Abstract
Objective
Behaviorally based therapies for the treatment of perpetrators who initiate intimate partner violence (IPV) have generally shown minimal therapeutic efficacy. To explore a new treatment approach for IPV, we examined the effects of a selective serotonin reuptake inhibitor on the irritability subscale score of the Modified Overt Aggression Scale. This score served as a surrogate marker for the anger and physical aggression that characterize perpetrators of IPV.
Method
A 12-week, double-blind, randomized, placebo-controlled intervention study employing fluoxetine, alcohol treatment, and cognitive-behavioral therapy was performed. Sixty (46 men) non–court-mandated, DSM-IV–diagnosed alcoholic perpetrators of IPV with a history of at least 2 episodes of IPV in the year prior to participation in the study were evaluated. The primary outcome measure was the score on the irritability subscale of the Modified Overt Aggression Scale. Secondary measures included anxiety, depression, and ratings by the perpetrator's spouse/significant other. The study was conducted from January 2002 through December 2007.
Results
A repeated-measures analysis of variance using the irritability subscale scores obtained from perpetrators who completed the 12-week study (n = 24) showed a significant drug effect (F1,21 = 12.09, P = .002). Last observation carried forward (F1,32 = 4.24, P = .048) as well as intent-to-treat analysis (F1,54 = 5.0, P = .034) also showed a significant drug effect. Spouses'/significant others' physical and nonphysical Partner Abuse Scale ratings showed a significant reduction of abuse over time (F1,11 = 10.2, P = .009 and F1,11 = 24.2, P = .0005, respectively).
Conclusion
This is the first controlled study to show that a pharmacologic intervention employing a selective serotonin reuptake inhibitor, in conjunction with alcohol treatment and cognitive-behavioral therapy, can reduce measures of anger and physical aggression in alcoholic perpetrators of IPV.
Alcohol usage is strongly correlated with intimate partner violence (IPV).1 It is estimated that between 50% and 70% of perpetrators of IPV have an alcohol problem,2,3 and, according to some studies, 60%–90% of the perpetrators of IPV are under the influence of alcohol at the time of the violence.4,5 Studies examining the effect of alcohol treatment on IPV show aggression decreases with alcohol treatment.6,7
Descriptive studies show that individuals with high trait anger are the most likely to exhibit alcohol-associated aggression.8–10 Anger is an emotion that is associated with both verbal and physical aggression and is typically treated with behaviorally based initiatives. For example, the Attorney General's Task Force on Family Violence recommended that perpetrators attend court-mandated batterer intervention programs as a possible alternative to incarceration.11 These programs traditionally utilize the Duluth Model,12 which is designed to change men's sexist and patriarchal views toward women, as well as use psychoeducational and cognitive-behavioral therapies (CBTs) to improve anger control and communication techniques. However, results from a meta-analysis of 10 studies involving court-mandated batterer intervention programs show that these treatment programs are largely ineffective in reducing the likelihood of future violence.11 Similarly, a meta-analysis of 22 studies involving mostly non–court-mandated batterer intervention programs shows only a nominal effect in decreasing the rate of recidivism among batterers.13
There has been minimal research directed toward understanding biologic factors that contribute to IPV. To address this deficiency, George et al14 developed a biologic model, based on a series of studies,15–17 that was aimed at understanding the link between neuropathways and the behaviors evidenced by perpetrators of IPV. Perpetrators are hyperresponsive to environmental stimuli15 and have decreased correlations between cortical structures and the amygdala.17 Changes in neurotransmitter systems such as serotonin16 could result in the perpetrators' heightened sensitivity to environmental stimuli and affect the neuro-connections between the cortex and the amygdala.
In this study, we examined a group of perpetrators of IPV with diagnosis of alcohol dependence, who demonstrated significant levels of physical aggression toward their significant others. The perpetrators were randomly assigned according to a double-blind, placebo-controlled design to receive either fluoxetine or placebo treatment. A selective serotonin reuptake inhibitor was chosen due to serotonin's ability to modulate the processing of environmental stimuli,18,19 to increase orbital frontal cortex function,20 and to reduce impulsive types of aggression.21–23 We hypothesized that fluoxetine would be more effective than placebo in decreasing scores on the irritability subscale (IS) of the Modified Overt Aggression Scale (MOAS).24 The IS score served as a surrogate marker for anger and physical aggression. The physical and nonphysical Partner Abuse Scale25 ratings were used as another measure to corroborate the effects of treatment.
Method
Subject Selection
Sixty perpetrators of IPV were obtained from a population of treatment-seeking, alcohol-dependent patients who were admitted to the National Institute on Alcohol Abuse and Alcoholism Clinical Center Research Unit at the National Institutes of Health. All patients were in good health, were not taking any medications, and had a negative history for major head trauma (ie, no periods of unconsciousness lasting longer that 1 hour). Assessment included a general medical and psychiatric evaluation, including a routine laboratory assessment; a structured clinical interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) diagnoses26; and a brain magnetic resonance imaging. Patients with a history of at least 2 episodes of physical aggression (eg, hitting, pushing, punching, choking) toward their significant others in the year prior to their hospitalization were recruited to participate in this study. All perpetrators were also required to have some acts of IPV when they were not under the influence of alcohol and to have a minimum score of 3 on the Straus Conflict Tactics Scales physical violence subscale.27,28
Approval for the study was obtained from the National Institute on Alcohol Abuse and Alcoholism Institutional Review Board. The study results and participants' safety were monitored by a data safety monitoring board. Signed informed consent was obtained by the principal investigator (D.T.G.) or his designee. The study was conducted from January 2002 through December 2007.
Therapeutic Interventions
All perpetrators of IPV participated in standard cognitive and motivational therapies in addition to self-help groups (eg, Alcoholics Anonymous) for the treatment of their alcoholism. In addition, perpetrators received individualized CBT, which stressed the seriousness of domestic violence. The goal of therapy was to help the perpetrators verbalize perceived threats from environmental stimuli and to find appropriate nonviolent solutions to these threats. Each perpetrator was presented with information that outlined how biologic factors could facilitate many of the behaviors associated with IPV.14 Conjoint therapy with significant others was designed to focus on couple dynamics, communication skills, and conflict resolution. However, none of the significant others elected to participate in the offered couples therapy.
Study Design
Perpetrators were randomly assigned, according to a double-blind design, to receive either the selective serotonin reuptake inhibitor fluoxetine (maximum of 40 mg/d) or placebo. Forty milligrams of fluoxetine was chosen because it has been shown to be effective in decreasing other types of impulsive violence.21 Perpetrators were started on 1 capsule per day (ie, 10 mg of fluoxetine or placebo) for 3 days and then increased to 2 capsules. On days 14 and 21, the number of capsules was increased to 3 and 4, respectively. Perpetrators who experienced untoward side effects were maintained on the highest dose that they could tolerate. The majority of perpetrators were maintained on 40 mg of fluoxetine per day. The minimum dose tolerated was 30 mg per day. Plasma levels of fluoxetine were obtained monthly to assess drug compliance.
A power analysis was performed for an analysis of variance with 2 groups to detect an effect size of 0.8 with a 30% dropout rate. Following randomization by the National Institutes of Health pharmacy, the perpetrators were carefully monitored in the outpatient clinic over a 3-month period. The decision to employ a 3-month treatment period represented a balance between the time necessary to establish the effectiveness of fluoxetine and the desire to decrease the exposure time that significant others were at risk for abuse. Perpetrators were seen on a weekly basis during the first month and then every 2 weeks thereafter. During each clinic visit, perpetrators were assessed for their response to medication, drug side effects, breath alcohol concentrations, use of illicit drugs, and the seriousness of their aggression. If perpetrators missed regularly scheduled appointments, they were contacted by phone to encourage continued study participation. Perpetrators who relapsed to alcoholic drinking and remained in contact with the National Institute on Alcohol Abuse and Alcoholism were required by the data safety monitoring board to be readmitted to the inpatient unit for detoxification from alcohol and additional CBT; in addition, they were placed back on treatment with medication for the remainder of their study period.
To characterize the effects of fluoxetine or placebo as subjectively experienced by the perpetrators, rating scales were administered once every 4 weeks. We used the IS score of the MOAS24 as a surrogate marker for anger and physical aggression. The IS score is composed of (1) subjective irritability, measuring feelings of anger and annoyance and (2) overt irritability, measuring argumentativeness, shouting, loss of temper, and physical aggression. We selected the IS as a surrogate marker to assess the perpetrators' responses to treatment on the basis of our previous work15 showing that perpetrators are hypersensitive to environmental stimuli (ie, irritable) and clinical interviews indicating that perpetrators often feel angry independent of any specific antecedent. We also measured anxiety (ie, Spielberger State Anxiety Inventory29) and depression (ie, Hamilton Depression Rating Scale30).
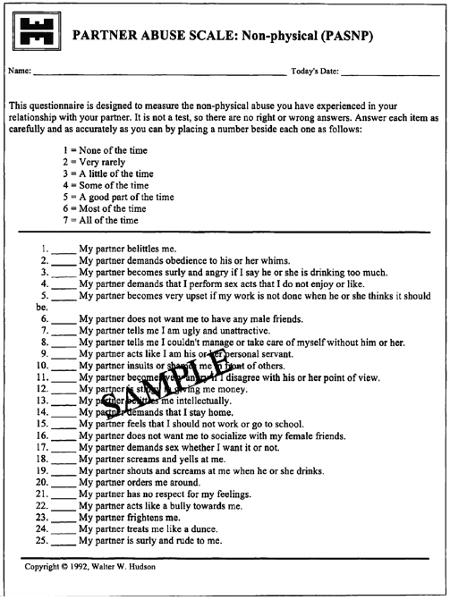
Spouses/significant others who were willing to participate in the study were administered the Partner Abuse Scale25 (Appendices 1 and 2) at the beginning and at the end of the 12-week study. The scale provided the significant others' perspectives on the perpetrators' behavioral responses to treatment.
Results
All 60 perpetrators of IPV met DSM-IV diagnostic criteria for alcohol dependence. Four alcoholic perpetrators were missing the baseline IS and were dropped from analysis. Thirty-two alcoholic perpetrators had unexcused missed assessments. Ten of these 32 perpetrators had stopped their medication for prolonged periods of time, had missed multiple assessments, and were readmitted to the hospital as described under the Study Design section. The remaining 22 alcoholic perpetrators failed to complete the 12 weeks of the study and were completely lost to follow-up (Figure 1).
Figure 1. Study Profile.
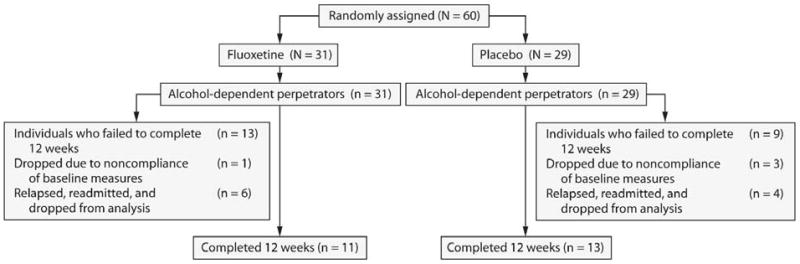
The 10 readmitted perpetrators were eliminated from all analyses except the intent-to-treat analysis because of the possible confound of being off medication treatment for a prolonged period of time and the additional treatment that they received. There were no baseline IS (F1,59 = 1.43, P = .24) or alcohol lifetime consumption (F1,59 = 0.01, P = .92) differences between these 10 perpetrators and those retained for analysis. All analyses were performed using STATISTICA, version 7.1 (StatSoft, Inc, Tulsa, Oklahoma).31
Table 1 shows the lifetime characteristics of all 60 alcoholic perpetrators. There were no significant differences between the treatment groups. Table 2 shows the number of missing perpetrators at weeks 4, 8, and 12 as a function of fluoxetine versus placebo for the 22 lost-to-follow-up perpetrators.
Table 1. Lifetime Characteristics of 60 Alcohol-Dependent Perpetrators of Intimate Partner Violencea.
| Characteristic | Placebo, n = 29 | Fluoxetine, n = 31 |
|---|---|---|
| Age, y | 39.1 ± 6.7 | 38.8 ± 7.7 |
| Male, n (%) | 22 (76) | 24 (77) |
| Female, n (%) | 7 (24) | 7 (23) |
| Lifetime drinking, kg | 812.8 ± 649.6 | 707.1 ± 539.8 |
| Years of heavy drinking | 12.3 ± 7.5 | 12.9 ± 8.2 |
| Age at onset, y | 19.9 ± 6.2 | 21.6 ± 7.6 |
| Michigan Alcohol Screening Test | 52.3 ± 39.1 | 47.3 ± 19.3 |
| Straus Conflict Tactics Scales | ||
| Verbal reasoning subscale scoreb | 30.2 ± 19.5 | 28.0 ± 18.1 |
| Verbal aggression subscale scorec | 61.4 ± 23.8 | 64.5 ± 23.2 |
| Physical violence subscale scorec | 16.3 ± 13.4 | 15.7 ± 11.2 |
Values shown as mean ± SD unless otherwise noted.
Higher scores represent greater reasoning ability.
Higher scores represent more severe verbal and physical aggression.
Table 2. Number of Perpetrators Who Failed to Complete 4, 8, and 12 Weeks of Treatment.
| Treatment Group | Week 4 | Week 8 | Week 12 |
|---|---|---|---|
| Placebo | 4 | 4 | 1 |
| Fluoxetine | 8 | 3 | 2 |
| Total | 12 | 7 | 3 |
Irritability Subscale of the Modified Overt Aggression Scale
The IS score was analyzed using 3 different statistical methods. These methods utilized the completer analysis, the last-observation-carried-forward analysis, and the intent-to-treat analysis.
Completer data analysis, utilizing scores from baseline through week 12, was a repeated-measures analysis of covariance with the IS score as the repeated measure and the baseline IS score as the covariate. The between-groups measure was the drug factor. There was no significant repeated-measures effect (F2,42 = 0.85, P = .43) or interaction effect (F2,42 = 0.02, P = .98). There was a significant drug effect (F1,21 = 12.09, P = .002) (Figure 2).
Figure 2. Effect on the IS Score of the MOAS for Perpetrators Who Completed 12 Weeks of Treatmenta.
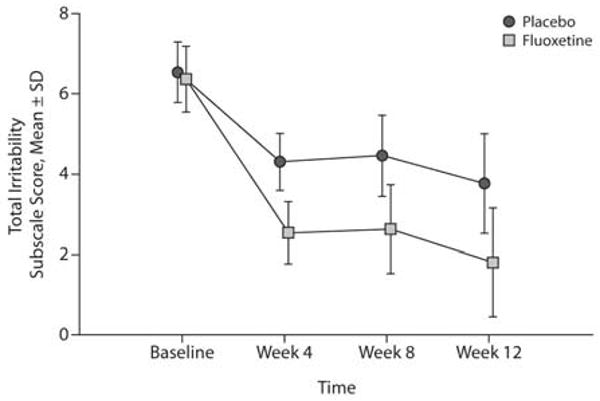
aBaseline scores were used as the covariate. There was no significant repeated-measures effect (F2,42 = 0.85, P = .43) or interaction effect (F2,42 = 0.02, P = .98). There was a significant drug effect (F1,21 = 12.09, P = .002).
Abbreviations: IS = irritability subscale, MOAS = Modified Overt Aggression Scale.
The last-observation-carried-forward-analysis, in which the last observations from week 4 and week 8 were carried forward to week 12, was utilized to account for missing data. We performed an analysis of covariance with the IS score as the dependent variable at week 12 and the IS score at baseline as the covariate. The drug factor was the between-groups variable. There was a significant drug effect (F1,32 = 4.24, P = .048).
To examine the possibility that the missing IS scores were missing completely at random, we performed the following analyses. Using an analysis of variance model with a drug factor, with a missing value factor, and with the IS score at baseline as the dependent variable, it was found that the missing value at week 4 could not be predicted from the data at baseline (F1,42 = 0.10, P = .75). A multivariate test was performed for missing data at week 8 using data from baseline and week 4. The missing data at week 8 could not be predicted from values of the observed data at baseline and week 4 (F2,29 = 0.34, P = .71). A multivariate test was performed for missing data at week 12 using data from baseline, week 4, and week 8. The missing data at week 12 could not be predicted from values of the observed data at baseline, week 4, and week 8 (F3,22 = 0.66, P = .59). We conclude that, with respect to the IS score, the data may be missing completely at random.
To examine the possibility that the missing data were related to alcoholic drinking, we performed an analysis of variance with total lifetime alcohol consumption as the dependent variable and the missing data factor at week 4 and found a trend for missing data at week 4 (F1,40 = 3.83, P = .057). This finding suggests that alcohol may be a contributing cause of the missing data. There was no significant correlation between total lifetime drinking and baseline IS scores, further suggesting that the missing data mechanism is unrelated to the IS score. Comparison of fluoxetine versus placebo for missing data was not significant (χ22 = 1.68, P = .43) (Table 2). These results provide some evidence that the missing IS scores may be considered as missing completely at random.
All 60 alcoholic perpetrators were entered into an intent-to-treat analysis utilizing scores from baseline through week 12. A repeated-measures analysis of covariance was performed with the IS score as the repeated measure and the baseline IS score as the covariate. The between-groups measure was the drug factor. There was no significant repeated-measures effect (F2,54 = 0.70, P = .50) or interaction effect (F2,54 = 0.04, P = .96). There was a significant drug effect (F1,54 = 5.0, P = .034).
Spielberger State Anxiety Inventory and Hamilton Depression Rating Scale
To examine whether depression and anxiety could have influenced the drug effect on the IS score, we performed a repeated-measures analysis of covariance for the Hamilton Depression Rating Scale scores and Spielberger State Anxiety Inventory scores (Table 3). Using the baseline measures as covariates, there was no significant drug effect, repeated-measures effect, or interaction effect.
Table 3. Behavioral Ratings (mean ± SD) During 12 Weeks of Fluoxetine or Placebo Treatment.
| Behavioral Rating | Baseline | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| Spielberger State Anxiety Inventory score | ||||
| Placebo (n = 10) | 44.6 ± 13.3 | 39.7 ± 14.2 | 41.7 ± 11.2 | 38.2 ± 13.9 |
| Fluoxetine (n = 10) | 36.9 ± 9.8 | 35.8 ± 11.4 | 39.5 ± 12.4 | 37.9 ± 9.9 |
| Hamilton Depression Rating Scale score | ||||
| Placebo (n = 12) | 12.8 ± 7.7 | 11.1 ± 7.6 | 11.4 ± 7.7 | 11.5 ± 7.2 |
| Fluoxetine (n = 10) | 5.2 ± 4.0 | 7.1 ± 5.5 | 8.3 ± 7.3 | 8.4 ± 4.6 |
Fluoxetine Levels
All perpetrators randomly assigned to receive fluoxetine had measurable drug levels with the following group means ± SDs for fluoxetine plus its metabolite norfluoxetine (ng/mL): week 4 = 129.8 ± 59.1 ng/mL, week 8 = 181.5 ± 90.4 ng/mL, and week 12 = 212.3 ± 116.1 ng/mL.
Spouse and Significant Other Evaluation
A repeated-measures analysis of variance for time points baseline and week 12 was performed with drug treatment as the between-groups factor. Both the nonphysical and physical Partner Abuse Scale ratings showed a significant time effect (F1,11 = 24.2, P = .0005 and F1,11 = 10.2, P = .009, respectively), with no significant interaction effect and no significant drug effect (Table 4).
Table 4. Scores on Partner Abuse Scale Completed by Spouses/Significant Othersa.
| Partner Abuse Scale Score | Beginning, mean ± SD | End, mean ± SD | F1,11 | P |
|---|---|---|---|---|
| Nonphysical (n = 13) | 83.8 ± 26.5 | 46.9 ± 14.0 | 24.2 | .0005 |
| Physical (n = 13) | 44.8 ± 16.7 | 28.0 ± 4.5 | 10.2 | .009 |
The designations “Beginning” and “End” represent the time points that the spouses/significant others answered the questionaires. Spouses'/significant others' testing dates were not always the same testing dates as the perpetrators'.
Safety and Tolerability
Fluoxetine was well tolerated. Only 2 perpetrators were maintained on less than 40 mg/d of fluoxetine. There were no serious adverse events.
Discussion
In this study, perpetrators of IPV who received fluoxetine, in addition to CBT and alcohol treatment, showed a greater reduction in the IS score on the MOAS than perpetrators who received just CBT and alcohol treatment. This decrease in the IS score indicates improvement in both the emotional (eg, feelings of anger, irritability, annoyance) as well as the behavioral aspects (eg, argumentativeness, shouting, loss of temper, and physical aggression) of IPV. Of note, this decrease in the IS score occurred in perpetrators who had minimal levels of depression, as measured by the Hamilton Depression Rating Scale, and who showed no significant change in their Spielberger State Anxiety Inventory scores during the 12-week study.
In this study, we recruited the perpetrators of IPV from a cohort of treatment-seeking alcoholics. Alcohol treatment programs potentially provide a large source of perpetrators of IPV, since 50%–70% of perpetrators of IPV have an alcohol-related diagnosis.2,3 Nonconfrontational structured interactions, consistent with the recommendations by Stuart et al,32 served to decrease their defensiveness and anxiety. Presenting the biologically based medical model (see Introduction and George et al14) to the perpetrators provided a nonthreatening means to confront their behavior and help them understand their overreactivity to perceived threats. In our experience, the model was universally well received by perpetrators and contributed to both a reduction in their need to project blame and a willingness to assume responsibility for their behavior and participate in IPV treatment. All of the perpetrators identified from our alcohol treatment facility freely and willingly enrolled in the study.
A major problem in IPV research and treatment is the high dropout rate found among alcoholic perpetrators. Previous studies show that there is a 40%–60% dropout rate even for court-ordered perpetrators.13 This dropout rate is consistent with the findings of a large study for depression involving a selective serotonin reuptake inhibitor, which also showed a high attrition rate, especially in patients with comorbid alcoholism, drug abuse, and anxiety disorders.33 Statistical analyses performed on our missing data suggest that the dropouts were best explained by relapse to alcoholic drinking. This is corroborated by the subset of perpetrators who relapsed and required readmission for stabilization. These perpetrators stated that relapse to alcohol drinking was a major factor in missed follow-up appointments. They reported that they became apathetic, demoralized, and embarrassed and stopped taking the medication when they relapsed. In future studies, patient retention may be improved by adding naltrexone or other similar medications shown to be effective in decreasing alcohol consumption.
The small number of significant others participating in the protocol typifies the difficulty of engaging significant others in the treatment of the perpetrators of IPV. Before the start of the study, a significant proportion of the significant others had separated from the perpetrators, in large part, because of their violence and alcoholism. Similar to the findings of previous studies,34 the significant others who remained with the perpetrators were very bitter and did not participate in conjoint therapy. In spite of their resentments, ratings by the significant others at week 12 showed that the perpetrators had significant improvements on both the verbal and physical aggression scales and suggest that a reduction in the perpetrators' IS scores is a valid surrogate marker for detecting a reduction in physical and nonphysical aggression in the home environment. This reduction in physical and nonphysical aggression represents contributions from alcohol treatment, CBT, and fluoxetine. A much larger study with a different design would be required to determine the contribution of each of these factors.
To our knowledge, this is the first controlled study examining the effects of a pharmacologic intervention to treat perpetrators of IPV. Our results show that alcoholic perpetrators who received fluoxetine, in addition to CBT and alcohol treatment, had a greater reduction in the IS score on the MOAS than perpetrators who received just CBT and alcohol treatment. Clinical interviews with the perpetrators at the end of the study substantiated the fact that those taking fluoxetine were less reactive to environmental stimuli and had “more time to think” before reacting to the environmental stimuli. Since anxiety and depression scores were the same under drug and placebo, the IS score changes were not due to differences in anxiety and depression. Because studies show that 50%–70% of perpetrators of IPV have an alcohol diagnosis, our findings, if replicated in a larger patient sample, have important implications for improving current treatments employed to treat perpetrators of IPV. Studies are now underway using functional magnetic resonance imaging to examine the effects of fluoxetine on brain function in perpetrators of IPV.
Acknowledgments
Special thanks to Kathryn Rice, BA, for many hours of collecting and entering data for this manuscript and Shellie-Anne Levy, BA, for assisting with data quality assurance. In addition, we are indebted to Vijay Ramchandani, PhD, and Markus Heilig, MD, PhD, for reviewing the manuscript. All acknowledged individuals are from the Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, Bethesda, Maryland, and they have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None reported.
Appendix 1. Partner Abuse Scale—Physical

Appendix 2. Partner Abuse Scale—Nonphysical
Footnotes
Drug names: fluoxetine (Prozac and others), naltrexone (Vivitrol, ReVia, and others).
Potential conflicts of interest: None reported.
Previous presentation: This study was presented in poster form at the National Institutes of Health Research Festival, October 6–9, 2009, Masur Auditorium, Natcher Conference Center, Bethesda, Maryland.
Publisher's Disclaimer: Disclaimer: The work was performed in accordance with the official duties as government employees, and, therefore, the contents of this manuscript are considered to be within public domain.
References
- 1.Kantor GK, Straus MA. The ‘drunken bum’ theory of wife beating. In: Straus MA, Gelles RJ, editors. Physical Violence in American Families. New Brunswick, NJ: Transaction Publishers; 1990. pp. 203–224. [Google Scholar]
- 2.Leonard KE, Blane HT. Alcohol and marital aggression in a national sample of young men. J Interpers Violence. 1992;7(1):19–30. doi: 10.1177/088626092007001002. [DOI] [Google Scholar]
- 3.Stuart GL, Moore TM, Kahler CW, et al. Substance abuse and relationship violence among men court-referred to batterers' intervention programs. Subst Abus. 2003;24(2):107–122. doi: 10.1080/08897070309511539. [DOI] [PubMed] [Google Scholar]
- 4.Brookoff D, O'Brien KK, Cook CS, et al. Characteristics of participants in domestic violence: assessment at the scene of domestic assault. JAMA. 1997;277(17):1369–1373. doi: 10.1001/jama.277.17.1369. [DOI] [PubMed] [Google Scholar]
- 5.Fals-Stewart W. The occurrence of partner physical aggression on days of alcohol consumption: a longitudinal diary study. J Consult Clin Psychol. 2003;71(1):41–52. doi: 10.1037/0022-006X.71.1.41. [DOI] [PubMed] [Google Scholar]
- 6.O'Farrell TJ, Murphy CM, Stephan SH, et al. Partner violence before and after couples-based alcoholism treatment for male alcoholic patients: the role of treatment involvement and abstinence. J Consult Clin Psychol. 2004;72(2):202–217. doi: 10.1037/0022-006X.72.2.202. [DOI] [PubMed] [Google Scholar]
- 7.Stuart GL, Ramsey SE, Moore TM, et al. Reductions in marital violence following treatment for alcohol dependence. J Interpers Violence. 2003;18(10):1113–1131. doi: 10.1177/0886260503255550. [DOI] [PubMed] [Google Scholar]
- 8.Giancola PR, Parrott DJ, Roth RM. The influence of difficult temperament on alcohol-related aggression: better accounted for by executive functioning? Addict Behav. 2006;31(12):2169–2187. doi: 10.1016/j.addbeh.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Parrott DJ, Giancola PR. A further examination of the relation between trait anger and alcohol-related aggression: the role of anger control. Alcohol Clin Exp Res. 2004;28(6):855–864. doi: 10.1097/01.alc.0000128226.92708.21. [DOI] [PubMed] [Google Scholar]
- 10.Eckhardt CI. Effects of alcohol intoxication on anger experience and expression among partner assaultive men. J Consult Clin Psychol. 2007;75(1):61–71. doi: 10.1037/0022-006X.75.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Feder L, Wilson DB. A meta-analytic review of court-mandated batterer intervention programs: can courts affect abusers' behavior? J Exp Criminol. 2005;1(2):239–262. doi: 10.1007/s11292-005-1179-0. [DOI] [Google Scholar]
- 12.Pence E, Paymar M. Education Groups for Men Who Batter: The Duluth Model. New York, NY: Springer-Verlag; 1993. [Google Scholar]
- 13.Babcock JC, Green CE, Robie C. Does batterers' treatment work? a meta-analytic review of domestic violence treatment. Clin Psychol Rev. 2004;23(8):1023–1053. doi: 10.1016/j.cpr.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 14.George DT, Phillips MJ, Doty L, et al. A model linking biology, behavior and psychiatric diagnoses in perpetrators of domestic violence. Med Hypotheses. 2006;67(2):345–353. doi: 10.1016/j.mehy.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 15.George DT, Hibbeln JR, Ragan PW, et al. Lactate-induced rage and panic in a select group of subjects who perpetrate acts of domestic violence. Biol Psychiatry. 2000;47(9):804–812. doi: 10.1016/S0006-3223(99)00300-5. [DOI] [PubMed] [Google Scholar]
- 16.George DT, Umhau JC, Phillips MJ, et al. Serotonin, testosterone and alcohol in the etiology of domestic violence. Psychiatry Res. 2001;104(1):27–37. doi: 10.1016/S0165-1781(01)00292-X. [DOI] [PubMed] [Google Scholar]
- 17.George DT, Rawlings RR, Williams WA, et al. A select group of perpetrators of domestic violence: evidence of decreased metabolism in the right hypothalamus and reduced relationships between cortical/subcortical brain structures in position emission tomography. Psychiatry Res. 2004;130(1):11–25. doi: 10.1016/S0925-4927(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 18.Marks GA, Speciale SG, Cobbey K, et al. Serotonergic inhibition of the dorsal lateral geniculate nucleus. Brain Res. 1987;418(1):76–84. doi: 10.1016/0006-8993(87)90964-4. [DOI] [PubMed] [Google Scholar]
- 19.Cools R, Calder AJ, Lawrence AD, et al. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology (Berl) 2005;180(4):670–679. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- 20.New AS, Buchsbaum MS, Hazlett EA, et al. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology (Berl) 2004;176(3–4):451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- 21.Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54(12):1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 22.Salzman C, Wolfson AN, Schatzberg A, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. J Clin Psychopharmacol. 1995;15(1):23–29. doi: 10.1097/00004714-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Fava M, Rosenbaum JF, McCarthy M, et al. Anger attacks in depressed outpatients and their response to fluoxetine. Psychopharmacol Bull. 1991;27(3):275–279. [PubMed] [Google Scholar]
- 24.Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3(2):S44–S51. [PubMed] [Google Scholar]
- 25.Hudson WW. Partner Abuse Scale. Tempe, AZ: Walmyr Publishing Company; 1990. [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 27.Straus MA. Physical Violence in American Families. New Brunswick, NJ: Transactions Publishers; 1990. Measuring Intrafamily Conflict and Violence. The Conflict Tactics (CT) Scales; pp. 29–47. [Google Scholar]
- 28.Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics Scales. J Marriage Fam. 1979;41(1):75–88. doi: 10.2307/351733. [DOI] [Google Scholar]
- 29.Spielberger CD, Gorsuch RL, Lushene RD. Test Manual of the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Statsoft, Inc. STATISTICA, version 7.1 (data analysis software system) Tulsa, OK: 2005. [Google Scholar]
- 32.Stuart GL, Temple JR, Moore TM. Improving batterer intervention programs through theory-based research. JAMA. 2007;298(5):560–562. doi: 10.1001/jama.298.5.560. [DOI] [PubMed] [Google Scholar]
- 33.Warden D, Trivedi MH, Wisniewski SR, et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry. 2007;164(8):1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 34.Office of Justice Programs. National Institute of Justice. Batterer Intervention Programs: Where Do We Go From Here? [February 13, 2008];NCJ195079. 2003 :1–29. http://www.ncjrs.gov/pdffiles1/nij/195079.pdf.


