Abstract
Both DNA and RNA can serve as powerful building blocks for bottom-up fabrication of nanostructures. A pioneering concept proposed by Ned Seeman 30 years ago has led to an explosion of knowledge in DNA nanotechnology. RNA can be manipulated with simplicity characteristic of DNA, while possessing noncanonical base-pairing, versatile function and catalytic activity similar to proteins. However, standing in awe of the sensitivity of RNA to RNase degradation has made many scientists flinch away from RNA nanotechnology. Here we report the construction of stable RNA nanoparticles resistant to RNase digestion. The chemically modified RNA retained its property for correct folding in dimer formation, appropriate structure in procapsid binding, and biological activity in gearing phi29 nanomotor to package viral DNA and producing infectious viral particles. Our results demonstrate that it is practical to produce RNase resistant, biologically active and stable RNA for application in nanotechnology.
Keywords: 2’-F modification, pRNA, RNase resistant, dimer formation, phi29 DNA-packaging nanomotor
Living organisms produce a wide variety of highly-ordered or patterned structures such as smart nanomachines and elegant arrays that are made up of macromolecules to perform diverse biological functions. RNA and DNA share certain common features via their unique properties of strand complementarities and self-assembly, which can serve as powerful building blocks for bottom-up fabrication of nanostructures and nanodevices. A pioneering concept introduced by Ned Seeman 30 years ago has led to an explosion of knowledge in DNA nanotechnology.1–3 RNA can be manipulated with simplicity characteristic of DNA, while possessing noncanonical base-pairing, versatile function, and catalytic activity similar to proteins. Typically, RNA molecules contain a large variety of single-stranded stem-loops for inter- and/or intra-molecular interactions. These loops can serve as mounting dovetails, and thus, external linking dowels might not be needed in fabrication and assembly.4–8
Although the concept of RNA nanotechnology has been developed for more than ten years6,9–13 (for review, see14), standing in awe of RNA to RNase degradation has made many scientists hesitant to apply RNA nanotechnology. The popularity in the study of RNA nanostructure has emerged only recently as reflected in the fact that 90% of publications in RNA nanostructure were published on or after the year of 2006. However, RNA instability and degradation has continued to significantly hinder the advancement of this field.
Discovery of siRNA has provided another venue for RNA–mediated therapeutics.15 Several RNA-based therapeutic approaches using small interfering RNA (siRNA),16–20 ribozymes21–24 and anti-sense RNA25,26 have been shown to down regulate specific gene expression in cancerous or viral-infected cells. Recent discoveries of novel functionality of small regulators or noncoding RNA such as ribozyme, tRNA, mRNA, rRNA, snoRNAs, microRNAs, siRNAs, piRNAs, snRNA, SmY RNA, scaRNA, gRNA, Y RNA, telomerase RNA, and riboswitch also appears as a result of the expanding interest for the RNA field.27–30 Although gene silencing with high efficacy and specificity has been achieved in vitro, the effective use of RNA as a therapeutic in clinical application remains challenging due to degradation of RNA in serum after entering the body.
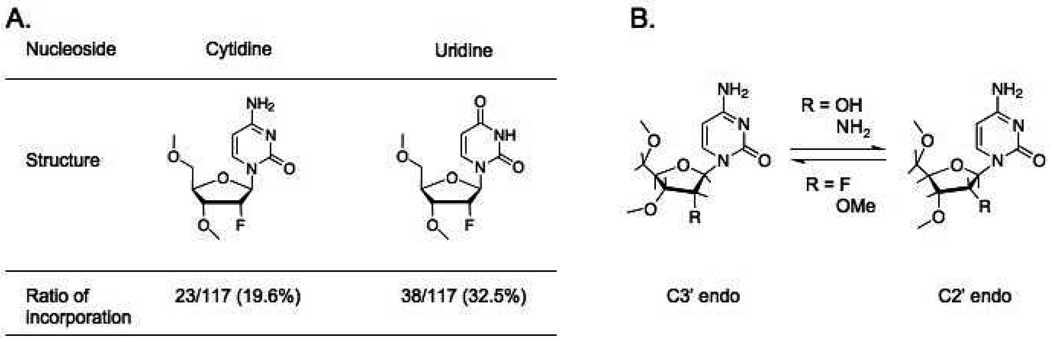
Recently, it was reported that 2’-modifications can enhance the serum stability of siRNA31,32 without compromising double-helix formation and the effect of siRNA in gene silencing.33–39 Earlier studies showed that, while the A-form helix is very important, the 2’-OH is not required for siRNA activity40. Most studies on 2’-hydroxyl group modification38,39,41–43 (Fig. 1A) focused on double stranded siRNA.33–36,44 Even though the use of 2’-modifications in siRNA gene silencing studies has been popular and 2’-deoxy-2’-fluoro modification (2’-F) has also been applied to SELEX,45,46 their effects on the folding and biological function of modified RNA related to the unique properties of RNA, which involve non-canonical base pairing, have not been demonstrated.
Figure 1.
Structure of the 2’-modified ribonucleotides. (A) Location of modification and ratio of incorporation in the pRNA Aa’ sequence. (B) Illustration of the equilibrium between the C2’ endo and C3’ endo conformation of the sugar ring following the 2’ substituent.
2’-F modification has been demonstrated to support the C3’ endo conformation of the sugar ring that plays a role in the formation of A-form configuration in RNA (Fig. 2B).47 This explains why 2’-F modification enhances the stability of RNA/RNA duplex as long as the annealing temperature (Tm) is monitored. Besides the alternation of ribose structure, which is involved in ribonuclease action, the indirect resistance against nuclease or base degradation of the phosphodiester bond is also correlated with its potential to increase the duplex stability.48
Figure 2.
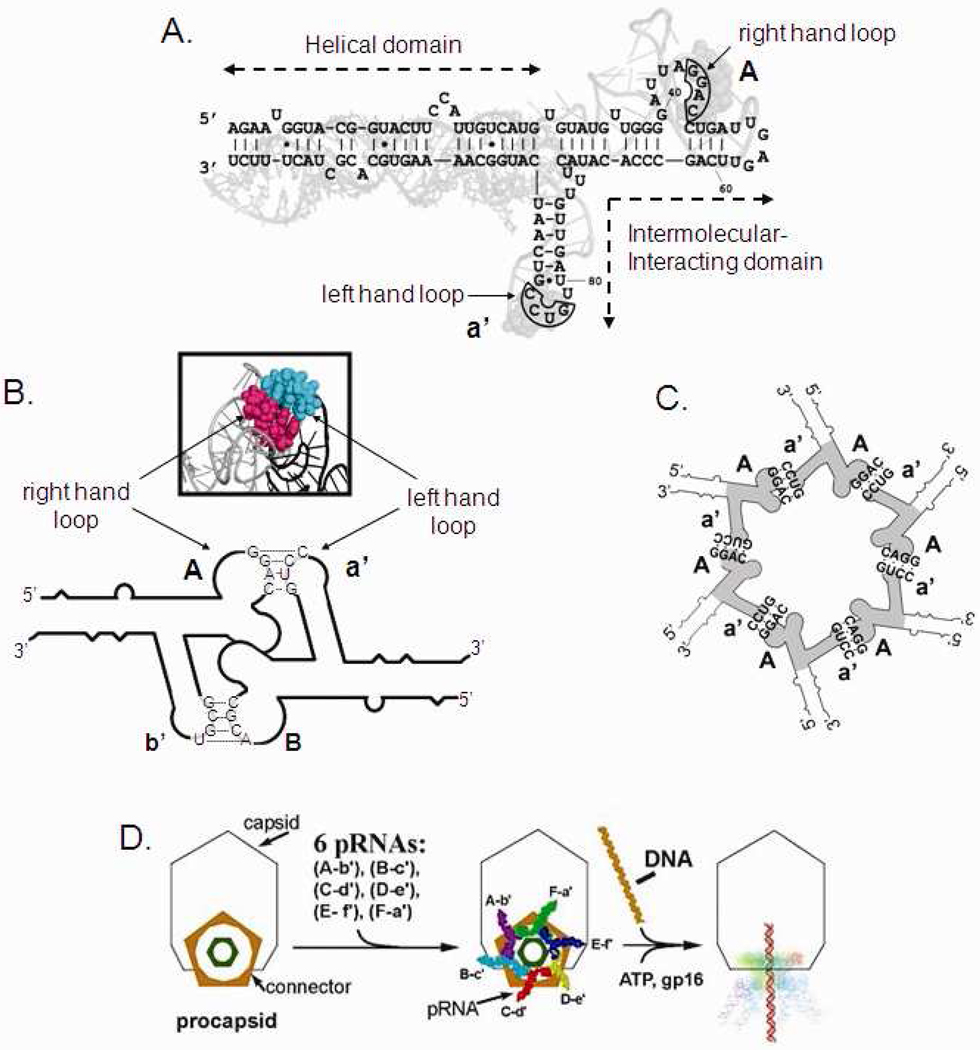
Sequence and secondary structural of phi29 DNA-packaging RNA (pRNA). Upper-case letters represent the upper loop and lower-case letters the lower loop of pRNA. A pair of upper and lower case for a same letters indicate a pair of complementary loops, whereas a pair of upper and lower case from different letters indicate non-complementary loops. For example, pRNA Aa’ refers to a pRNA with complementary right loop A (5’G45G46A47C48) and left loop a’ (3’C85C84U83G82), which can form homo-dimer by itself. pRNA Ae’ containing non-complementary upper loop A (5’G45G46A47C48) and lower loop e’ (3’C85G84G83U82) can form hetero-dimer with pRNA Ea’ containing left-hand loop a’ (3’C85C84U83G82) and right-hand loop E (5’G45C46C47A48). (A) Superposition of the primary and secondary structure of the pRNA Aa’. The four bases in the right and left loops, which are responsible for inter-RNA interactions are boxed. (B) Diagram depicting pRNA dimer formation and 3D model of the hand-in-hand interaction. (C) pRNA hexamer. (D) Packaging of phi29 DNA through the motor geared by six pRNA.
Though the improved nuclease resistance provided by 2’-modification is now well established, there is still a discrepancy about the effect of such modifications on the activity of chemically modified siRNA. Some reports demonstrate that 2’-modification of the sense strand generates a stable complex, that retain gene silencing function.33–37 Other reports show that a large number of 2’-modifications (in either strand) decreases siRNA activity.39 The mechanism of siRNA action involves the unwinding of the siRNA in the RISC complex and the binding of the antisense strand to the mRNA.49 It is expected that due to requirement in siRNA double strand formation, 2’-modifications on the sense strand and not the antisense strand did not affect helix formation and protects the siRNA from degradation in vivo during the delivery process, thus enhancing the siRNA function. However, the escort of the siRNA from degradation during in vivo transportation would not justify a conclusion that 2’modification does not affect the protein/RNA interaction in the RISC complex, since the sense strand is not present in the protein/RNA complex. Others have shown that 2’-modification of the antisense strand also resulted in a gene silencing effect. However, all of these studies used only 50% (U and C, not G and A) of 2’-modified nucleotide, the discrepancy might be due to the case by case effect, since some locations of modification might affect the protein/RNA interaction, while others locations might not.
All previous studies on 2’-modification of siRNA only proved that 2’-modifications increase the thermal stability of the helical structures of the double-stranded RNA. The question of whether the 2’-modified RNA fold correctly as wild type RNA that is unique in noncanonical nucleotide interaction and if the 2’-modified RNA retained biological function has not been reported. To address these questions, we synthesized 2’-F modified RNA to study the effect of such modifications on the RNA structure, folding, and biological function; taking advantage of our highly sensitive in vitro bacteriophage phi29 virion assembly assay.
The bacteriophage phi29 DNA-packaging nanomotor50 is comprised of 1) a central protein core,51,52 2) six copies of packaging RNA (pRNA),9,10,53,54 and 3) an energy conversion enzyme gp16 (Fig. 2). The central core of the motor is called the connector, containing a dodecamer channel that acts as a path for the translocation of dsDNA (Fig. 2D). The pRNA forms dimers via the inter-RNA interaction with one pair of the right and left hand interlocking loops (Fig. 2A, B). The dimer configuration is the building block in hexamer formation9,10,55–57 (Fig. 2C). The unique features of the pRNA9,53 of bacteriophage phi29 DNA packaging motor to form dimers, trimers, hexamers, and patterned superstructures via loop-loop interactions,9,10,55–57 make it a promising vehicle for escorting therapeutics such as siRNA for targeting to specific cancer or viral infected cell.7,8,14,58–60
Here we report the production of highly stable pRNA molecules, even in the presence of fetal bovin serum (FBS), through the 2’-modification. The 2’-modified pRNA was synthesized by in vitro transcription with Y639F mutant T7 RNA polymerase61–63 in the presence of 2’-modified pyrimidine. Our data illustrated that incorporation of 2’-F modified pyrimidine in the pRNA sequence didn’t affect its folding for dimer formation and motor binding. The resulting modified pRNAs retained biological function close to its non-modified counterpart in gearing the phi29 DNA-packaging nanomotor and generated the infectious virion even in the presence of high concentration of RNase.
Results and discussion
The effects of pyrimidine modifications with 2’-F derivates were evaluated using a highly sensitive phi29 DNA packaging and virion assembly system. The phi29 packaging RNA (pRNA) forms a hexameric ring through inter-RNA interactions to gear the bacterial virus phi29 DNA packaging motor via defined steps of conformational changes (Fig. 2). The six pRNAs form a hexagon via intermolecular base-pairing between the right loop (bases 42–45) and the left loop (bases 82–85) (Fig. 2B).9,10,57,64–66 pRNA dimers are the building blocks of hexamers10,67 (Fig. 2C), with the pathway for building a hexamer being: dimer→tetramer→hexamer.67,68 Phylogenetic analyses of pRNAs69 from several phages show few conserved bases but similar secondary structures55 and single base mutation in the helical domain renders the pRNA inactive,70 suggesting the high specificity of pRNA structure. The indicators for assessing RNA folding, structure, and function is dimer formation, procapsid binding, and pRNA biological activity in DNA packaging and phi29 virion assembly.
pRNA dimer formation is not affected by 2’-F modification
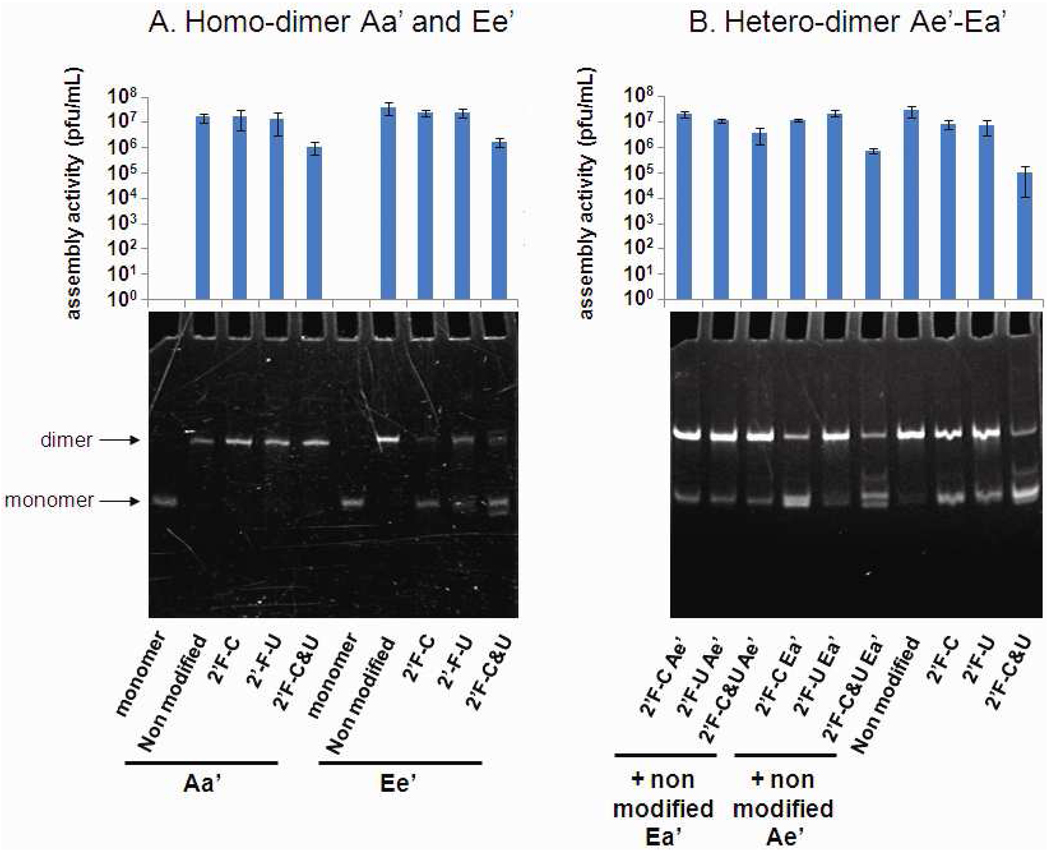
Since dimer formation relies on correct folding of individual RNA subunits and the chemical structure of these two loops for interaction, the efficiency in dimer formation could serve as a parameter to evaluate the effect of 2’-modification in RNA folding, structure, and inter-RNA interactions. In this study, both homo-dimers and hetero-dimers were evaluated. Homo-dimers are composed of two identical pRNA subunits such as pRNA Aa’ or Ee’. Heterodimer is composed of two different interlocking pRNA such as pRNA Ae’ and Ea’ (see Fig. 2). Our results indicate that pRNAs with 2’-F pyrimidine modification were competent in pRNA dimer formation, with only C or U modified or both C and U modified (Fig. 3). In addition, their migration profile in native PAGE gel was found to be very similar to their unmodified counterpart, which also indicates proper folding (Fig. 3).
Figure 3.
Native PAGE gel showing the formation of dimer nanoparticle of pRNA and histogram of the virion assembly activities associated to these nanoparticle (A) homodimer Aa’ and Ee’. (B) Hetero-dimer Ae’/Ea’.
2’-F modification of pRNA has minimal impact on phi29 virion assembly
1. Viral assembly of 2’-F pRNA homo-dimer
Aa’ and Ee’ pRNA homo-dimers that are either 2’-F-C or 2’-F-U modified exhibited 107 pfu/ml activity, which was comparable with non-modified Aa’ and Ee’ homo-dimers. Also, 2’-F-C&U modified homo-dimers displayed an assembly activity around 106 pfu/ml (Fig. 3A).
2. Viral assembly of 2’-F pRNA hetero-dimer
To further verify the modification’s impact on pRNA functional activity, various combinations of Ae’ and Ea’ monomers with and without modification were analyzed by the in vitro assembly system. Hetero-dimers formed by a non-modified monomer and monomer with 2’-F-C or 2’-F-U pyrimidine modified Ae’ or Ea’ had comparable activity to non-modified hetero-dimer at 107 pfu/ml. The same observation has been made when both monomers were 2’-F-C or 2’-F-U modified. Hetero-dimers with one 2’-F-C&U modified monomer and one non-modified monomer also had an activity of 106–107 pfu/ml. A slight decrease in virion assembly activity (105 pfu/ml) was observed in the case of hetero-dimer formed with two 2’-F-C&U monomer Ae’ and Ea’.
The number of plaque-forming units per milliliter (pfu/mL) produced in the viral assembly assay70–72 was used as a measure of the biological activity of the modified pRNA which is in direct correlation with the retention of their correct folding. Furthermore, homo-dimers and hetero-dimers formed by two 2’-F-C&U modified monomers displayed only a slight decrease in virion assembly activity despite their high incorporation of modified ribonucleotides. In our model, such virion assembly activities are indicative of fully functional and correctly folded pRNA molecule.
2’-F modified pRNA Aa’ remains stable in presence of RNase A or fetal bovin serum
Phi29 pRNA made of natural nucleotides is sensitive to degradation in vivo and in vitro by RNases or serum, which contains RNA degradation enzymes. Such instability limits its potential to be used as a building block in bottom-up therapeutic nanoparticle assembly.6,56
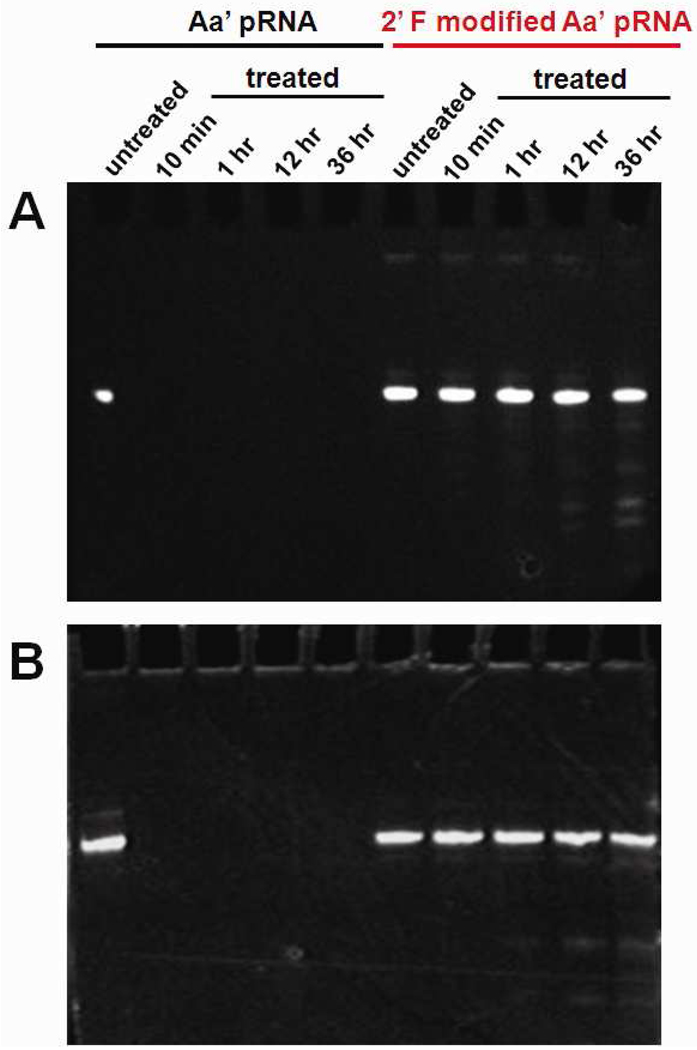
We examined the stability of different 2’-F modified pRNAs Aa’ (2’-F-C, 2’-F-U and 2’-F-C&U) in the presence of RNase A or in the presence of fetal bovin serum (FBS), which mimics in vivo conditions (Fig. 4). Although 2’-F-C and 2’-F-U didn’t show a significant increase in stability in presence of either RNase A or FBS (Supplementary Figure S1), 2’-F-C&U modified pRNAs demonstrated resistance to both RNase A and FBS digestion for up to 16 hours of incubation (Fig. 4).
Figure 4.
Urea-PAGE denatured gel showing the stability between non-modified pRNA Aa’ and 2’-F-C&U pRNA Aa’ after incubation at different time point in the presence of (A) RNase A (1 mg/ml), and (B) fetal bovine serum (10%).
2’-F modifications have been demonstrated to support the C3’ endo conformation of the sugar ring that play a role in the formation of A-form configuration in RNA. This explains why 2’-F modification did not interfere or even enhance the stability of RNA/RNA duplex as long as the Tm is concern. Besides the alternation of ribose structure, which is involved in ribonuclease action, the indirect resistance against nuclease of the phosphodiester bond is often correlated with the potential of 2’-modified nucleotide to increase the duplex stability.48 Our data suggests that both 2’-F-U and 2’-F-C are required to achieve enhanced improvement in RNase resistance. 2’-F nucleosides were able to be incorporated at a high ratios (2’F-C&U) in the pRNA sequence by the mutant Y639F of the T7 RNA polymerase,61–63 leading to highly resistant pRNA even in the presence of FBS. Furthermore, regarding to the slight decrease in virion assembly activity discussed above, such a high ratio of 2’-F nucleotide incorporation appears not to be detrimental for the pRNA function or folding. For these reasons, 2’-F-C&U-pRNA was identified as our best option to provide RNase resistant pRNA that can be used to gear the phi29 DNA packaging nanomotor or as building block for nanoparticle assembly. Thus, our study proceeded using only 2’-F-C&U modified pRNA.
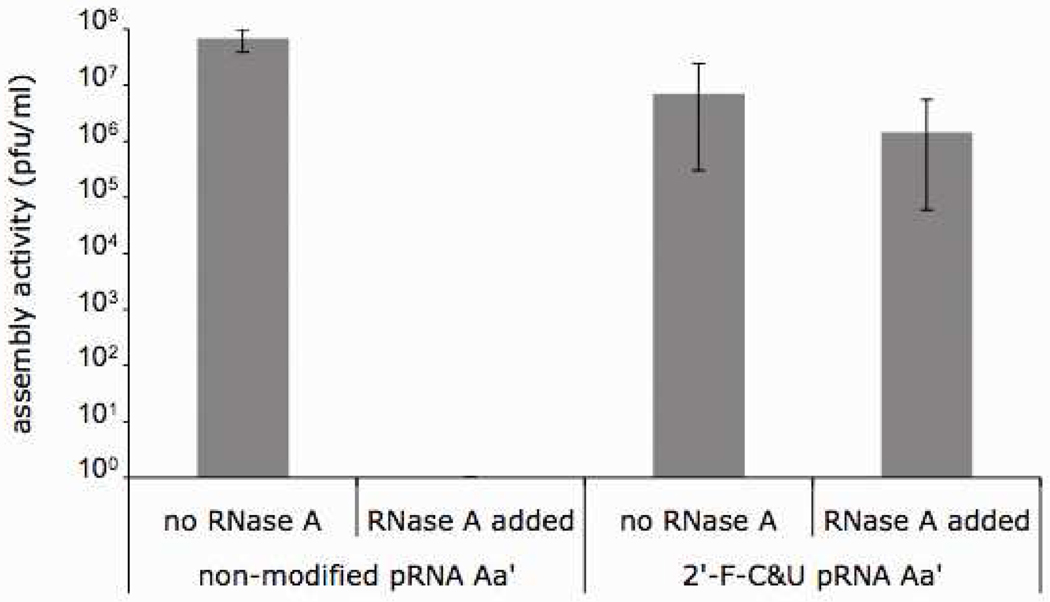
Activity of the 2’F-C&U-pRNA in RNase environment
The stability of 2’-F modified pRNA was further tested by an in vitro viral assembly. In the presence of RNase A, the activity of non-modified pRNA was completely abolished. However, the 2’-F-C&U modified pRNA retained most of the assembly activity (106 pfu/ml in the presence of RNase A, as compared to 107 pfu/ml in the absence of RNase A) (Fig. 5). This results support the conclusion that 2’-F-C&U modification renders pRNA with RNase A resistance while not interfering with the appropriate folding and biological function of pRNA.
Figure 5.
Assembly activities of the 2’-F-C&U pRNA Aa’ and its non-modifed counterparts in the presence or absence of RNase A.
Effect of pRNA 2’-F modification in DNA packaging
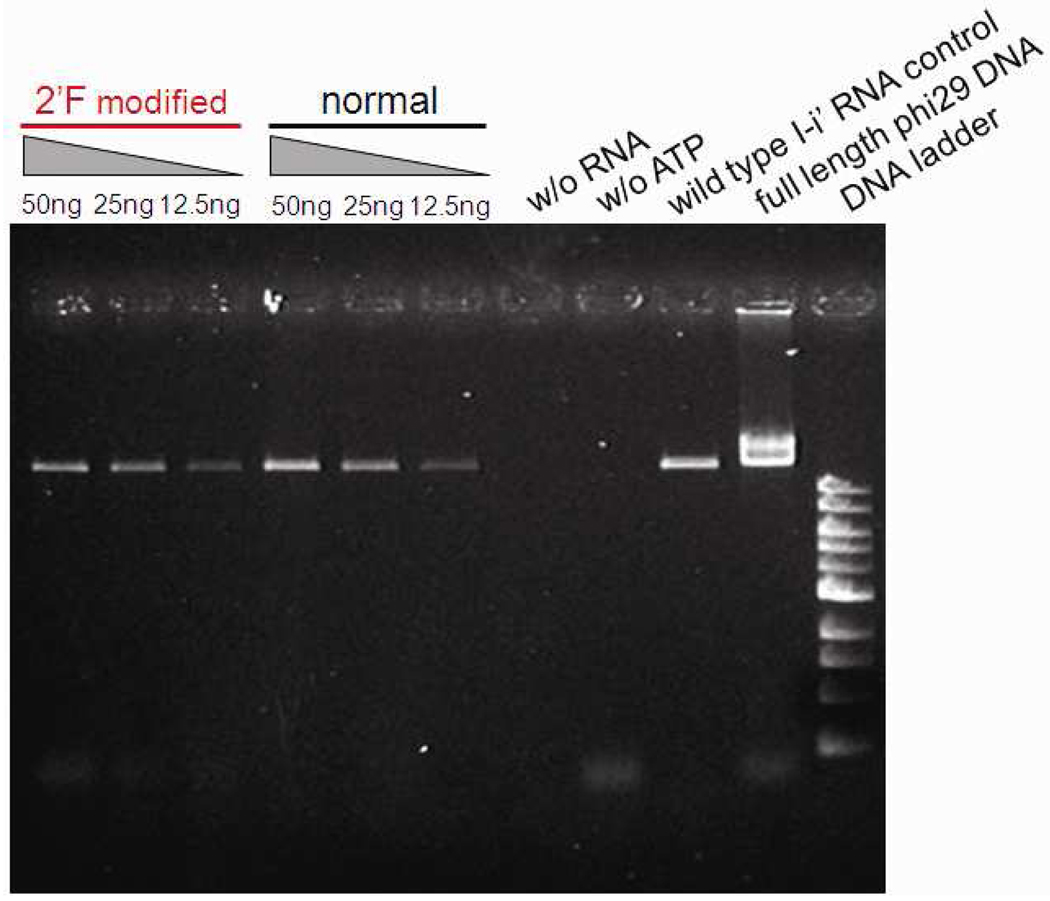
We also compared DNA packaging efficiencies between 2’-F-C&U pRNA and non-modified pRNA with a phi29 DNA packaging assay. 1% agarose gel electrophoresis showed that DNA packaging activity mediated by 2’F-C&U pRNA was dose-dependent, and the packaging efficiency was similar to or slightly lower than that of non-modified pRNA (Fig. 6). This indicated that 2’-F-C&U pRNA retained correct folding and was competent in DNA packaging.
Figure 6.
Agarose gel showing the efficiency of the 2’-F-C&U pRNA Aa’ in DNA packaging compared to its non-modified counterparts.
Characterization of 2’F-C&U pRNA: pH stability, sucrose gradient sedimentation, titration of the dimer formation, and atomic force microscopy (AFM)
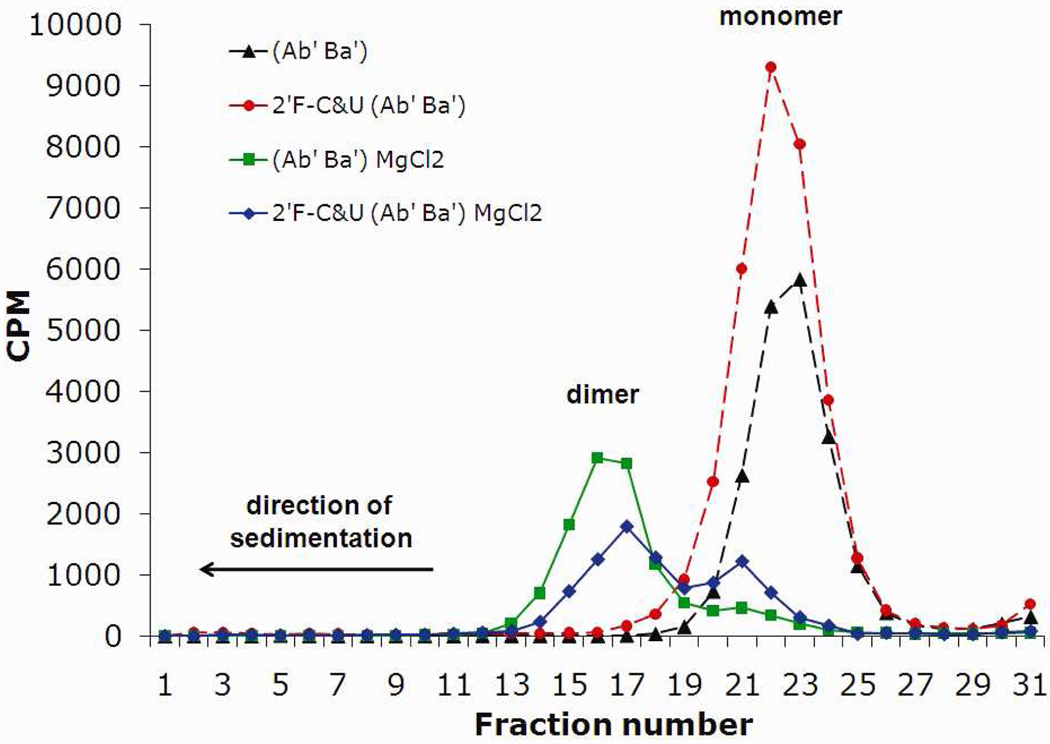
The effect of divalent metal ions and pH of the solution on the dimer formation of 2’F-C&U pRNA molecules was investigated. Sucrose gradient sedimentation of non-modified pRNA revealed a peak at fraction number 22, representing the monomer67 in the absence of divalent metal ions (Fig. 7). In the presence of MgCl2, the peak was shifted to the fraction number 16 corresponding to the dimer67 with a higher molecular weight (pRNA dimer, Fig. 5). The same profile was obtained with 2’-F-C&U pRNA, except that the dimer formation efficiency was reduced slightly (Fig. 5). This data suggest that the incorporation of 2’-F modification slightly decreased the stability of the resulting pRNA dimer complex. Previously, it was demonstrated that pRNA dimer formation was not exclusive to the presence of Mg2+ but could also occur in the presence of other divalent metal such as Sr2+ and Mn2+.67 Interestingly, we also found that Mn2+ and Sr2+ led to the formation of dimer of 2’-F-C&U pRNA (Supplementary Figure S2), but this was not further investigated.
Figure 7.
Sucrose gradient sedimentation analysis of the formation of [3H]pRNA dimer in presence or absence of MgCl2.
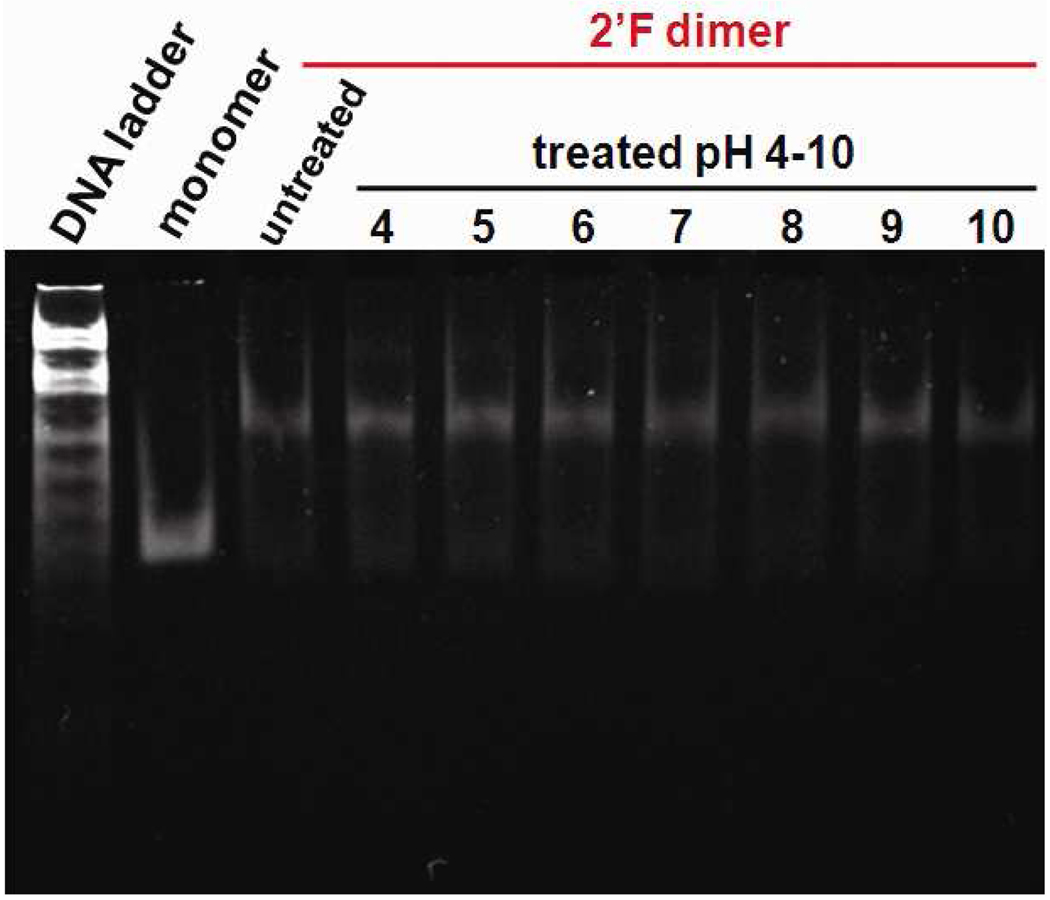
RNA molecules generally hydrolyze in basic environments due to the 2’-OH, which acts as a nucleophilic moiety to attack the phosphodiester backbone. The alkaline hydrolysis of RNA requires a linear geometry between the 2’-OH and the phosphate group, which is prevented by the 3’-endo sugar pucker of helical region.48 It has been previously reported that pRNA molecules present an unusual stability in a broad range of pH from 4 to 10 due to its tight and stable folding.6,56 The stability of 2’-F-C&U pRNA dimer particles was also explored in a similar range of pH from 4 to 10. In native PAGE, no lower bands, relating to partial hydrolysis of the modified pRNA, were found indicating that the 2’-F-C&U-pRNA is highly stable, even in a basic environment, up to pH 10 (Fig. 8). Moreover, the dimer particle didn’t dissociate in these conditions indicating that the 2’-F-C&U pRNA retained its self-assembling property in this broad range of pH (Fig. 8).
Figure 8.
Native PAGE gel showing the stability of the 2’-F-C&U pRNA Aa’ homo-dimer after incubation at different pH ranging from 4 to 10.
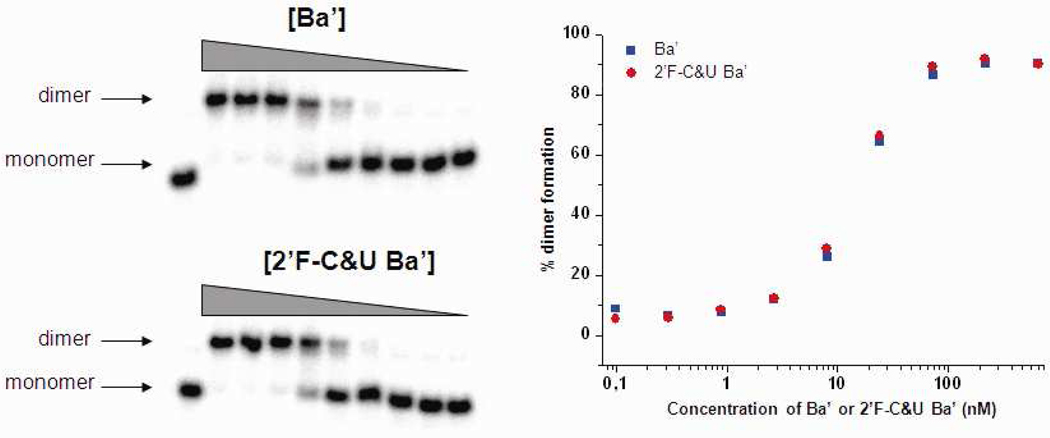
To continue the characterization of 2’-F-C&U pRNA properties, association constants relative to the dimer formation of [32P]pRNA Ab’ monomer and cold 2’-F-C&U or non-modified pRNA Ba’ were compared (Fig. 9). A plot of the dimer formation with radiolabelled non-modified pRNA Ab’ function of the concentration of 2’-F modified and non-modified pRNA Ba’ showed exactly the same profile and revealed an association constant between 24 nM and 8 nM for both. This data suggests that 2’-F-C&U pRNA preserved the dimer formation property of pRNA. Consequently, the decrease in stability observed during sucrose gradient sedimentation should not be attributed to a decrease in affinity for hand-in-hand recognition and dimer formation, but rather to a slight increase of the dissociation constant.
Figure 9.
Titration of dimer formation between [32P] prNA Ab’ and cold 2’-F-C&U and non-modified pRNA Ba’ (0.1, 0.3, 0.9, 2.6, 8, 24, 72, 216 and 650 nM). (A) Native PAGE gel. (B) Plot showing the percentage of dimer formation function of the concentration of cold 2’-F-C&U and non-modified pRNA Ba’.
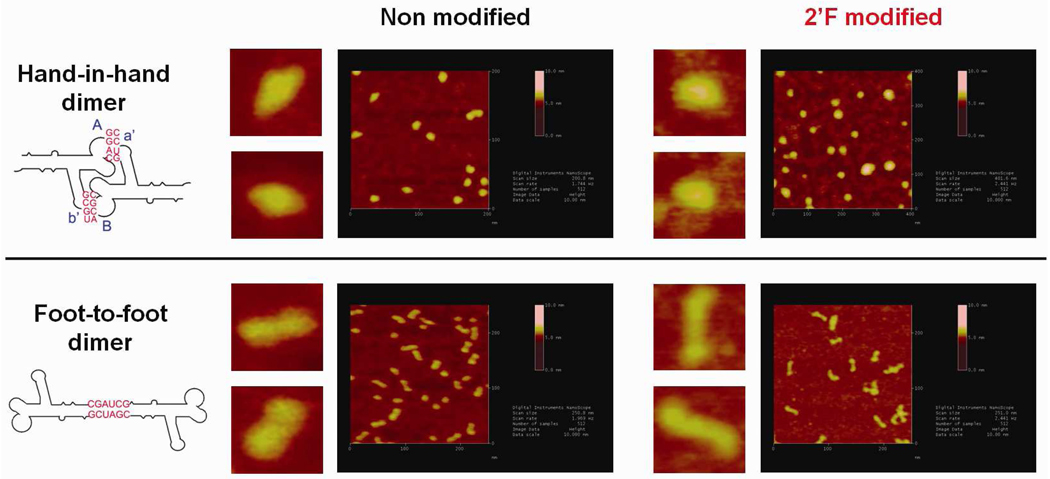
Finally, the shape of dimer nanoparticles made of 2’-F-C&U pRNA have been visualized by atomic force microscopy (AFM, Fig. 10). 2’-F-C&U hand-in-hand or 2’F-C&U foot-to-foot dimer nanoparticles imaged by AFM showed a similar shape comparatively to the respective nanoparticles prepared with non-modified pRNA. This data showed that 2’-F-C&U pRNA can be used as an efficient building block for nanoparticle assembly, while preserving the original shape of the non-modified pRNA.
Figure 10.
Atomic force microscopy images showing hand-in-hand and foot-to-foot dimer nanoparticle of non-modified pRNA and 2’-F-C&U pRNA respectively.
Since ribose 2’-OH group frequently plays critical roles (hydrogen-bonding and metal ion-coordination) in 3D structure formation of functional RNAs, it is surprising that pRNA retains biological activity even after substitution of its C&U with their 2’-F derivatives. Although, there is still a discrepancy considering fluorine can establish hydrogen-bonding, it is generally admitted that fluorine has a lower capacity to form a hydrogen bond than oxygen.73 It would be interesting to know the effect of 2’-F substitutions, on the 3D structure of the pRNA monomer (Fig 1A), dimer and hexamer (Fig 1D). However, without studies by X-ray diffraction or NMR, the 3D structure of the modified pRNA remains elusive. In this study, we used biological activity assay of the pRNA (dimer formation, virion assembly, and DNA packaging) as an indicator of the effect of 2’-F substitution on the global structure of pRNA. Moreover, 2’-F-C&U modified pRNA retains its ability to form a dimer in the presence of Mg2+, which demonstrates that 2’-F modified pRNA retains the capacity of metal ion-coordination to a certain extent. Besides the considerations in terms of hydrogen-bonding and metal ion coordination, the 2’-OH plays an important role by inducing a steric hinderance that gives RNA its specificity in terms of helical conformation (A-form), and the 2’-F substitution has already been reported to favor the A-form conformation.73,74 Our results suggest that maintaining the A-form helical conformation is more important for pRNA activity than the chemical properties of the 2’-group.
Conclusion
The presence of 2’-F modification did not affect the folding and self-assembling property of pRNA in the presence of divalent ions. The modified pRNA was stable between pH 4 and 10, and resistant to degradation by RNase A or other degradation enzymes in FBS. 2’-F-C&U modified pRNA molecule was also demonstrated to efficiently gear the phi29 DNA-packaging nanomotor for DNA packaging and virion assembly, even in the presence of high concentration of RNase. In conclusion, RNase resistant and biologically active stable RNA can be constructed by chemical modification for application in nanotechnology.
Experimental procedure
In vitro synthesis of chemically modified pRNAs
Chemically modified pRNAs were synthesized following our previously described method for production of pRNA mutant75 with some modifications. Briefly, linear plasmid DNA was used as template to generate PCR DNA fragments with the appropriate primer pair (TAATACGACTCACTATTAGAATGGTACGGTACTTCC and AGAAAGTAGCGTGC). The purified PCR DNA fragments were used as templates to synthesize the pRNA Aa’ by in vitro transcription using Y639F mutant T7 RNA polymerase in a final volume of 20 µl containing 40 mM Tris-acetate pH 8.0, 5 mM DTT, 1 mM EDTA, 10 mM Mg-acetate, 0.5 mM MnCl2, 8 mM spermidine, 2 µg of DNA template, 2’-F modified dCTP and/or dUTP (each at 5 mM final, Trilink), ATP, and GTP (each at 5 mM final). After overnight incubation at 37°C, the reaction was terminated with the addition of 1 µl of RNase free DNase I (1mg/ml, Sigma). Chemically modified pRNAs were then resolved in 8% polyacrylamide gel with 8M urea. The corresponding bands were excised and eluted from the gel over 1.5 h at 37°C in the elution buffer (0.5 M NH4OAC, 0.1 mM EDTA, 0.1% SDS, and 0.5 mM MgCl2). pRNAs were then precipitated overnight at −20°C after addition of 2.5 X volume of 100% ethanol and 1/10 volume of 3 M NaOAc (pH 5.2). The precipitate was pelleted by centrifugation (16500 × g, 10 min), washed with 70% ethanol, and dried by speed vacuum. Finally, dried RNA was resolved in 0.05% DEPC aqueous solution to constitute our stock solution of pRNA.
Native TBM PAGE for dimer detection
Stock solutions of pRNA were diluted in TBM buffer (89 mM Tris, 200 mM Boric acid, 5 mM MgCl2, pH 7.6) to achieve a final concentration of 30 µg/ml. Equal molar ratio of each pRNAs was applied to study the formation of dimers, while keeping the total amount of pRNA constant in each lane. 12% native polyacrylamide gels were prepared in TBM buffer. 5 mM Mg2+ was included in all buffers to maintain the folding of pRNA and the formation of dimers.67,76 After running at 4°C for 3 hrs, gels were stained with ethidium bromide and images were captured with an Eagle Eye II system (Stratagene).
RNA activity assay with the highly sensitive phi29 assembly system
The activity of pRNAs was assayed with the highly sensitive phi29 in vitro assembly system71,72,77. The purification of procapsids,78,79 gp16,80 DNA-gp3, and the preparation of neck and tail protein extracts has been described previously.71,72,77 In vitro phi29 assembly was performed as described previously71. Briefly, 10 µl of purified procapsid (0.013 µM) was dialyzed on a 0.025-µm VS filter membrane (Millipore Corp.) against TBE buffer (89 mM Tris, 200 mM Boric acid, 2 mM EDTA, pH 7.6) for 15 min at RT. 1.5 µl of TMS buffer (50 mM Tris, 100 mM NaCl, 10 mM MgCl2, pH 7.8) was added to stock solution of pRNAs (50ng/µL) to provide Mg2+ necessary for the dimer formation. Procapsid solution was added to each pRNA solution and the mixtures were further dialyzed against TMS for 30 min. The pRNA-enriched procapsid solution was then mixed with gp1680,81 and DNA-gp371 in the reaction buffer (10 mM ATP, 6 mM 2-mercaptoethanol, 3 mM spermidine in TMS) to initiate DNA packaging. After 30 min of incubation, neck, tail, and morphogenic proteins72,77 were added to the DNA packaging reactions to complete assembly of infectious virions. The activity of each pRNA was measured solely based on the number of plaque formations per unit (pfu/ml) produced in the viral assembly assay.70–72
Stability assay in presence of RNase
In a final volume of 20 µl, 2 µg of non-modified and 2’-F-C&U pRNA Aa’ were incubated at 37°C in RMPI-1640 medium (Gibco) containing either Fetal Bovin Serum (Gibco) or RNase A (RPA Grade, Ambion) at a final concentration of 10% and 1 mg/ml respectively. Aliquots (4 µl) were taken at multiple time points (0, 10 min, 1 h, 12 h, and 36 h) and ran for 2 h at RT in 8% urea PAGE in TBE buffer. After running, gels were stained by ethidium bromide and images were captured with an Eagle Eye II system (Stratagene).
In vitro phi29 DNA-packaging
The in vitro DNA packaging efficiency of phi29 virion in presence of pRNA, ATPase gp16, phi29 DNA-gp3, procapsid, and with supply of final 1 mM ATP in TMS buffer has been assayed as previously described.71,72 Briefly, the 10 µl of purified procapsid (0.13 µM) were mixed with pRNA and dialyzed on a 0.025-µm VS filter membrane (Millipore Corp.) against TBE buffer for 15 min at RT and then against TMS for another 30 min. The pRNA-enriched procapsid solution were then mixed with gp16,80,81 DNA-gp3,71 and ATP reaction buffer to initiate the DNA packaging step. After 1 hr, the packaging mixture was treated by 1 µl of 1 mg/ml DNase (Worthington Biochemical Co.) at RT for 20 min, followed by the treatment of 1 µl 0.5 M EDTA at 75°C for 15~20 min, 0.5 µl 0.1 mg/ml RNase A (RPA Grade, Ambion) at RT for 10~20 min and 1 µl 20 mg/ml Proteinase K at 55°C for 1 h. The final reaction mixture was assayed by 0.8% agarose electrophoresis at 100 V for 1 h. After running, gels were stained by ethidium bromide and images were captured with an Eagle Eye II system (Stratagene).
Effect of pH on 2’-F-C&U pRNA dimer formation
About 250 ng of 2’-F-C&U pRNA Aa’ were incubated in Phosphate-Citrate buffer at different pH values (4, 5, 6, 7, 8, 9, and 10) for 5 minutes at room temperature and then ran in a native PAGE in TBM buffer. After running, the gel was then stained by ethidium bromide and imaged with an Eagle Eye II system (Stratagene).
Dimer formation of 2’-F RNA in various conditions detected by sucrose gradient
Linear 5–20% sucrose gradients were prepared in TB buffer (89 mM Tris, 200 mM Boric acid, pH 7.6), with the addition or not of 10 mM MgCl2 (Fischer). The pRNA mixtures were then loaded onto the top of the gradient. Samples were spun at 45,000 rpm for 14.5 h at 4 °C in a SW55 rotor. After sedimentation, fractions were collected and subjected to scintillation counting (Packard 1900TR Liquid Scintillation Analyzer).
pRNA association
[32P]pRNA Ab’ at a final concentration of 16.25 nM was incubated with increasing concentration of 2’-F-C&U and non-modified pRNA Ba’ (0.1, 0.3, 0.9, 2.6, 8, 24, 72, 216 and 650 nM) for 30 min and then separated by a TBM native gel PAGE. After running, gels were imaged with Cyclone Storage Phosphor Screen system (Packard) and analyzed with OptiQuant software.
AFM picture of hand-in-hand and foot-to-foot pRNA dimer
For all samples, the specially modified mica surface (APS mica) was used. The APS mica was obtained by incubation of freshly cleaved mica in 167 nM 1-(3-aminopropyl)silatrane. The details of APS mica surface modification is described in details in literature.82,83 Samples were diluted with TMS buffer up to 3–5 nM final concentration. Then the droplet of samples (5 µl–10 µl) were immediately deposited on APS mica. After 2 min incubation on the surface the excess of the sample was washed with DEPC treated water and dried with the flow of Ar gas. AFM images in air were acquired using MultiMode AFM NanoScope IV system (Veeco/Digital Instruments, Santa Barbara, CA) operating in tapping mode. Two type of AFM probes were used for tapping mode imaging in air: 1) regular tapping Mode Silicon Probes (Olympus from Asylum Research, Santa_Barbara, CA) with a spring constant of about 42 N/m and a resonant frequency between 300–320 kHz. 2) non contact NSG01_DLC probes (K-Tek Nanotechnology, Wilsonville, OR) with a spring constant of about 5.5 N/m and a resonance frequency between 120–150 kHz.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health (R01-GM59944, R01-EB003730). Jing Liu is the recipient of a scholarship under the State Scholar Fund by China Scholarship Council (CSC). For the AFM images, we thank Dr. Luda Shlyakhtenko and the Nanoimaging Core Facility and grants from NIH (SIG program), UNMC Program of Excellence (POE) and Nebraska Research Initiative (NRI). We also thank Dan Shu and Feng Xiao for assistance in RNA preparation.
Footnotes
Supporting Information Available:
RNase stability datas for 2’-F-C and 2’-F-U pRNA Aa’, sucrose sedimentation profiles of pRNA in presence of Mg2+, Mn2+ and Sr2+. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lin C, Liu Y, Yan H. Designer DNA Nanoarchitectures. Biochemistry. 2009;48:1663–1674. doi: 10.1021/bi802324w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaye FA, Palmer AL, Sleiman HF. Assembling Materials With DNA As the Guide. Science. 2008;321:1795–1799. doi: 10.1126/science.1154533. [DOI] [PubMed] [Google Scholar]
- 3.Seeman NC. Nanomaterials Based on DNA. Annu. Rev. Biochem. 2010;79:65–87. doi: 10.1146/annurev-biochem-060308-102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glotzer SC. Materials Science. Some Assembly Required. Science. 2004;306:419–420. doi: 10.1126/science.1099988. [DOI] [PubMed] [Google Scholar]
- 5.Gates BD, Xu Q, Stewart M, Ryan D, Willson CG, Whitesides GM. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005;105:1171–1196. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- 6.Shu D, Moll WD, Deng Z, Mao C, Guo P. Bottom-Up Assembly of RNA Arrays and Superstructures As Potential Parts in Nanotechnology. Nano Lett. 2004;4:1717–1723. doi: 10.1021/nl0494497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo S, Tschammer N, Mohammed S, Guo P. Specific Delivery of Therapeutic RNAs to Cancer Cells Via the Dimerization Mechanism of Phi29 Motor PRNA. Hum Gene Ther. 2005;16:1097–1109. doi: 10.1089/hum.2005.16.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khaled A, Guo S, Li F, Guo P. Controllable Self-Assembly of Nanoparticles for Specific Delivery of Multiple Therapeutic Molecules to Cancer Cells Using RNA Nanotechnology. Nano Letters. 2005;5:1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo P, Zhang C, Chen C, Trottier M, Garver K. Inter-RNA Interaction of Phage Phi29 PRNA to Form a Hexameric Complex for Viral DNA Transportation. Mol. Cell. 1998;2:149–155. doi: 10.1016/s1097-2765(00)80124-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Lemieux S, Wu X, St.-Arnaud S, McMurray CT, Major F, Anderson D. Function of Hexameric RNA in Packaging of Bacteriophage Phi29 DNA in Vitro. Mol. Cell. 1998;2:141–147. doi: 10.1016/s1097-2765(00)80123-9. [DOI] [PubMed] [Google Scholar]
- 11.Jaeger L, Leontis NB. Tecto-RNA: One Dimensional Self-Assembly Through Tertiary Interactions. Angew. Chem Int. Ed Engl. 2000;39:2521–2524. doi: 10.1002/1521-3773(20000717)39:14<2521::aid-anie2521>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger L, Westhof E, Leontis NB. TectoRNA: Modular Assembly Units for the Construction of RNA Nano-Objects. Nucleic Acids Res. 2001;29:455–463. doi: 10.1093/nar/29.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chworos A, Severcan I, Koyfman AY, Weinkam P, Oroudjev E, Hansma HG, Jaeger L. Building Programmable Jigsaw Puzzles With RNA. Science. 2004;306:2068–2072. doi: 10.1126/science.1104686. [DOI] [PubMed] [Google Scholar]
- 14.Guo P. RNA Nanotechnology: Engineering, Assembly and Applications in Detection, Gene Delivery and Therapy. Journal of Nanoscience and Nanotechnology. 2005;5:1964–1982. doi: 10.1166/jnn.2005.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Li WX, Ding SW. Induction and Suppression of RNA Silencing by an Animal Virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 17.Brummelkamp TR, Bernards R, Agami R. A System for Stable Expression of Short Interfering RNAs in Mammalian Cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 18.Jacque JM, Triques K, Stevenson M. Modulation of HIV-1 Replication by RNA Interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The Polycomb Group Protein EZH2 Is Involved in Progression of Prostate Cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael GG. Medicine: Silencing Viruses With RNA. Nature. 2002;418:379–380. doi: 10.1038/418379a. [DOI] [PubMed] [Google Scholar]
- 21.Sarver NA, Cantin EM, Chang PS, Zaia JA, Ladne PA, Stephens DA, Rossi JJ. Ribozymes As Potential Anti-HIV-1 Therapeutic Agents. Science. 1990;247:1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- 22.Chowrira BM, Berzal-Herranz A, Burke JM. Novel Guanosine Requirement for Catalysis by the Hairpin Ribozyme. Nature. 1991;354:320–322. doi: 10.1038/354320a0. [DOI] [PubMed] [Google Scholar]
- 23.Forster AC, Symons RH. Self-Cleavage of Virusoid RNA Is Performed by the Proposed 55- Nucleotide Active Site. Cell. 1987;50:9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- 24.Sarver Nava, Cantin Edouard M, Chang Pairoj S, Zaia John A, Ladne Paula A, Stephens Delilah A, Rossi John J. Ribozymes As Potential Anti-HIV-1 Therapeutic Agents. Science. 1990;24:1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- 25.Coleman J, Hirashima A, Inocuchi Y, Green PJ, Inouye MA. Novel Immune System Against Bacteriophage Infection Using Complementary RNA (MicRNA) Nature. 1985;315:601–603. doi: 10.1038/315601a0. [DOI] [PubMed] [Google Scholar]
- 26.Knecht DA, Loomis WF. Antisense RNA Inactivation of Myosin Heavy Chain Gene Expression in Dictyostelium Discoideum. Science. 1987;236:1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- 27.Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghildiyal M, Zamore PD. Small Silencing RNAs: an Expanding Universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-Coding RNAs: Regulators of Disease. J. Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 30.Mattick JS. The Genetic Signatures of Noncoding RNAs. PLoS. Genet. 2009;5:e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de PD, Bentley MV, Mahato RI. Hydrophobization and Bioconjugation for Enhanced SiRNA Delivery and Targeting. RNA. 2007;13:431–456. doi: 10.1261/rna.459807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behlke MA. Chemical Modification of SiRNAs for in Vivo Use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 33.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. RNA Interference in Mammalian Cells by Chemically-Modified RNA. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 34.Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T. Sequence, Chemical, and Structural Variation of Small Interfering RNAs and Short Hairpin RNAs and the Effect on Mammalian Gene Silencing. Antisense Nucleic Acid Drug Dev. 2003;13:83–105. doi: 10.1089/108729003321629638. [DOI] [PubMed] [Google Scholar]
- 35.Elmen J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Orum H, Koch T, Wahlestedt C. Locked Nucleic Acid (LNA) Mediated Improvements in SiRNA Stability and Functionality. Nucleic Acids Res. 2005;33:439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In Vivo Activity of Nuclease-Resistant SiRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czauderna F, Fechtner M, Dames S, Aygun H, Klippel A, Pronk GJ, Giese K, Kaufmann J. Structural Variations and Stabilising Modifications of Synthetic SiRNAs in Mammalian Cells. Nucleic Acids Res. 2003;31:2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieken WA, Olsen DB, Benseler F, Aurup H, Eckstein F. Kinetic Characterization of Ribonuclease-Resistant 2'-Modified Hammerhead Ribozymes. Science. 1991;253:314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- 39.Watts JK, Deleavey GF, Damha MJ. Chemically Modified SiRNA: Tools and Applications. Drug Discovery Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Chiu YL, Rana TM. SiRNA Function in RNAi: a Chemical Modification Analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rusckowski M, Qu T, Roskey A, Agrawal S. Biodistribution and Metabolism of a Mixed Backbone Oligonucleotide (GEM 231) Following Single and Multiple Dose Administration in Mice. Antisense Nucleic Acid Drug Dev. 2000;10:333–345. doi: 10.1089/oli.1.2000.10.333. [DOI] [PubMed] [Google Scholar]
- 42.Jaeger L, Verzemnieks EJ, Geary C. The UA_Handle: a Versatile Submotif in Stable RNA Architectures. Nucleic Acids Res. 2009;37:215–230. doi: 10.1093/nar/gkn911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki AM, Casper MD, Freier SM, Lesnik EA, Zounes MC, Cummins LL, Gonzalez C, Cook PD. Uniformly Modified 2'-Deoxy-2'-Fluoro Phosphorothioate Oligonucleotides As Nuclease-Resistant Antisense Compounds With High Affinity and Specificity for RNA Targets. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- 44.Williams AA, Darwanto A, Theruvathu JA, Burdzy A, Neidigh JW, Sowers LC. Impact of Sugar Pucker on Base Pair and Mispair Stability. Biochemistry. 2009;48:11994–12004. doi: 10.1021/bi9014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keefe AD, Pai S, Ellington A. Aptamers As Therapeutics. Nat. Rev. Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JF, Stovall GM, Ellington AD. Aptamer Therapeutics Advance. Curr. Opin. Chem. Biol. 2006;10:282–289. doi: 10.1016/j.cbpa.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Kreutz C, Kahlig H, Konrat R, Micura R. Ribose 2'-F Labeling: a Simple Tool for the Characterization of RNA Secondary Structure Equilibria by 19F NMR Spectroscopy. J. Am. Chem. Soc. 2005;127:11558–11559. doi: 10.1021/ja052844u. [DOI] [PubMed] [Google Scholar]
- 48.Motorin Y, Helm M. TRNA Stabilization by Modified Nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 49.Hannon GJ. RNA Interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 50.Guo P. Structure and Function of Phi29 Hexameric RNA That Drive Viral DNA Packaging Motor: Review. Prog in Nucl Acid Res & Mole Biol. 2002;72:415–472. doi: 10.1016/s0079-6603(02)72076-x. [DOI] [PubMed] [Google Scholar]
- 51.Simpson AA, Leiman PG, Tao Y, He Y, Badasso MO, Jardine PJ, Anderson DL, Rossman MG. Structure Determination of the Head-Tail Connector of Bacteriophage Phi29. Acta Cryst. 2001;D57:1260–1269. doi: 10.1107/s0907444901010435. [DOI] [PubMed] [Google Scholar]
- 52.Guasch A, Pous J, Ibarra B, Gomis-Ruth FX, Valpuesta JM, Sousa N, Carrascosa JL, Coll M. Detailed Architecture of a DNA Translocating Machine: the High- Resolution Structure of the Bacteriophage Phi29 Connector Particle. J. Mol. Biol. 2002;315:663–676. doi: 10.1006/jmbi.2001.5278. [DOI] [PubMed] [Google Scholar]
- 53.Guo P, Erickson S, Anderson D. A Small Viral RNA Is Required for in Vitro Packaging of Bacteriophage Phi29 DNA. Science. 1987;236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- 54.Shu D, Zhang H, Jin J, Guo P. Counting of Six PRNAs of Phi29 DNA-Packaging Motor With Customized Single Molecule Dual-View System. EMBO J. 2007;26:527–537. doi: 10.1038/sj.emboj.7601506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Zhang C, Guo P. Sequence Requirement for Hand-in-Hand Interaction in Formation of PRNA Dimers and Hexamers to Gear Phi29 DNA Translocation Motor. RNA. 1999;5:805–818. doi: 10.1017/s1355838299990350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu D, Huang L, Hoeprich S, Guo P. Construction of Phi29 DNA-Packaging RNA (PRNA) Monomers, Dimers and Trimers With Variable Sizes and Shapes As Potential Parts for Nano-Devices. J. Nanosci. Nanotechnol. 2003;3:295–302. doi: 10.1166/jnn.2003.160. [DOI] [PubMed] [Google Scholar]
- 57.Hendrix RW. Bacteriophage DNA Packaging: RNA Gears in a DNA Transport Machine (Minireview) Cell. 1998;94:147–150. doi: 10.1016/s0092-8674(00)81413-0. [DOI] [PubMed] [Google Scholar]
- 58.Hoeprich S, ZHou Q, Guo S, Qi G, Wang Y, Guo P. Bacterial Virus Phi29 PRNA As a Hammerhead Ribozyme Escort to Destroy Hepatitis B Virus. Gene Ther. 2003;10:1258–1267. doi: 10.1038/sj.gt.3302002. [DOI] [PubMed] [Google Scholar]
- 59.Guo S, Huang F, Guo P. Construction of Folate-Conjugated PRNA of Bacteriophage Phi29 DNA Packaging Motor for Delivery of Chimeric SiRNA to Nasopharyngeal Carcinoma Cells. Gene Ther. 2006;13:814–820. doi: 10.1038/sj.gt.3302716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Huifang M, Su Yue, Guo Songchuan, Yuan Ji, Lim Travis, Liu Jing, Guo Peixuan, Yang Decheng. Target Delivery of Anti-Coxsachievirus SiRNAs Using Ligand-Conjugated Packaging RNAs. Antiviral Res. 2009;83:307–316. doi: 10.1016/j.antiviral.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sousa R, Padilla R. A Mutant T7 RNA Polymerase As a DNA Polymerase. EMBO J. 1995;14:4609–4621. doi: 10.1002/j.1460-2075.1995.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Padilla R, Sousa R. Efficient Synthesis of Nucleic Acids Heavily Modified With Non- Canonical Ribose 2'-Groups Using a MutantT7 RNA Polymerase (RNAP) Nucleic Acids Res. 1999;27:1561–1563. doi: 10.1093/nar/27.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Y, Eckstein F, Padilla R, Sousa R. Mechanism of Ribose 2'-Group Discrimination by an RNA Polymerase. Biochemistry. 1997;36:8231–8242. doi: 10.1021/bi962674l. [DOI] [PubMed] [Google Scholar]
- 64.Chen C, Guo P. Sequential Action of Six Virus-Encoded DNA-Packaging RNAs During Phage Phi29 Genomic DNA Translocation. J. Virol. 1997;71:3864–3871. doi: 10.1128/jvi.71.5.3864-3871.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trottier M, Zhang CL, Guo P. Complete Inhibition of Virion Assembly in Vivo With Mutant PRNA Essential for Phage Phi29 DNA Packaging. J. Virol. 1996;70:55–61. doi: 10.1128/jvi.70.1.55-61.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trottier M, Guo P. Approaches to Determine Stoichiometry of Viral Assembly Components. J. Virol. 1997;71:487–494. doi: 10.1128/jvi.71.1.487-494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C, Sheng S, Shao Z, Guo P. A Dimer As a Building Block in Assembling RNA: A Hexamer That Gears Bacterial Virus Phi29 DNA-Translocating Machinery. J Biol Chem. 2000;275:17510–17516. doi: 10.1074/jbc.M909662199. [DOI] [PubMed] [Google Scholar]
- 68.Hoeprich S, Guo P. Computer Modeling of Three-Dimensional Structure of DNA-Packaging RNA(PRNA) Monomer, Dimer, and Hexamer of Phi29 DNA Packaging Motor. J Biol. Chem. 2002;277:20794–20803. doi: 10.1074/jbc.M112061200. [DOI] [PubMed] [Google Scholar]
- 69.Pecenkova T, Benes V, Paces J, Vlcek C, Paces V. Bacteriophage B103: Complete DNA Sequence of Its Genome and Relationship to Other Bacillus Phages. Gene. 1997;199:157–163. doi: 10.1016/s0378-1119(97)00363-6. [DOI] [PubMed] [Google Scholar]
- 70.Zhang CL, Tellinghuisen T, Guo P. Confirmation of the Helical Structure of the 5'/3' Termini of the Essential DNA Packaging PRNA of Phage Φ29. RNA. 1995;1:1041–1050. [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CS, Guo P. A Highly Sensitive System for the in Vitro Assembly of Bacteriophage Phi29 of Bacillus Subtilis. Virology. 1994;202:1039–1042. doi: 10.1006/viro.1994.1434. [DOI] [PubMed] [Google Scholar]
- 72.Lee CS, Guo P. In Vitro Assembly of Infectious Virions of Ds-DNA Phage Φ29 From Cloned Gene Products and Synthetic Nucleic Acids. J. Virol. 1995;69:5018–5023. doi: 10.1128/jvi.69.8.5018-5023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reif B, Wittmann V, Schwalbe H, Griesinger C, Worner K, JahnHofmann K, Engels JW, Bermel W. Structural Comparison of Oligoribonucleotides and Their 2'-Deoxy-2'-Fluoro Analogs by Heteronuclear NMR Spectroscopy. Helvetica Chimica Acta. 1997;80:1952–1971. [Google Scholar]
- 74.Luy B, Marino JP. Measurement and Application of 1H-19F Dipolar Couplings in the Structure Determination of 2'-Fluorolabeled RNA. J. Biomol. NMR. 2001;20:39–47. doi: 10.1023/a:1011210307947. [DOI] [PubMed] [Google Scholar]
- 75.Zhang CL, Lee C-S, Guo P. The Proximate 5' and 3' Ends of the 120-Base Viral RNA (PRNA) Are Crucial for the Packaging of Bacteriophage Φ29 DNA. Virology. 1994;201:77–85. doi: 10.1006/viro.1994.1267. [DOI] [PubMed] [Google Scholar]
- 76.Mat-Arip Y, Garver K, Chen C, Sheng S, Shao Z, Guo P. Three-Dimensional Interaction of Phi29 PRNA Dimer Probed by Chemical Modification Interference, Cryo-AFM, and Cross-Linking. J. Biol. Chem. 2001;276:32575–32584. doi: 10.1074/jbc.M100045200. [DOI] [PubMed] [Google Scholar]
- 77.Lee CS, Guo P. Sequential Interactions of Structural Proteins in Phage Phi29 Procapsid Assembly. J. Virol. 1995;69:5024–5032. doi: 10.1128/jvi.69.8.5024-5032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo P, Rajogopal B, Anderson D, Erickson S, Lee C-S. SRNA of Bacteriophage Phi29 of B.Subtilis Mediates DNA Packaging of Phi29 Proheads Assembled in E. Coli. Virology. 1991;185:395–400. doi: 10.1016/0042-6822(91)90787-c. [DOI] [PubMed] [Google Scholar]
- 79.Guo P, Erickson S, Xu W, Olson N, Baker TS, Anderson D. Regulation of the Phage Φ29 Prohead Shape and Size by the Portal Vertex. Virology. 1991;183:366–373. doi: 10.1016/0042-6822(91)90149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo P, Grimes S, Anderson D. A Defined System for in Vitro Packaging of DNA-Gp3 of the Bacillus Subtilis Bacteriophage Phi29. Proc. Natl. Acad. Sci. USA. 1986;83:3505–3509. doi: 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo P, Peterson C, Anderson D. Prohead and DNA-Gp3-Dependent ATPase Activity of the DNA Packaging Protein Gp16 of Bacteriophage Φ29. J Mol Biol. 1987;197:229–236. doi: 10.1016/0022-2836(87)90121-5. [DOI] [PubMed] [Google Scholar]
- 82.Shlyakhtenko LS, Gall AA, Filonov A, Cerovac Z, Lushnikov A, Lyubchenko YL. Silatrane-Based Surface Chemistry for Immobilization of DNA, Protein-DNA Complexes and Other Biological Materials. Ultramicroscopy. 2003;97:279–287. doi: 10.1016/S0304-3991(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 83.Lyubchenko YL, Shlyakhtenko LS. AFM for Analysis of Structure and Dynamics of DNA and Protein-DNA Complexes. Methods. 2009;47:206–213. doi: 10.1016/j.ymeth.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.