Abstract
Although the hippocampus is critical for the formation and retrieval of spatial memories, it is unclear how subregions are differentially involved in these processes. Previous high-resolution functional magnetic resonance imaging (fMRI) studies have shown that CA2, CA3, and dentate gyrus (CA23DG) regions support the encoding of novel associations, while the subicular cortices support the retrieval of these learned associations. Whether these subregions are employed in humans during encoding and retrieval of spatial information has yet to be explored. Using high-resolution fMRI (1.6 mm × 1.6 mm in-plane), we found that activity within the right CA23DG increased during encoding compared to retrieval. Conversely, right subicular activity increased during retrieval compared to encoding of spatial associations. These results are consistent with previous studies illustrating dissociations within human hippocampal subregions and further suggest these regions are similarly involved during the encoding and retrieval of spatial information.
Keywords: hippocampus, CA3, subiculum, fMRI, encoding, retrieval, spatial learning
Learning and recalling spatial information is critical for the ability to form and retrieve memories for events. Furthermore, processing the spatial context in which these memories are formed may assist in the binding of items into a stable episodic memory representation (Eichenbaum, 2004; Burgess et al. 2002; Holscher, 2003). An intact medial temporal lobe (MTL) is necessary for the formation of episodic memory (Scoville and Milner, 1957). It has been suggested that the MTL may processes spatial and non-spatial components of episodic memory independently (Burgess et al. 2002). By this view, spatial and nonspatial information may engage different MTL subregions during encoding and/or retrieval due to the special status of spatial information in the hippocampus.
The MTL is composed of the hippocampus proper (CA fields 1, 2, and 3; dentate gyrus [DG]; and subiculum) and adjacent cortices (parahippocampal [PHC], entorhinal [ERC], and perirhinal [PRC]). Although substantial evidence supports a role for the MTL in declarative learning, it is still unclear how specific subregions are involved (Squire 2004). Several neuroimaging studies support spatial dissociations along the anterior-posterior axis of the hippocampus during encoding and retrieval (Lepage et al., 1998; Schacter and Wagner, 1999; Zeineh, et al. 2003). However, several other studies fail to find differences (for review see Schacter and Wagner, 1999). High-resolution functional magnetic resonance imaging (fMRI) studies have shown that the CA2, CA3, and dentate gyrus (CA23DG) support encoding of novel face-name and object-object associations, whereas the subicular cortices support retrieval of these learned associations (Zeineh et al. 2003, Eldridge et al. 2005). Whether these subregions are employed during spatial memory tasks has yet to be explored. Furthermore, previous data targeting the hippocampus as a whole suggest that the left is more specialized for verbal learning (Frisk and Milner, 1990), while the right for nonverbal learning (e.g. visuospatial [Smith and Milner 1989]), which should be reflected in hemispheric differences in patterns of activity within specific hippocampal subregions. To determine which subregions are involved during encoding and retrieval of spatial information we used high-resolution functional magnetic resonance imaging (fMRI) while subjects learned and recalled spatial information (store locations) within 3D virtual environments.
The current study is a new analysis of data that has been presented in part (Suthana et al., 2009). The current study includes additional data (retrieval activity), which were not presented in the previous study. We therefore describe our subjects and methods briefly here (see Suthana et al., 2009 for full details). Our previous study (2009) focused on different types of spatial learning. We looked solely at differences in encoding during egocentric (viewpoint-dependent) and allocentric (viewpoint-independent) navigation. In the current study we sought to determine whether there are encoding vs. retrieval differences for spatial information. Subjects were eighteen normal, right-handed (nine male, nine female) between the ages of 20 and 31 (24.89 + 0.72 years), all of who provided informed consent and participated in the study, which was performed under UCLA Institutional Review Board (IRB) testing protocols.
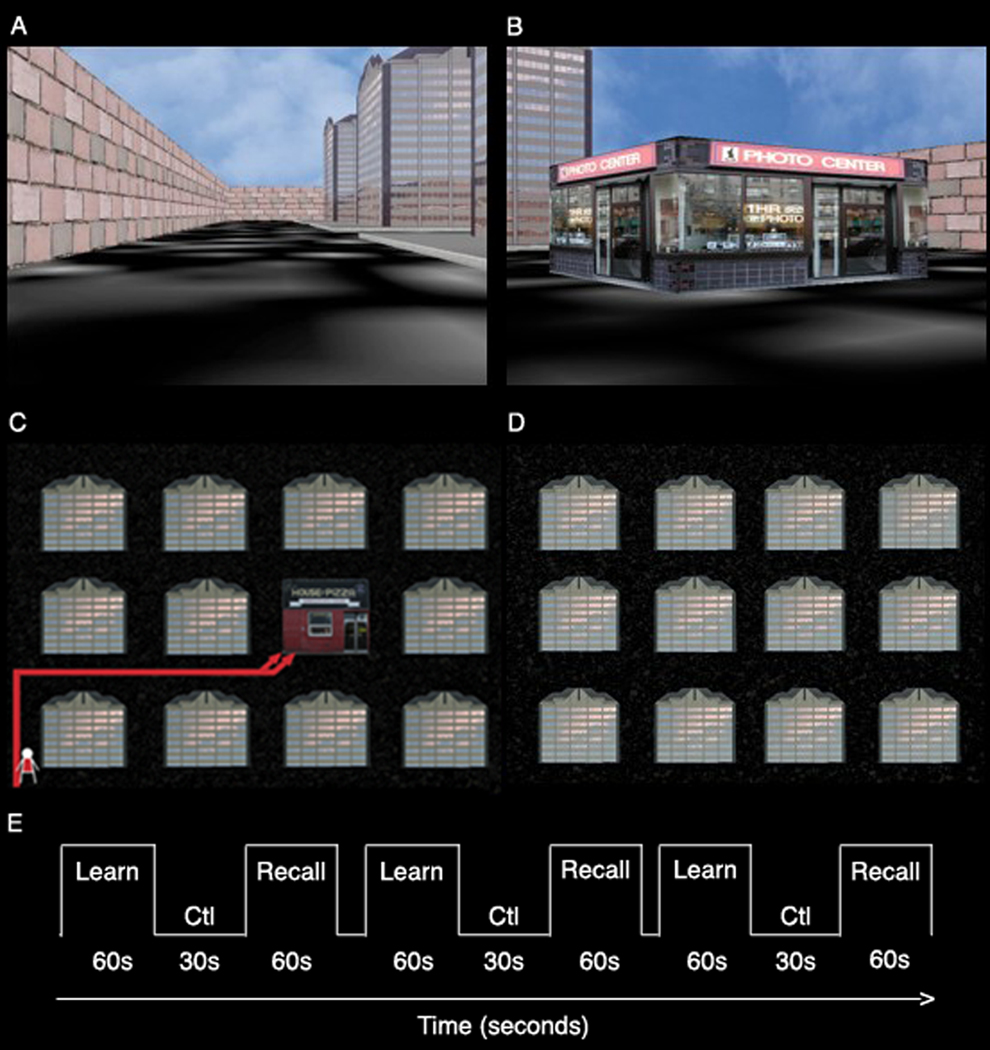
Subjects viewed videos of navigation through novel spatial environments using a single starting point. They were instructed to learn store locations (Fig 1). Navigation videos were previously recorded with continuously refreshed (60Hz) virtual reality environments surrounded by a wall with a 4 × 3 grid design (Fig. 1) containing buildings, roads, and stores. The task was presented in a blocked design of alternating encoding, control, and retrieval conditions (Fig. 1E). During encoding, subjects viewed navigation to novel stores (Fig. 1B) where store locations were repeated across encoding blocks. In half of the encoding blocks, navigation began at a single starting point (egocentric condition), while in the other encoding blocks, subjects were trained using multiple starting points (allocentric condition). These two conditions were matched for difficulty as assessed by performance on the retrieval test (Suthana et al 2009). Retrieval blocks consisted of viewing of navigation to old previously learned (target) or novel (lure) store locations (same store stimuli were used); subjects had to determine if the stores were in old or new locations within the city and respond by pressing one of two assigned buttons. The retrieval blocks were the same for locations encoded in the egocentric and allocentric conditions. In the direction-pressing control condition (Fig. 1D), subjects viewed navigation through the same cities however without stores (just buildings and roads), and were instructed to press the corresponding button on the keypad every time the direction was changed (left and right). Navigation videos were displayed and recorded using pyepl (http://pyepl.sourceforge.net/), Snapz Pro X (Ambrosia software; Rochester, NY), and an adapted version of yellowcab2 (original download http://memory.psych.upenn.edu/Software). iMovie was used to edit videos (Apple Inc; Cupertino, CA) and stimuli were presented using MacStim 3.2.1 software (WhiteAnt Occasional Publishing; Melbourne, AUS).
Figure 1. Virtual city snapshot.
(A) Shown is a snapshot of a virtual city from a sample starting point and a (B) sample store stimulus. (C) Subjects’ learned store locations from an initial starting point within a city. (D) Layout of city without stores used in the direction-pressing control baseline condition. (E) The task consisted of alternating blocks of encoding (learn) and retrieval (recall) interspersed with blocks of control (Ctl).
Subjects were scanned at the University of California, Los Angeles Ahmanson-Lovelace Brain Mapping Center using a Siemens Allegra head-only 3 Tesla scanner. High in-plane resolution structural images (spin echo, matrix size = 512 × 512, TR = 5200 ms, TE = 105 ms, 19 slices, contiguous; voxel size: 0.391 × 0.391 × 3 mm) and echo-planar images (TR = 3000 ms, TE = 39 ms, 128 × 128, 19 slices, contiguous; voxel size = 1.6 × 1.6 × 3 mm) were acquired in the same plane and registered using a matched-bandwidth coplanar sequence (TR = 5000 ms, TE = 66 ms, 19 slices, contiguous; voxel size = 1.6 × 1.6 × 3 mm). Headphones and 512 × 512 resolution magnet-compatible 3-D goggles were used to present auditory and visual stimuli to the subject (Resonance Technologies, Inc.). A Macintosh G4 Powerbook computer was used to present stimuli and key presses were recorded for behavioral analysis.
FEAT (fMRI Expert Analysis Tool) part of FMRIB Software Analysis (FSL version 3.3, www.fmrib.ox.ac.uk/fsl) was used for fMRI analysis. Motion correction was applied to functional images using MCFLIRT (Jenkinson et al., 2002) (FMRIB's motion correction linear image registration tool). The brain surfaces were extracted using BET (brain extraction tool) (Smith, 2002). Images were temporally high-pass filtered (Gaussian-weighted least-squares straight line fitting, with sigma = 100.0s), intensity normalized, and spatially smoothed using a Gaussian kernel of FWHM 3mm. Time-series statistical analysis was carried out using FILM (FMRIB’s improved linear model) with local autocorrelation correction (Woolrich et al., 2001). Functional, high-resolution coplanar and structural images were aligned using FLIRT (FMRIB’s Linear Image Registration Tool) via an affine transformation. In order to optimize registration to MTL structures, weighted masks of each subjects’ MTL regions were used for registration of EPI to structural images. We also manually checked each subject’s registration using landmarks that are visible on both types of images (i.e. blood vessels). Supplementary figure 1 shows example individuals’ structural images with co-registered visible landmarks drawn on the EPI images (red lines). Regressors of interest were created by convolving a delta function (time onsets) with a canonical (gamma) hemodynamic response function and temporal derivative.
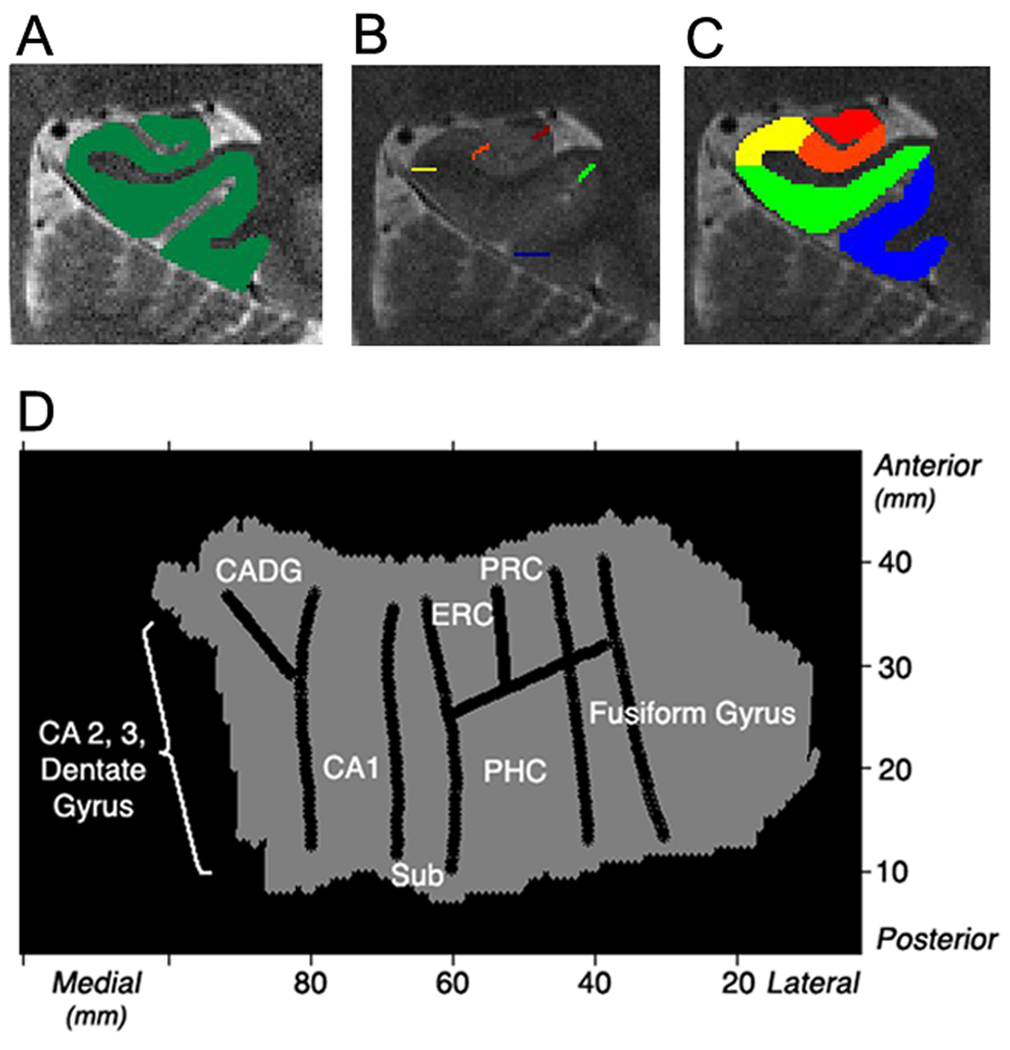
The 3-D gray matter of the MTL subregions was created (Fig. 2A), computationally unfolded and interpolated to improve segmentation using mrUnfold software (Teo et al., 1997; Engel et al., 1997). This yielded a final voxel size of 0.391 × 0.391 × 0.429 mm (Fig. 2D). The position of the various CA fields, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus were demarcated (Fig. 2B) on the structural images based on the atlas by Amaral and Insausti (1990), and Duvernoy (1998). The dentate gyrus is not distinguishable from adjacent CA fields with our current human imaging methods, and therefore labeled the encompassing region CA23DG. With the unfolding methods used anterior CA fields 1–3 and dentate gyrus are not separable from each other and therefore included as an encompassing region (Ant CADG) on the unfolded flat map. However, anterior CA1 is distinguishable from CA23DG using our regions of interest (ROI) methods. For the ROI analysis, anatomical ROIs were created a priori by defining voxels in 2D space and then projecting them into 3D space (Fig. 2C). However, the anterior CADG ROI was separated into CA1 and CA23DG in 3D space using the same atlases by Amaral and Duvernoy. ROIs consisted of anterior and posterior CA2, 3 and dentate gyrus (CA23DG), anterior and posterior CA1, anterior and posterior subiculum, ERC, PRC, PHC, and fusiform gyrus. In FSL, the average percent signal change was computed for each ROI using the average parameter estimates with the height of an isolated event as the scaling factor relative to the voxel mean (see http://mumford.bol.ucla.edu/perchange_guide.pdf).
Figure 2. Unfolding method.
(A) Each subjects’ gray matter (green) is created by segmenting white matter and cerebral spinal fluid. The gray matter is then computationally unfolded and boundaries (B) between regions are projected onto the unfolded flat map. (D) An averaged group flat map (shown is the left) is created showing regions CA2, 3, and dentate gyrus, CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus. (C) Voxels in 2D space are projected into 3D space to create anatomical regions of interests showing posterior regions (left): CA23DG (red), CA1 (orange), subiculum (yellow), PHC (green), and fusiform gyrus (blue).
Shown in Figure 3 is a group averaged unfolded map created based on the eighteen individual subject anatomical images and boundaries. Each subjects’ individual anatomical and time functional data was warped into the template (for details on group analysis, see Thompson et al. 2000). Subject’s fMRI signal (e.g., beta values) was then compared across subjects for each voxel using a mixed-effects t-test (t ≥ 2.4, p < 0.05, corrected). Some subjects’ flat maps were larger than others and therefore, there is an absence of significant fMRI group activity on the outer edges of the flat group map (Figure 3). For more details on methods, see Ekstrom et al. (2009), Thompson et al. (2000), Zeineh et al. (2001), and Suthana et al. (2009). Correction for multiple comparisons for single subject contrasts were cluster corrected at Z > 2.3 and p < 0.05. Group activation maps were corrected using a Bonferroni correction for 10 ROIs (p < 0.0005). For ROI analysis, post hoc comparisons were made only if global analysis indicated a statistically significant (p < 0.05, corrected) effect of condition (Encoding vs. Retrieval).
Figure 3. Unfolding Results.
(A) Group voxel based mixed-effects unfolded t-test maps (N = 18, statistical maps of significantly activated and deactivated regions; −2.4 ≥ t ≥ 2.4, p < 0.05 corrected) for the left and right MTL regions during the encoding and retrieval of locations compared to baseline. (B) Group voxel based mixed-effects unfolded t-test maps (N = 18, statistical maps of significantly difference in activity between encoding and retrieval conditions; −2.4 ≥ t ≥ 2.4, p < 0.05 corrected) for the left and right MTL regions. Regions shown include CA2,3 and dentate gyrus, CA1, subiculum (sub), entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus.
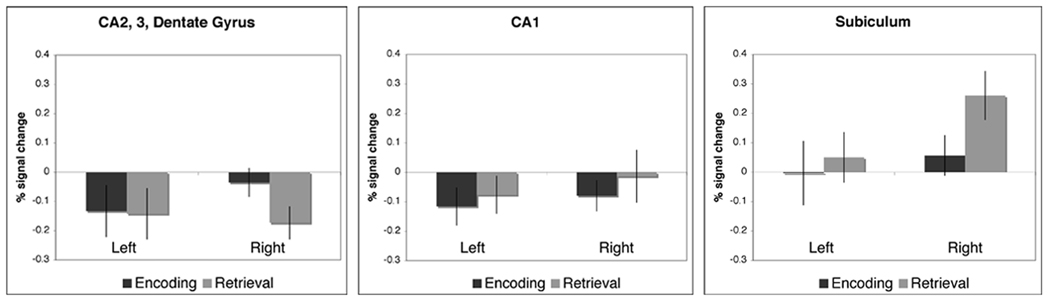
Subjects successfully encoded store-place associations, as reflected in their ability to accurately recognize store locations during retrieval (egocentric % correct: mean ± s.e.m., 73.33 ± 3.89; allocentric % correct: mean ± s.e.m., 68.33 ± 5.18). A voxel-wise analysis, showed significant clusters of increased and decreased activation within various MTL subregions during both encoding and retrieval during navigation with stores compared to baseline (Fig. 3A). In the single start-point (egocentric) condition, directly contrasting encoding and retrieval blocks yielded significant differences in activation (t ≥ 2.4) within the right CA23DG for encoding versus retrieval and within the right subiculum for retrieval versus encoding (Fig. 3B). We also completed an independent analysis based solely on anatomical regions of interests (ROIs). ROIs were determined a priori, and average percent signal change was then calculated for each region. These ROIs were based on the anatomical definitions of CA2, 3 and dentate gyrus (CA23DG), anterior and posterior CA1, anterior and posterior subiculum, entorhinal cortex (ERC), perirhinal cortex (PRC), parahippocampal cortex (PHC), and fusiform gyrus. Average percent signal change compared to baseline within hippocampal regions are shown in Figure 4. A three-way ANOVA revealed a significant main effect of condition (encoding vs. retrieval), (F (3,238) = 8.03, p < 0.005)). Post-hoc analyses yielded significant differences within the subiculum (retrieval > encoding, t(17) = 2.60; p < 0.05; right > left, t(17) = 2.88; p < 0.05), and CA23DG (encoding > retrieval, t(17) = 2.23; p < 0.05) (Fig. 4). For the subiculum the posterior anatomical ROI is shown. Although activity increased compared to baseline during both encoding and retrieval, no significant condition differences were found within extrahippocampal ROIs between conditions (Supplementary Fig. 2).
Figure 4. Egocentric Encoding versus Retrieval.
Shown are the hippocampal ROIs average % signal changes (n = 18) during encoding and retrieval in (A) CA23DG, (A) CA1, and (C) subiculum. Within the CA23DG we found a significant difference between conditions (encoding > retrieval, t(17) = 2.60; p < 0.05). Within the subiculum we found a significant difference between conditions (encoding > retrieval, t(17) = 2.23; p < 0.05).
In contrast, in the multiple start-point (allocentric) condition, there were no significant differences in activation when comparing the encoding and retrieval blocks (Supplementary Fig. 3). Similar to the egocentric condition no significant differences were found within extrahippocampal ROIs (Supplementary Fig. 4). We did see significant increase in activity within the right posterior CA1 as reported previously (Suthana et al., 2009). These results are consistent with the idea that encoding an allocentric map of the environment is accompanied by retrieval of previously taken routes. Thus, encoding and retrieval phases are intermixed to a greater extent during the multiple start-point condition, which may explain the lack of differences during encoding versus retrieval detected during this condition.
The present results demonstrate a functional dissociation within hippocampal subregions during encoding and retrieval of spatial information when subjects learned to navigate from a single start point. When subjects are encoding this spatial information compared to when they are retrieving this information, activity within the CA23DG subregion of the hippocampus is significantly increased. Conversely, the subiculum is preferentially engaged during recall. Furthermore, this dissociation was localized to the right hemisphere consistent with neuropsychological evidence from patients with spatial memory deficits related to right hemispheric MTL damage (Smith and Milner 1981). Our results are consistent with previous high-resolution fMRI studies (Zeineh et al., 2003; Eldridge et al., 2005), which also show activity within CA23DG and subiculum during the learning and recall of face-name and object-object associations suggesting hippocampal subregions process spatial and non-spatial information similarly.
Because the hippocampus is a small and variable structure, it becomes challenging to detect changes in activity averaged across several subjects. It is vulnerable to misregistration because of its variability and convolution in structure. Additionally, it consists of functionally distinct subregions, which may have opposite responses during a given task. Averaging across these subregions may thus tend to mask a detectable signal during a given cognitive function. We address these difficulties by employing a higher resolution imaging method combined with computational unfolding in order to separately investigate smaller hippocampal regions.
Our results are consistent with rodent electrophysiological data and computational models, which suggest functional dissociations within hippocampal subregions. Specifically, it is thought the CA3 may be involved in long-term memory formation and storage of new information entering via ERC (McNaughton and Morris 1987; Rolls 1996; Levy 1989; Vinogradova 2001; Mizumori et al., 2004). However, these studies may speak to the neural activity (firing rate) within hippocampal subfields regions. Studies show that the BOLD signal reflects both local field potentials (synaptic input activity; Mitzdorf 1985; Ekstrom et al., 2009) and local changes in neural firing rate, (Logothetis et al. 2001; Mukamel et al. 2005). Therefore, it is possible that during certain tasks BOLD increases in CA3 are evident during encoding, when novel information enters via synaptic input from ERC. Conversely, during retrieval, BOLD activity within the subiculum should increase reflective of increased synaptic activity from CA3 input. However, several models suggest encoding and retrieval occur within the same subregions (O’Reilly and Rudy, 2001; Rolls, 1996). Recent findings suggest that the involvement of different hippocampal subregions in memory encoding may depend on the type of information being encoded in addition to its novelty (Suthana et al., 2009; Bakker et al., 2008; Preston et al., 2010). Future studies will be needed to tease apart the exact involvement of the hippocampal circuit during different stages of mnemonic processing.
Results from recent high-resolution fMRI studies also parallel our and previous data from non-human electrophysiological studies. Olsen and colleagues (2009) found CA23DG activity dominated study and delay phases of a delayed-match-to-sample task whereas CA1 and subiculum activation dominated probe phases. They also found higher hippocampal activity associated with correct compared to incorrect trials. In our study, because navigation trials are so long, we were unable to fit in enough trials to directly compare correct versus incorrect trials. However, future navigation studies designed specifically to address this question would presumably find similar results. Also, consistent with our results, other recent high-resolution fMRI studies find encoding related activity in CA23DG and subicular activity during retrieval of episodic information (Carr et al., 2010; Viskontas et al., 2009). For a complete review of high-resolution fMRI of the human MTL, see Carr, Rissman, & Wagner, (2010).
Encoding and retrieval are challenging to separate during a given cognitive task. Encoding also occurs during retrieval where one has to determine if the information is new or previously learned, especially during novel (lure) retrieval trials. Similarly, retrieval processes are invoked during learning, especially during late encoding trials. Each condition is presumably dominated by one process (encoding or retrieval). Therefore, studies are able to successfully detect differences between these two conditions. In our study, for the egocentric (single start point) condition, there were clear differences between encoding and retrieval phases. However, when subjects learned locations from multiple start points in the allocentric condition, there was little difference between encoding and retrieval activity, presumably because the formation of a cognitive map of the environment likely requires the retrieval of previously learned route information during the learning of new routes. Future studies will help clarify the exact mechanisms of encoding/retrieval processes within the MTL and relate them directly to computational models (O’Reilly and Rudy, 2001; Rolls, 1996; McClelland and Goddard, 1996; O’Reilly and McClelland, 1994; Levy et al., 1989).
These results extend our previous findings showing posterior CA1 involvement during the encoding of viewpoint-independent (allocentric) spatial information compared to encoding egocentric spatial information (Suthana et al. 2009). However, in the current study, activation differences in encoding and retrieval only emerged when subjects learned and recalled spatial locations from a repeated viewpoint-dependent (egocentric) starting point and thus were not reliant on forming a cognitive map of space. We did not find comparable differences in the allocentric condition, presumably because the process of learning a map of the environment from multiple start points would combine both encoding the current trial and retrieving previous trials, and thus these processes are intermixed in the encoding phase. Encoding from multiple starting points involves both encoding and retrieval: encoding of novel routes from new starting points in addition to integrating or incorporating this information with retrieval of previously learned routes. It is likely that this characteristic of the allocentric encoding phase resulted in the similarity between the encoding and retrieval phases.
As previously reported (Suthana et al., 2009), we saw CA23DG activity in the absence of CA1 activity during encoding of egocentric spatial information. We extend these findings by detecting subiculum activity during retrieval of egocentric spatial information. The subregional signal changes we detect are localized to the posterior hippocampus consistent with previous findings implicating posterior hippocampus in spatial memory (Maguire et al. 2006; Gabrieli et al., 1997; Fernandez et al., 1998; Hassabis et al., 2009). With our ROI analysis method we did not see significant increases from baseline during encoding within hippocampal structures. We did however, see voxels of significant activity during encoding and retrieval compared to baseline with our voxel-wise analysis method that may have been masked with our ROI method, which includes non-responsive voxels and thus may have masked our signal. Furthermore, we employed an active baseline condition rather than simple fixation, which may have enhanced our ability to detect hippocampal activity changes (Stark and Squire, 2001).
Overall, our results suggest dissociations within hippocampal subregions during spatial learning and memory. Furthermore, consistent with previous findings of right hippocampal involvement during spatial processing, activity differences were localized to the right hemisphere. High-resolution fMRI combined with computational cortical unfolding techniques provides a unique opportunity to investigate models of hippocampal function. This approach has allowed for the functional separation of encoding and retrieval processes in the MTL. Future research using these methods may provide insight into human memory processes at the circuit level.
Supplementary Material
A weighted mask of each individual subjects’ MTL regions were used to optimize the registration to these structures. Each subject’s registration was checked manually using landmarks that are visible on both types of images (i.e. blood vessels). Shown in red are example landmarks drawn on EPIs co-registered to structural images.
Shown are the extrahippocampal ROIs average % signal changes (n = 18; error bars correspond to the standard error across subjects)) during encoding and retrieval in the regions: (a) entorhinal cortex (ERC), (b) perirhinal cortex (PRC), (c) parahippocampal cortex (PHC), and (d) fusiform gyrus. No significant differences between encoding and retrieval conditions were found.
Shown are the hippocampal ROIs average % signal changes (n = 18) during encoding and retrieval in (A) CA23DG, (A) CA1, and (C) subiculum. No significant differences between encoding and retrieval conditions were found.
Shown are the extrahippocampal ROIs average % signal changes (n = 18; error bars correspond to the standard error across subjects)) during encoding and retrieval in the regions: (a) entorhinal cortex (ERC), (b) perirhinal cortex (PRC), (c) parahippocampal cortex (PHC), and (d) fusiform gyrus. No significant differences between encoding and retrieval conditions were found.
ACKNOWLEDGEMENTS
We thank Michael J. Kahana for sharing and support of the virtual navigation task “yellowcab”. We also thank Michael Zeineh and Paul Thompson for assistance with group unfolding scripts and Michael Jones and James Kyle for technical assistance. This work was supported by the National Institute of Mental Health 5T32 MH015795, F32 NS50067-03, and by NIDA 5T90DA022768-02. For generous support the authors we thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. Finally, we also wish to thank all of the subjects for their participation in this study.
REFERENCES
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cerebral Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. The human nervous system. San Diego: Academic Press; 1990. The hippocampal formation; pp. 711–755. [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. The Journal of Neuroscience. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002a;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002b;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. Berlin: Springer; 1998. [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Bazih AJ, Suthana NA, Al-Hakim R, Ogura K, Zeineh M, et al. Advances in high-resolution imaging and computational unfolding of the human hippocampus. NeuroImage. 2009;47:42–49. doi: 10.1016/j.neuroimage.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. The Journal of Neuroscience. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fernández G, Weyerts H, Schrader-Bölsche M, Tendolkar I, Smid HG, Tempelmann C, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. The Journal of Neuroscience. 1998;18:1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain: A Journal of Neurology. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- Frisk V, Milner B. The relationship of working memory to the immediate recall of stories following unilateral temporal or frontal lobectomy. Neuropsychologia. 1990;28:121–135. doi: 10.1016/0028-3932(90)90095-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Chu C, Rees G, Weiskopf N, Molyneux PD, Maguire EA. Decoding neuronal ensembles in the human hippocampus. Current Biology. 2009;19:546–554. doi: 10.1016/j.cub.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C. Time, space and hippocampal functions. Reviews in the Neurosciences. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proceedings of the National Academy of Sciences. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Mellet E, Briscogne S, Tzourio-Mazoyer N, Ghaëm O, Petit L, Zago L, et al. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. NeuroImage. 2000;12:588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. "I have often walked down this street before": fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner BJ. Loss of recent memory after bilateral hippocampal lesions. Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. Journal of Neuroscience. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo PC, Sapiro G, Wandell BA. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Transactions on Medical Imaging. 1997;16:852–863. doi: 10.1109/42.650881. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Woods RP, Mega MS, Toga AW. Mathematical/computational challenges in creating deformable and probabilistic atlases of the human brain. Human Brain Mapping. 2000;9:81–92. doi: 10.1002/(SICI)1097-0193(200002)9:2<81::AID-HBM3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Unfolding the human hippocampus with high resolution structural and functional MRI. The Anatomical Record. 2001;2:111–120. doi: 10.1002/ar.1061. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A weighted mask of each individual subjects’ MTL regions were used to optimize the registration to these structures. Each subject’s registration was checked manually using landmarks that are visible on both types of images (i.e. blood vessels). Shown in red are example landmarks drawn on EPIs co-registered to structural images.
Shown are the extrahippocampal ROIs average % signal changes (n = 18; error bars correspond to the standard error across subjects)) during encoding and retrieval in the regions: (a) entorhinal cortex (ERC), (b) perirhinal cortex (PRC), (c) parahippocampal cortex (PHC), and (d) fusiform gyrus. No significant differences between encoding and retrieval conditions were found.
Shown are the hippocampal ROIs average % signal changes (n = 18) during encoding and retrieval in (A) CA23DG, (A) CA1, and (C) subiculum. No significant differences between encoding and retrieval conditions were found.
Shown are the extrahippocampal ROIs average % signal changes (n = 18; error bars correspond to the standard error across subjects)) during encoding and retrieval in the regions: (a) entorhinal cortex (ERC), (b) perirhinal cortex (PRC), (c) parahippocampal cortex (PHC), and (d) fusiform gyrus. No significant differences between encoding and retrieval conditions were found.