balesar R, Unmehopa U, Bao A, Jockers R, van Heerikhuize J, Swaab DF. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol. 2006;499:897–910. doi: 10.1002/cne.21152. [DOI] [PubMed] [Google Scholar]
- 109.Ekmekcioglu C, Haslmayer P, Philipp C, Mehrabi MR, Glogar HD, Grimm M, Leibetseder VJ, Thalhammer T, Marktl W. Expression of the MT1 melatonin receptor subtype in human coronary arteries. J Recept Signal Transduct Res. 2001;21:85–91. doi: 10.1081/rrs-100107144. [DOI] [PubMed] [Google Scholar]
- 110.Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 111.Kräuchi K, Cajochen C, Möri D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advanced circadian phase and nocturnal regulation of core body temperature. Am J Physiol. 1997;272:R1178–R1188. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 112.Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- 113.Vener KJ, Szabo S, Moore JG. The effect of shift work on gastrointestinal (GI) function: A review. Chronobiologia. 1989;16:421–439. [PubMed] [Google Scholar]
- 114.Czeisler CA, Moore-Ede MC, Coleman RM. Rotating shift work schedules that disrupt sleep are improved by applying circadian principles. Science. 1982;217:460–463. doi: 10.1126/science.7089576. [DOI] [PubMed] [Google Scholar]
- 115.Czeisler CA, Dijk DJ. Use of bright light to treat maladaption to night shift work and circadian rhythm sleep disorders. J Sleep Res. 1995;4:70–73. doi: 10.1111/j.1365-2869.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 116.Czeisler CA, Chiasera AJ, Duffy JF. Research on sleep, circadian rhythms and aging: Applications to manned spaceflight. Exp Gerontol. 1991;26:217–232. doi: 10.1016/0531-5565(91)90014-d. [DOI] [PubMed] [Google Scholar]
- 117.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 118.Knutsson A. Shift work and coronary heart disease. Scand J Soc Med Suppl. 1989;44:1–36. [PubMed] [Google Scholar]
- 119.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 121.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS. Shift work and risk of colorectal cancer in the Nurses' Health Study. J Natl Cancer Inst. 2003 doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 122.Knutsson A, Åkerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 123.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 124.Tenkanen L, Sjoblom T, Kalimo R, Alikoski T, Harma M. Shift work, occupation and coronary heart disease over 6 years of follow-up in the Helsinki Heart Study. Scand J Work Environ Health. 1997;23:257–265. doi: 10.5271/sjweh.218. [DOI] [PubMed] [Google Scholar]
- 125.Karlsson B, Knutsson A, Lindahl B. Is there an association between shiftwork and having a metabolic syndrome? Results from a population based study of 27485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557–564. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- 127.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- 128.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 129.Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–454. doi: 10.1080/07420520802114193. [DOI] [PubMed] [Google Scholar]
- 130.Filipski E, King VM, Li X, Granda TG, Mormont M-C, Liu X, Claustrat B, Hastings MH, Lévi F. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 131.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 132.Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, norris F, Deacon S, Ribeiro D, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- 133.Ribeiro D, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- 134.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 135.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 136.Garcia GV, Freeman RV, Supiano MA, Smith MJ, Galecki AT, Halter JB. Glucose metabolism in older adults: a study including subjects more than 80 years of age. J Am Geriatr Soc. 1997;45:813–817. doi: 10.1111/j.1532-5415.1997.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 137.Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 138.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 139.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Annals of the NY Academy of Science. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- 142.Convit A, Rueger M, Wolf OT. Diabetes Type 2 and Stress: Impact on Memory and the Hippocampus. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford, UK: Academic Press; 2009. pp. 503–509. [Google Scholar]