Abstract
Previous studies suggest regulatory T cells (Tregs) inhibit graft versus host disease (GVHD) in mouse and human hematopoietic cell transplant (HCT) recipients. As the gastrointestinal tract represents one of the most common and severe sites of GVHD-related tissue damage, we sought to determine if a deficit in circulating or gastric mucosal Treg numbers correlates with the clinical onset of gastric GVHD. We used the marker FOXP3 to quantify Tregs in blood and in gastric antral biopsies in a cohort of 60 allogeneic HCT recipients undergoing endoscopy at a single center to evaluate symptoms suspicious for gastrointestinal GVHD. We show for the first time in the gastric mucosa and, contrary to existing reports, in the blood, that the percent of T cells expressing FOXP3 is at least as high in the presence as in the absence of GVHD involving the upper gut. There was no correlation of Treg frequency with the histological nor clinical severity of gastrointestinal GVHD. We conclude that Treg depletion is not a central feature in the pathogenesis of gastric GVHD in man.
Introduction
Graft-versus-host disease (GVHD) of the gastrointestinal tract represents one of the most serious and limiting complications of allogeneic hematopoietic cell transplantation (HCT)1. The pathogenesis of this disorder involves donor T cell recognition of host minor or major histocompatibility antigens, resulting in T cell activation, inflammation and ultimately the destruction of such host tissues as gastrointestinal mucosa, skin, and small bile ducts. In recent years, regulatory T cells (Tregs) have been found to play a central role in regulating T cell activation2 and preventing inflammation of the gastrointestinal tract3, 4. A relative deficiency of Tregs has been proposed to contribute to the uncontrolled inflammation of GVHD in both mouse models5–12 and humans13–18.
Tregs, which represent a small minority of CD4+ T cells, can be identified by their constitutive expression of CD25 and by nuclear expression of the transcription factor FOXP319. FOXP3 expression is necessary and sufficient for T cells to differentiate into a Treg phenotype20, 21, defined by the ability of these cells to inhibit the proliferation, cytokine secretion, and cytolytic activity of other T cells that they physically contact22, 23. Treg-mediated immune regulation appears to be particularly important in barrier organs such as the gut, as mice engineered to specifically lack Tregs4, 24 or humans born with FOXP3 mutations3, 25 develop severe inflammation of the gut that resembles gastrointestinal GVHD in man.
Murine models of GVHD have elegantly demonstrated that depletion of CD25+ Tregs promotes GVHD8, 12, while, conversely, infusion of fresh or in-vitro expanded CD4+CD25+ Tregs can either prevent or treat GVHD5, 9, 11. Prevention of GVHD required that Tregs be of donor origin7 and express CD62L6, 10. Treg infusions were able to prevent murine GVHD without compromising a desirable graft versus leukemia (GVL) effect26–28. Thus, allogeneic donor Treg infusion could represent an attractive way to treat or prevent GVHD in human HCT recipients. Similar to mouse models, Tregs in the peripheral blood of human HCT recipients have been reported to be lower in the presence than in the absence of acute GVHD14, 16, 18. Furthermore, a lower Treg content in the blood or grafts of donors, prior to infusion of donor cells, predicted the onset of GVHD13, 15–17. However, in chronic GVHD, this correlation between increased Tregs and decreased risk of GVHD has been absent29, 30 or even reversed31, although the latter study noted that Tregs expressed less CD62L in the setting of GVHD.
Most of the existing human studies have evaluated Treg content only in the peripheral blood, and did not focus specifically on gastrointestinal GVHD. However, only a very small fraction of peripheral blood Tregs bear the gut-homing integrin α4β732, and thus, peripheral blood may not accurately reflect Treg content in the gastrointestinal tract, where GVHD might presumably be influenced by Tregs. There have also been methodologic issues with some earlier studies, including the use of “high” expression levels of CD25 to identify Tregs, which must necessarily be chosen arbitrarily and identifies acutely activated T cells in addition to Tregs, as CD25 is up-regulated by T cells upon activation33. In the study reported here, we used an antibody recognizing the more specific marker FOXP3 for immunohistochemistry (IHC) and flow cytometry to identify Tregs in gastric mucosa and peripheral blood, respectively, from patients presenting for endoscopy with symptoms suspicious for gastrointestinal GVHD. We tested the hypothesis that histologic and endoscopic evidence of GVHD was correlated with a decrease in the number of Tregs in both gastrointestinal mucosa and peripheral blood. Contrary to our expectation and existing literature5–18, we found that the fraction of T cells that were Tregs was no lower in either the peripheral blood or the gastric mucosa of allograft recipients with GVHD than in those without.
Materials and Methods
Patient Selection
Under the aegis of a protocol approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center, allogeneic HCT recipients presenting for endoscopic evaluation of gastrointestinal symptoms consented to evaluation of their blood, gastrointestinal mucosal tissue samples, and medical records. Subjects with HIV or recurrence of malignancy were excluded. 79 blood and/or gastric biopsy specimens were obtained from 60 subjects, 46 having received myeloablative and 14 having received reduced-intensity conditioning regimens. Details of the subjects (treating each specimen as an independent subject) are summarized in Table 1. Indications for transplant included hematologic malignancy (n=57, comprised of acute or chronic leukemia, myelodysplastic syndrome, myeloma, or lymphoma); myelofibrosis (n=1); and aplastic anemia (n=2). Donors were related and HLA-matched (n=21) or unrelated (n=39). Of the unrelated donors, 22 were HLA-matched, and 17 mismatched.
Table 1.
| All Subjects | Histological gastric GVHD | Clinical GI GVHD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| never | at least once | never | at least once | |||||||
| total n= | 60 | 4 | 56 | 6 | 54 | |||||
| median | range | median | range | median | range | median | range | median | range | |
| Age | 54 | 21–74 | 54 | 36–60 | 53 | 21–74 | 54 | 40–62 | 53 | 21–74 |
| Day of GVHD onset | n/a | n/a | n/a | n/a | 35 | 11–976 | n/a | n/a | 36 | 11–986 |
| n | % | n | % | n | % | n | % | n | % | |
| Males | 35 | 58% | 4 | 100% | 31 | 55% | 5 | 83% | 30 | 56% |
| Myeloablative Conditioning | 46 | 77% | 2 | 50% | 44 | 79% | 3 | 50% | 43 | 80% |
| Donor: | ||||||||||
| Related | 21 | 35% | 0 | 0% | 21 | 38% | 1 | 17% | 20 | 37% |
| Unrelated, HLA-Matched | 22 | 37% | 1 | 25% | 21 | 38% | 2 | 33% | 20 | 37% |
| Unrelated with HLA-mismatch | 17 | 28% | 3 | 75% | 14 | 25% | 3 | 50% | 14 | 26% |
| Indication for HCT: | ||||||||||
| Acute Leukemia | 32 | 53% | 2 | 50% | 30 | 54% | 3 | 50% | 29 | 54% |
| Chronic Leukemia | 8 | 13% | 1 | 25% | 7 | 13% | 0 | 0% | 8 | 15% |
| Lymphoma | 3 | 5% | 1 | 25% | 2 | 4% | 1 | 17% | 2 | 4% |
| Myeloma | 3 | 5% | 0 | 0% | 3 | 5% | 1 | 17% | 2 | 4% |
| MDS | 11 | 18% | 0 | 0% | 11 | 20% | 0 | 0% | 11 | 20% |
| Myelofibrosis | 1 | 2% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 2% |
| Aplastic Anemia | 2 | 3% | 0 | 0% | 2 | 4% | 1 | 17% | 1 | 2% |
| Other GVHD sites: | ||||||||||
| Skin | 25 | 42% | 3 | 75% | 22 | 39% | 4 | 67% | 18 | 33% |
| Liver | 4 | 7% | 0 | 0% | 4 | 7% | 0 | 0% | 4 | 7% |
| GI GVHD prohylaxis: | ||||||||||
| CNI + MMF | 20 | 33% | 2 | 50% | 18 | 32% | 4 | 67% | 16 | 30% |
| CNI + MTX | 37 | 62% | 1 | 25% | 36 | 64% | 2 | 33% | 35 | 65% |
| CNI + MMF + Rapa | 3 | 5% | 1 | 25% | 2 | 4% | 0 | 0% | 3 | 6% |
Abbreviations: GVHD: graft versus host disease. GI: gastrointestinal. HLA: human leucocyte antigen. HCT: hematopoietic cell transplant. MDS: myelodysplastic syndrome. CNI: calcineurin inhibitor. MMF: mycophenolate mofetil. MTX: methotrexate. Rapa: rapamycin.
Gastrointestinal GVHD Evaluation
Patient signs and symptoms were prospectively recorded by the physician performing the endoscopy. At endoscopy, the presence or absence of focal abnormalities and diffuse mucosal pathology were noted. 1–6 biopsies were taken each from the gastric antrum, duodenum and/or rectosigmoid colon with 2.9–3.2 mm radial cut cold biopsy forceps (Boston Scientific). Biopsy specimens were placed in fresh neutral buffered formalin. Viral (CMV or adenovirus) were identified by histology, centrifugation viral culture, and immunohistology and Helicobacter pylori infections by by histological stains and CLO test., Fixed tissue specimens were oriented in paraffin blocks, serially sectioned at 4 microns, stained with hematoxylin and eosin, and evaluated by an experienced pathologist (RCH) blinded to clinical, endoscopic, and pathology information that had been reported for clinical purposes. When both gastric and colonic biopsy specimens were taken at the same endoscopy session, specimens were evaluated independently. A histologic diagnosis of GVHD was based on the presence of apoptotic destruction of mucosal crypt epithelial cells. A four-tier grading system extends from level I in which individual apoptotic epithelial cells are rare to level IV with extensive epithelial destruction associated with mucosal denudation, ulceration, hemorrhage and granulation tissue formation34, 35 Medical records were reviewed by a physician blinded to FOXP3 data, in order to determine if the clinicians caring for a given patient treated the patient for GVHD, what therapy was used, and how signs and symptoms responded to treatment. If present, GVHD was clinically considered “minimal” if symptoms resolved with topical glucocorticoid medication alone (oral beclomethasone dipropionate and/or enteric-coated budesonide); “mild” if symptoms resolved with 1 mg/kg/day prednisone or the equivalent dose of methylprednisolone; “moderate” if symptoms resolved with 2 mg/kg/day of systemic glucocorticoids; and “severe” if symptoms failed to respond to 2 mg/kg/day prednisone.
Flow Cytometry of Blood Cells
Immediately prior to the endoscopy in 59 patients, blood was taken from a central venous access line with a syringe and placed in a 10 mL heparin-containing tube. Ten patients had blood collected at two different times, and one patient had blood collected at three different times, so that a total of 70 blood samples was evaluated. Erythrocytes were lysed by calcium citrate, and residual leukocytes were stained extracellularly with fluorophor-conjugated antibodies to CD4, CD8, CD25 and CD62L (Becton Dickenson, San Jose CA) before fixation and permeabilization with a commercially available kit (eBioscience, San Diego CA) for intracellular staining with fluorophor conjugates of either an antibody to FOXP3 (clone 236A/E7, eBioscience) or an isotype-matched control antibody. Cells were scanned with a Becton Dickenson LSR2 flow cytometer and data was evaluated with FlowJo (Tree Star, Inc., Ashland OR) and Graph Pad Prism (Graph Pad Software, Inc., San Diego, CA) software. All acquisition and processing of flow cytometry data was performed by researchers blinded to clinical and histological data.
Immunohistochemistry (IHC) of gastrointestinal biopsy specimens
Paraffin blocks, containing 1 to 6 formalin-fixed endoscopic biopsies of gastric antral mucosa each, were retrieved for 25 subjects, including one from whom blood was not drawn Biopsies were evaluated at two or more time points in 15 of these individuals, resulting in a total of 43 independent evaluations. Sections were deparaffinized and subjected to antigen retrieval in neutral pH Target Retrieval Solution (DakoCytomation, Glostrup, Denmark), blocked, and then stained with either a monoclonal antibody to FOXP3 (clone 236A/E7, eBioscience) in Background Reducing Antibody Diluent (DakoCytomation), or a polyclonal antiserum to CD3 (Cell Marque, Rocklin, CA) in EDTA, using the Envision+ kit (DakoCytomation) on an automated immunostainer machine (DakoCytomation). Sections were digitally photographed in their entirety at low power, and images were processed with Photoshop software (Adobe, Woodinville, WA) to remove obvious blemishes and nonspecific IHC reaction product. Images were then analyzed with ImageJ software (NIH, Bethesda, MD) using a color deconvolution plugin to isolate DAB-stained cells and objectively count the number of FOXP3+ cells in each image. Because CD3+ cells are often too confluent to count as independent image objects, the total number of pixels staining positive for CD3 were quantified in each image and divided by the average number of positive pixels identified per CD3+ cell in non-confluent areas to estimate CD3+ T cell count. All staining and analysis was performed by investigators blinded to clinical or histological data.
Statistical Analyses
The outcome measures described in the figure legends were compared between groups of interest using generalized estimating equations (GEE) with a normal link function. This approach was required due to the fact that some patients contributed multiple samples and the within-patient correlation therefore needed to be accounted for in the estimation of the variance of the difference in measures. If only one sample were contributed per patient (as was the case for the majority of patients), this approach would function like an unpaired two-sample t-test. All p-values were for two-sided analyses.
Results
Patient Characteristics
Six subjects were found to have viral gastritis (n=5 for CMV, n=1 for adenovirus) at the time of endoscopy as an explanation for their GI symptoms, and so were excluded from GVHD+ cohorts. The remaining enrollees were determined to have GI GVHD based upon either GI biopsy histology (in the stomach and/or colon) or whether the physicians treated GI GVHD. In the latter case, GVHD severity was classified based upon patient response to treatment (see Materials and Methods). Details of patient cohorts thus classified are summarized in Table 1.
Peripheral blood Tregs in patients with gastrointestinal GVHD
Flow cytometry revealed that FOXP3 expression was restricted almost entirely to CD4+ T cells, as expected, and correlated with CD25 expression, although many FOXP3+ cells had moderate to low CD25 expression (Figure 1A). Peripheral blood Tregs from subjects in whom histologic evidence of GVHD was identified in biopsies of stomach, rectosigmoid colon, both, or neither were evaluated independently. We found the percent of CD4+ T cells that were FOXP3+ to be no lower in the blood of subjects with histologic evidence of gastrointestinal GVHD than in those without, the latter group consisting of patients with either viral (predominantly CMV) gastritis or no gastrointestinal inflammation. In fact, significantly more blood CD4+ cells were FOXP3+ if histologic evidence of GVHD was found in the stomach (p=0.003, Figure 1B). No correlation was observed between blood FOXP3+ Treg frequency and histological grade of GVHD severity in the upper or lower gastrointestinal tract, although the vast majority of specimens were grade I (data not shown).
Figure 1. Blood Tregs are not diminished in GVHD.
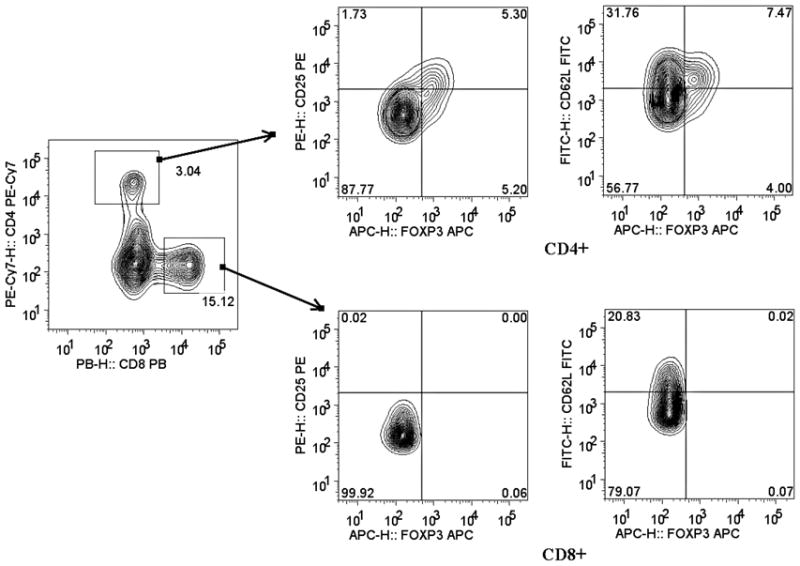
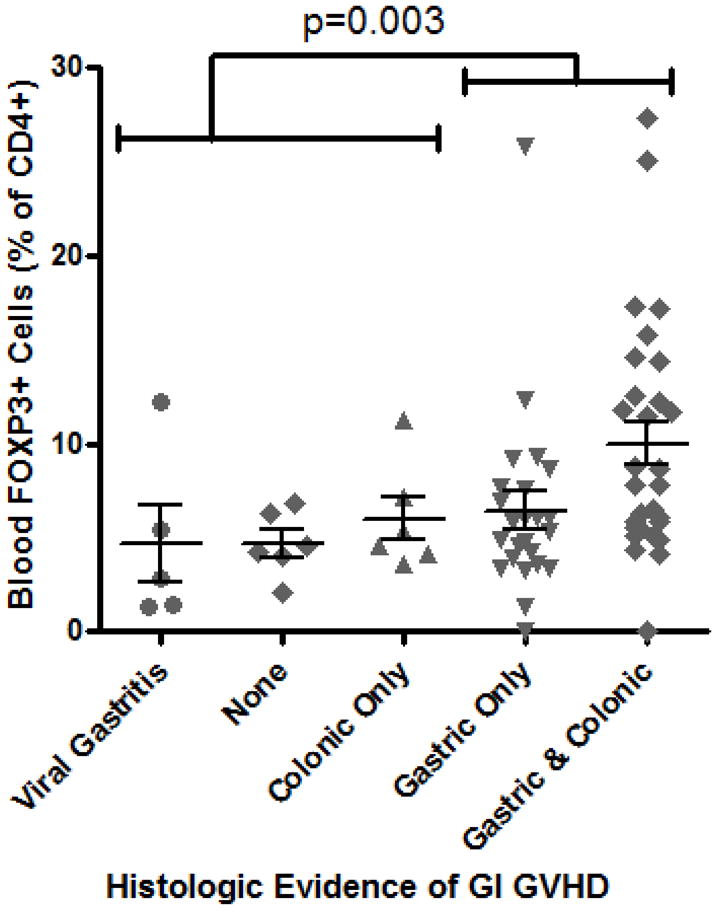
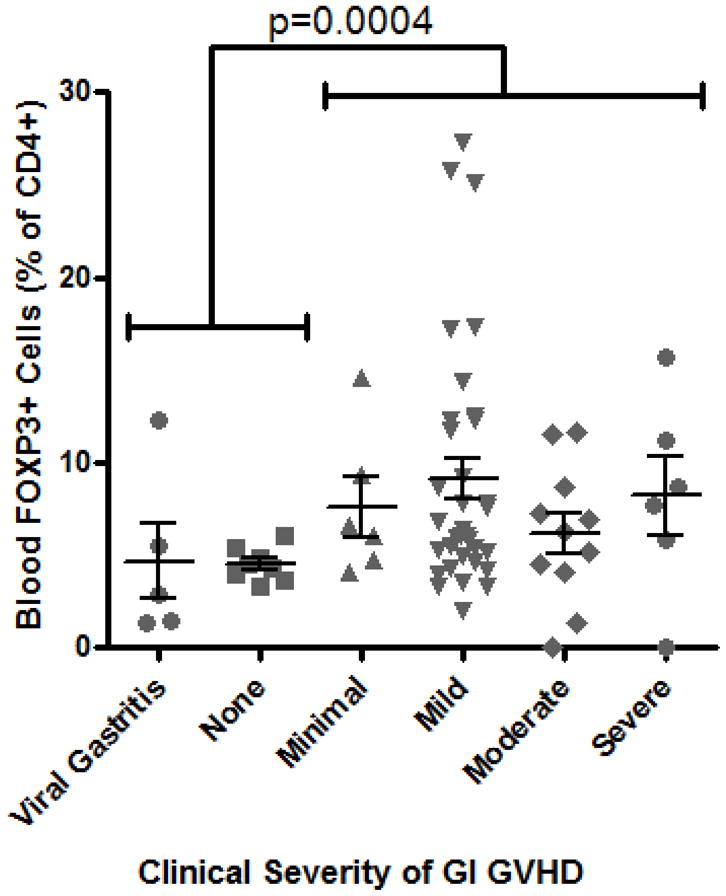
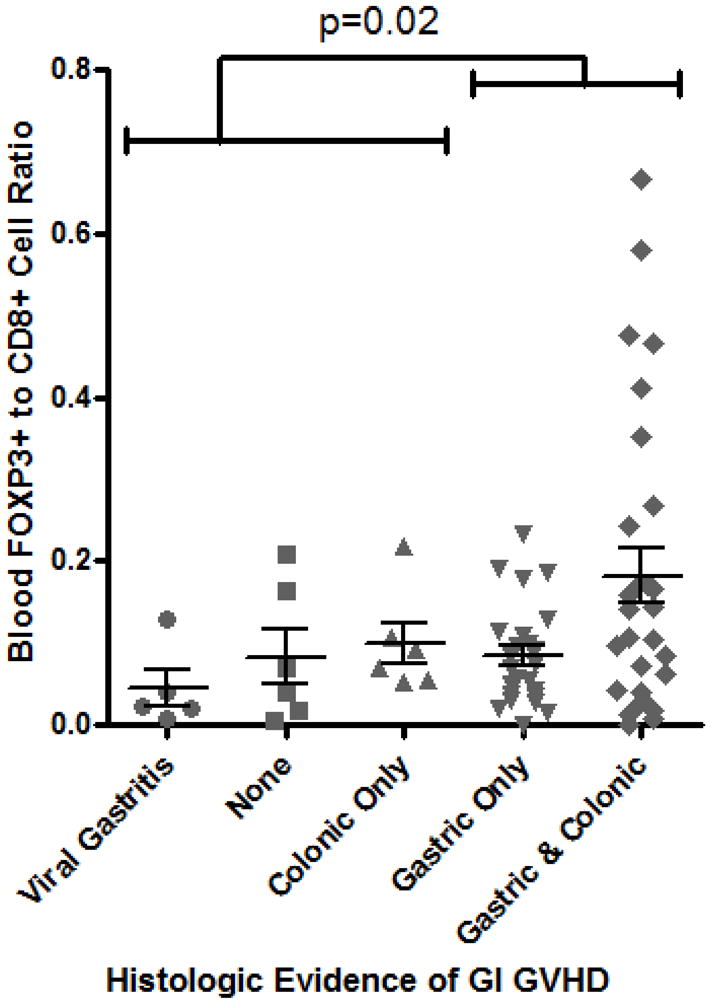
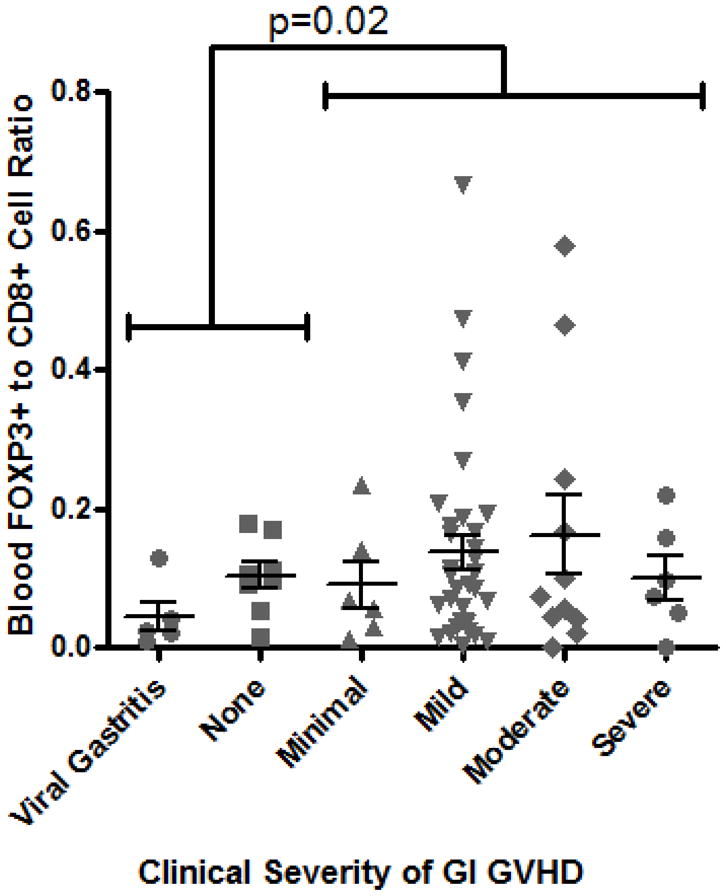
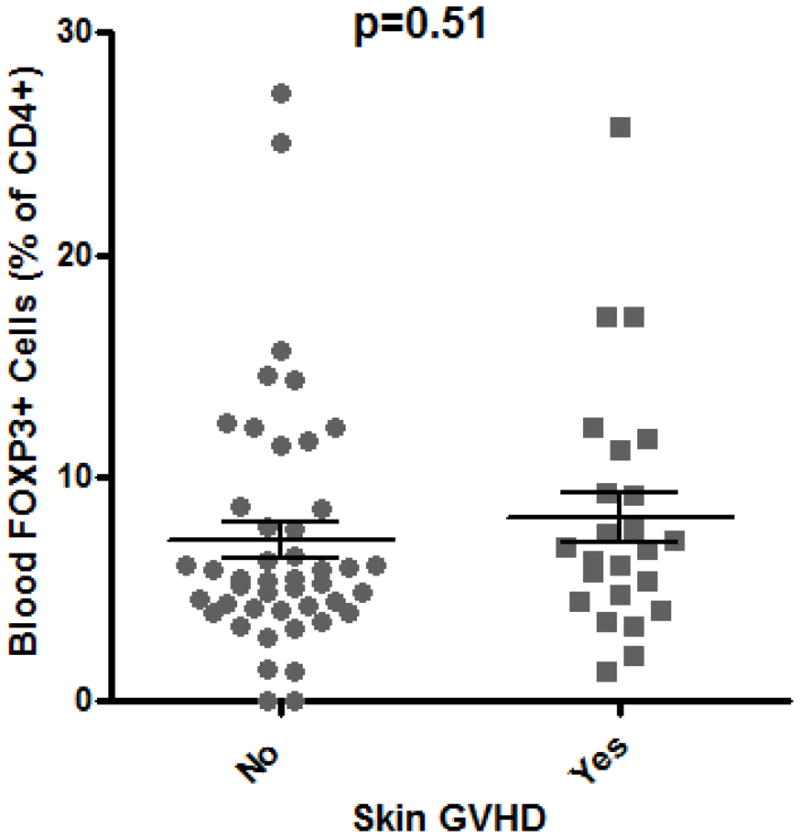
A: Representative plot of flow cytometric evaluation of blood, gated on live lymphocytes (left panel), and then gated on either CD4+ (upper panels) or CD8+ (lower panels) T cells. For each specimen, 4-quadrant gates were set to exclude >99% of cells stained with isotype control antibodies from positive quadrants (data not shown). B: The percent of CD4+ blood lymphocytes expressing FOXP3 by FACS is shown for patients with histologic evidence of GVHD in gastric biopsies (“upper GI only”), colorectal biopsies (“lower GI only”), or both (“upper & lower”) versus neither (“none”) or viral gastritis (CMV or adenovirus). The difference between all cases with versus without histologic evidence of GVHD in the stomach was analyzed to generate the p-value shown. C: FOXP3 expressing CD4+ lymphocytes were compared as in B between patients with varying clinical severity of GI GVHD, defined as described in “materials and methods”. All patients with any clinical evidence of GI GVHD were pooled for comparison with patients without clinical GI GVHD or with viral gastritis to generate the p-value shown. D, E: The ratio of blood FOXP3+ lymphocytes to CD8+ lymphocytes, determined by FACS, was compared between the cohorts described in B and C. F: FOXP3 expressing CD4+ lymphocytes were compared as in B between patients with versus without clinical evidence of skin GVHD.
Similarly, the percent of CD4+ cells in the bloodstream expressing FOXP3 did not correlate with clinical severity of gastrointestinal GVHD (as defined by treatment-responsiveness in Methods), and a paradoxically greater frequency of Tregs was identified in patients with gastrointestinal GVHD than in those without (p = 0.0004, Figure 1C). These observations were unchanged if we gated exclusively on cells that expressed both FOXP3 and CD25 (data not shown). Alloreactive donor CD8+ cytotoxic T lymphocytes (CTL) are thought to play a central role in mediating tissue damage in GVHD, so we additionally compared the relative ratio of CD4+, FOXP3+ Tregs to CD8+ CTL in these peripheral blood samples. However, the ratio of FOXP3+ to CD8+ cells was again paradoxically higher in patients with histological (Figure 1D) or clinical evidence of gastrointestinal GVHD (Figure 1E).
The presence of skin GVHD did not influence Treg frequency (Figure 1F), regardless of whether patients had histological or clinical evidence of gastrointestinal GVHD (data not shown). The shift in median fluorescent intensity between cells staining positive for FOXP3 and cells stained with an isotype-matched control antibody was used to estimate relative FOXP3 expression per Treg, and this was likewise found to be no lower in the presence than absence of histological or clinical evidence of GI GVHD (data not shown).
In mouse models, protection from GVHD has been associated specifically with Tregs that express CD62L (L-selectin) 6, 10. Therefore, we hypothesized that fewer FOXP3+ T cells would express CD62L in the circulation of patients with gastrointestinal GVHD. However, the percent of blood FOXP3+ T cells expressing CD62L was actually significantly higher in patients with histological evidence of gastrointestinal GVHD (p = 0.0004, Figure 2A) than in those without. A positive correlation between Treg CD62L expression and clinical evidence of gastrointestinal GVHD was less significant (p=0.21, Figure 2B), although the mean percent of CD62L+ Tregs was clearly no lower in the presence of GVHD. Thus, no quantitative deficiency of peripheral blood Tregs, or their expression of CD62L, was identified in GVHD.
Figure 2. Blood Treg CD62L expression are not decreased in GVHD.
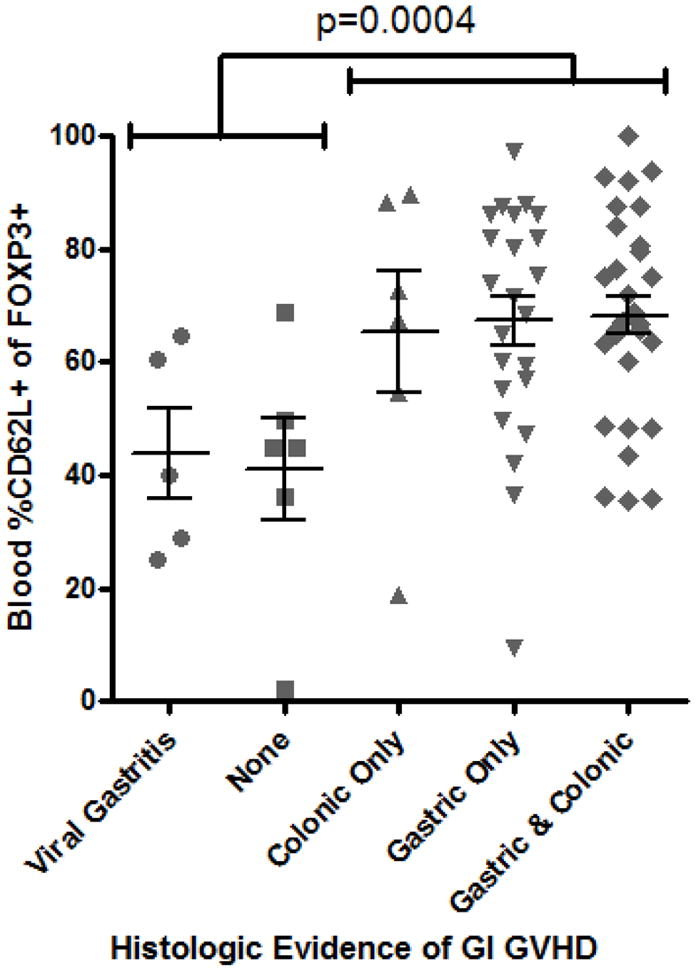
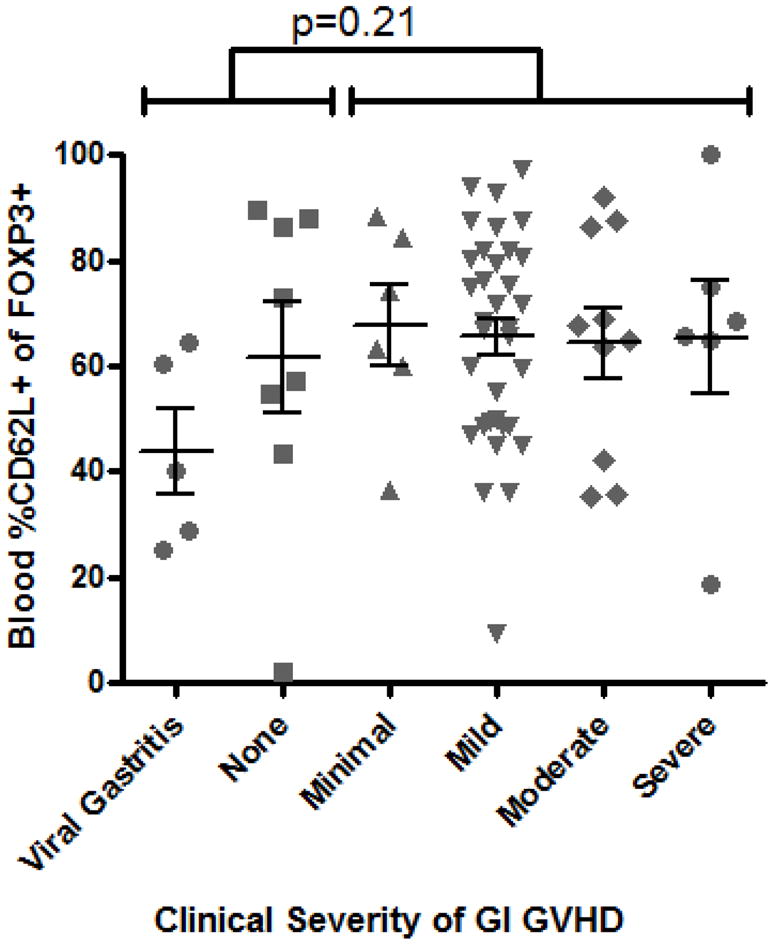
A: The percent of FOXP3+ lymphocytes expressing CD62L by FACS was compared between patients with versus without histological evidence of gastrointestinal GVHD. D: CD62L expression among FOXP3+ lymphocytes was compared, between patients with versus without any clinical evidence of GI GVHD, as described in “materials and methods”.
Mucosal Tregs in patients with gastrointestinal GVHD
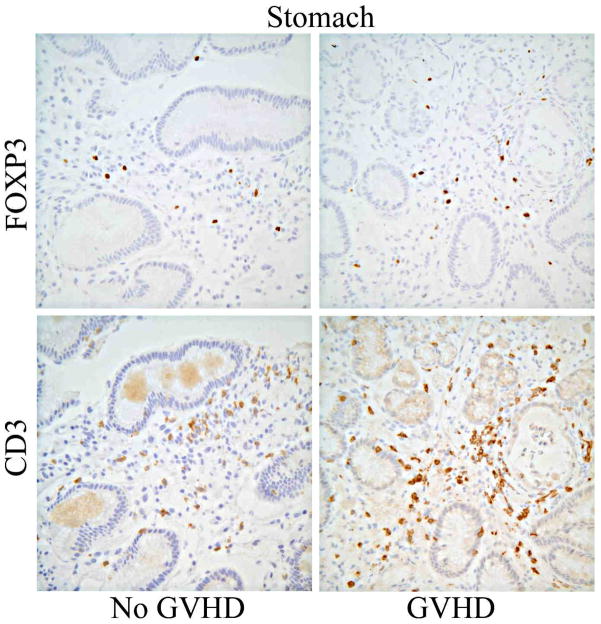
Gastric antral biopsies were evaluated for their Treg content by IHC of serial sections for FOXP3 or CD3 (Figure 3A). We found the number of FOXP3+ cells, relative to CD3+ T cells, was again paradoxically higher in gastric biopsies of subjects with gastric GVHD than in those without (p=0.02, figure 3B). Gastric Treg frequency did not drop in biopsies with a higher histological grade of GVHD, but only four specimens were read as higher than grade 1. Viral (predominantly CMV) gastritis was also associated with an increase in gastric FOXP3+ Treg frequency (p=0.02, Figure 3B).
Figure 3. Gastric mucosal Tregs are not decreased in GVHD.
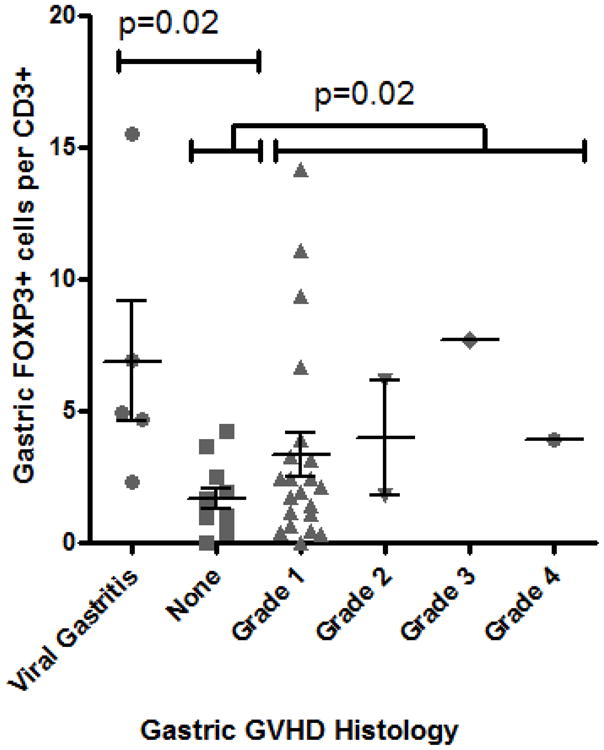
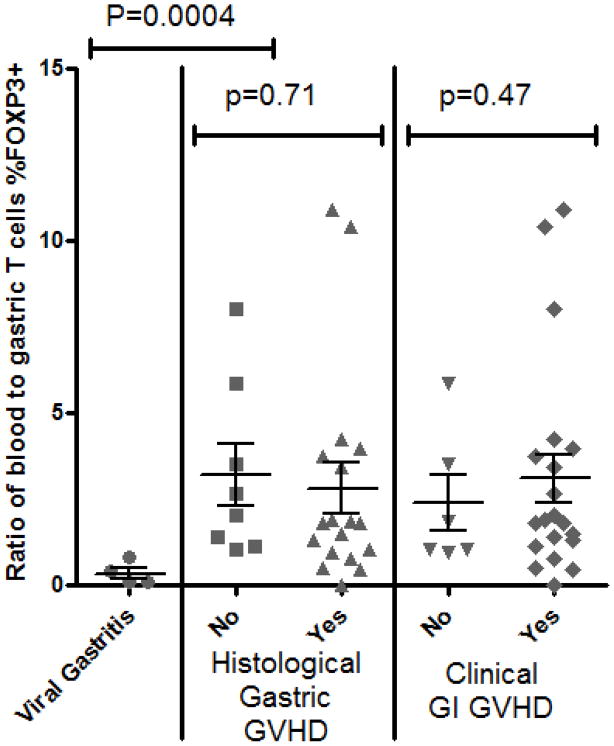
A: Representative histology of gastric biopsies from patients with (right panels) versus without (left panels) gastric GVHD, stained immunohistochemically for FOXP3 (upper panels) or CD3 (lower panels). B: The FOXP3+ to CD3+ ratio (expressed as a percent) of serial sections of gastric biopsies was compared based upon the histological grade of GVHD severity within those biopsies. P-value shown is for comparison between pooled specimens with grade I–IV GVHD, versus specimens with no GVHD (“none”) or viral gastritis. C: The percent of T cells expressing FOXP3 (per CD3+ cells in gastric biopsies, and per pooled CD4+ and CD8+ in blood) was expressed as a ratio between blood and biopsies, and compared between patients with or without histological evidence of gastric GVHD (middle) or clinical evidence of GI GVHD (right), or between patients with viral gastritis versus no histological evidence of gastric GVHD (left).
To test the hypothesis that Tregs may be sequestered from the blood into the mucosa in patients who do not develop gastrointestinal GVHD, we compared the ratio of blood to gastric Treg frequency between patients with or without GVHD in those subjects from whom both blood and mucosal biopsies had been analyzed at the same time. Treg frequency was defined as the percent of total T cells (T cells defined as CD3+ cells in mucosal biopsies, and the sum of CD4+ and CD8+ cells in peripheral blood) that expressed FOXP3. Evaluating all patients together, there was no correlation, positive or negative, between the percent of T cells expressing FOXP3 in the gastric mucosa versus blood (r2=0.001264, p=0.8369). There appeared to be more sequestration of FOXP3+ Tregs from blood to gastric mucosa in the few patients analyzed with viral gastritis than in patients without virus or gastric GVHD (p=0.0004). However, when viral gastritis patients were removed from the GVHD-negative cohort, the ratio of blood to mucosal Treg frequency did not correlate with the presence of gastrointestinal GVHD, defined either histologically or clinically (Figure 3C).
Tregs do not correlate with patient demographics, transplant characteristics, or medications
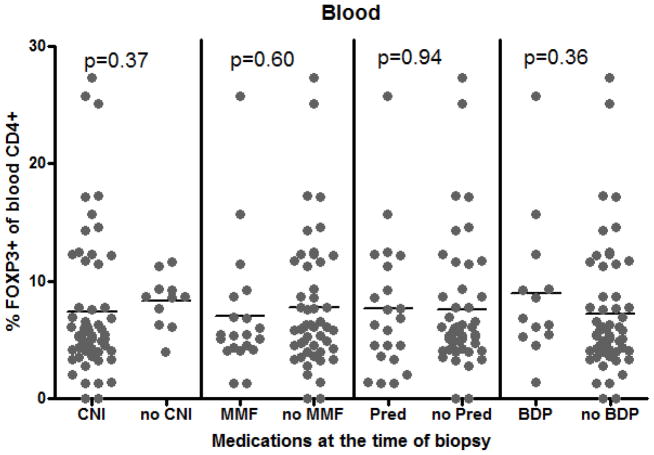
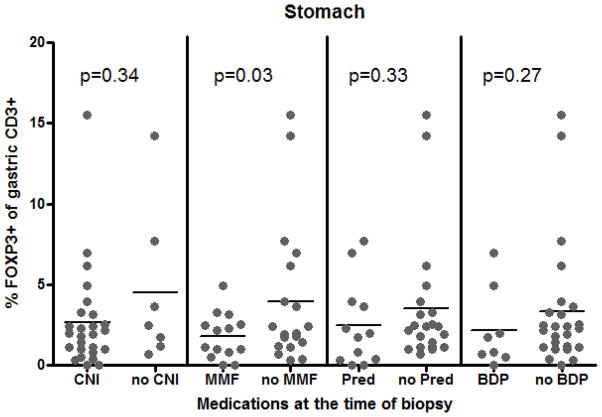
As this was a small observational study, there was necessarily considerable heterogeneity between patient comparison groups (see Table 1), raising the possibility that Treg frequency could be correlating with a characteristic other than the presence or absence of GI GVHD, so we tested a variety of potential confounders. By univariate analyses, we did not find subject age, gender, HCT indication, conditioning regimen, donor relationship, or time interval post HCT to correlate with Treg frequency in either blood or mucosal biopsies (data not shown). Whether or not patients were taking a calcineurin inhibitor (CNI), mycophenolate mofetil (MMF), or a glucocorticoid (either systemic or GI-specific) at the time of specimen acquisition had no effect on FOXP3+ Treg frequency in the blood (Figure 4A). A trend towards decreased gastric mucosal Tregs was noted in patients on any of the above (Figure 4B), but this was only significant for MMF (p=0.03). Previous use of systemic steroids post-HCT did not impact the blood or gastric FOXP3+ T cell frequency at the time of sampling (data not shown).
Figure 4. Tregs are not increased by immunosuppressant drugs.
The percent of CD4+ cells in blood expressing FOXP3 (A) or of CD3+ cells in gastric mucosa expressing FOXP3 (B) was compared between patients who were versus were not on a calcineurin inhibitor (CNI; tacrolimus or cyclosporine), mycophenolate mofetil (MMF), prednisone/methylprednisolone (Pred), or a combination of oral budesonide and beclomethasone dipropionate (BDP) at the time of biopsy.
CD3+ peripheral blood chimerism studies had been performed within 30 days of sampling for 32 of the 79 blood and/or gastric biopsy specimens used for this study; eight with reduced intensity conditioning (RIC), and 24 with myeloablative conditioning. Eighteen of these 32 specimens (and five specimens from RIC patients) were obtained approximately when there were >90% donor T cells in the peripheral blood, so only a minority of subjects had a significant number of recipient T cells still present at the time of sampling. When compared between subjects with versus without histological evidence of gastric GVHD, there was a nonsignificant trend towards more complete engraftment in patients with GVHD (p=0.1064). We also performed linear regression analyses of blood and gastric FOXP3+ cells versus chimerism, both for RIC and myeloablative regimen recipients, and found no significant correlations (data not shown).
Discussion
The principal findings of this prospective study were that FOXP3+ regulatory T cells in patients with the upper gut syndrome of acute GVHD were at least as common in both the peripheral blood and gastric mucosa as in patients without GVHD. That is, in contrast to a body of literature supporting a pivotal role for Tregs in limiting, preventing, or reversing GVHD in mice, our data do not indicate that a dearth of total FOXP3+ Tregs contributes to the pathogenesis of upper gut GVHD in humans. The technology employed in this study cannot exclude the possibility that a small subpopulation of these Tregs, perhaps with specificity to a certain antigen or allo-MHC, is depleted in GVHD. Furthermore, determining if these Tregs developed a functional defect in GVHD was outside the scope of this study. Similarly, we cannot exclude the possibility that a difference in the source of Tregs (donor versus recipient) correlates with GVHD, although this possibility would be unlikely in the peripheral blood of HCT recipients who underwent myeloablative conditioning, or otherwise were noted to have donor chimerism at or near 100% in their peripheral CD3+ cells. Indeed, in a subset of patients who were evaluated for donor-host chimerism within 30 days of study specimen acquisition, we found chimerism of CD3+ T cells to correlate with neither the presence of GVHD nor Treg frequency at any anatomic site evaluated (data not shown).
A number of differences between our study and existing reports enumerating peripheral blood Tregs in human GVHD may explain why we do not find an inverse correlation between GVHD and Treg frequency in the blood. First, our study focused primarily upon the gastrointestinal tract for the presence of GVHD, and cannot exclude a possible correlation between Tregs and GVHD involving the skin and liver. However, a substantial fraction of our subjects did have skin GVHD, with which blood Tregs showed no correlation (Figure 1E). Second, our subjects underwent initial phlebotomy and mucosal biopsy at the point of GVHD diagnosis, after the onset of symptoms and, in most cases, before the administration of systemic corticosteroids. For studies that reported a decreased Treg frequency subsequent to GVHD diagnosis13, 14, 16, 18, 36, it is possible that treatment of GVHD, particularly with systemic corticosteroids, altered Treg frequency relative to subjects without GVHD, although we did not see such an effect. Some of these studies reported a decreased Treg frequency in HCT donors or grafts or in the blood of recipients prior to GVHD onset. Therefore, it is possible that a decreased frequency of Tregs preceded the onset of clinical GVHD in our subjects, but was no longer evident by the time symptoms developed.
As noted previously, differences between our data and the existing literature may also reflect differences in the way that Tregs were identified. Many of the earlier studies of Tregs in GVHD, which were performed prior to the widespread availability of antibodies capable of recognizing FOXP3, instead used an arbitrarily high level of CD25 expression as a marker for Tregs in a population of CD4+ T cells18, 37, 38. We find that only a fraction of FOXP3+ cells express exceptionally high levels of CD25 (Figure 1A), making this marker fairly insensitive for FOXP3+ cells. Furthermore, CD25 expression is induced in all activated T cells, including FOXP3-cells, making this marker potentially nonspecific in the setting of acute inflammation. Some previous studies therefore corroborated CD25 data with FOXP3 mRNA expression levels14, 39. However, mRNA assays cannot differentiate low expression in a large fraction of cells from high expression in a small fraction of cells, and additionally may not accurately reflect protein expression. A more recent flow cytometric analysis of CD4+, CD25+, FOXP3+ cells in the blood of HCT recipients with versus without acute GVHD found no difference in relative Treg content, and additionally demonstrated that these Tregs are functional, with in vitro inhibitory activity against other T cells40..
Finally, almost all studies on Tregs in human GVHD have evaluated these cells exclusively in the peripheral blood. Only one study evaluated Tregs in colonic mucosa, finding an increased Treg frequency in HCT recipients who did not develop gastrointestinal GVHD relative to those who did, or to healthy controls41. However, the majority of patients in this study were on systemic corticosteroids at the time of biopsy, which may have altered results. We report the first evaluation of Treg frequency in the gastric mucosa of HCT recipients with the most common presentation of GVHD, that involving the upper gut42. Even in patients whose GVHD presents with diarrhea, there is more prominent apoptosis of epithelial cells in the bases of crypts in the gastric mucosa than in the colon43, suggesting that the stomach may be the more active area of alloreactivity in the GI tract, and hence a more sensitive organ in which to detect any deficiency in Tregs that might accompany GVHD. While a higher density of lymphocytes exists in the mid to distal small bowel, and might therefore be an even more representative mucosa in which to study GVHD, this area is not easily accessible with endoscopic biopsy.
Our observation that Tregs are increased in the setting of gastric GVHD seems counterintuitive, given the inhibitory role that FOXP3+ cells have been shown to have in mice. One difference between FOXP3 expression in mice and humans is that it can be induced de novo in most CD4+ T cells by in vitro activation in humans in the absence of exogenous TGF-β44–47. Therefore, the increased number of FOXP3+ T cells we have observed in GVHD may simply reflect acutely activated T cells. Consequently, the highest frequency of Tregs was seen in patients with both gastric and colonic GVHD (Figure 1B), perhaps reflecting a greater degree of systemic T cell activation in this population. Although some authors have found such activation-induced FOXP3 expression to confer no inhibitory activity44, others have reported that so-called “induced Tregs” have in vitro immuno-inhibitory activity similar to Tregs that stably express FOXP345–47, suggesting that both types of FOXP3+ T cells may be important for down-regulating inflammation. It is unclear why they would fail to do so in GVHD, but there may be differences between the in vitro and in vivo inhibitory potential of induced FOXP3+ T cells.
We conclude that upper gastrointestinal GVHD is not correlated with a global dearth of FOXP3+ Tregs in humans, in either the peripheral blood or gastric mucosa, at the time of symptomatic presentation. Consequently, enumeration of Tregs in these areas is unlikely to be of diagnostic or prognostic utility in the evaluation of upper gastrointestinal symptoms suspicious for GVHD. Our data also suggest that therapeutic infusion of Tregs may have limited efficacy in the treatment of established gastrointestinal GVHD, as the Tregs already present in patients with this disorder fail to inhibit mucosal inflammation. Indeed, the first published case reports of in vitro expanded Treg infusion in human HCT recipients were recently published48, and demonstrated no efficacy in a patient with severe acute GVHD, although their infusion may have had some effect in one with chronic GVHD. Future studies are needed to determine if there is a difference in the source (donor versus recipient) or alloreactivity of the Tregs present in patients with GVHD, in which case selective enrichment of Tregs with whichever characteristic is deficient in GVHD may provide a greater chance of therapeutic success. Alternatively, there may be conditions in the intestinal microenvironment of patients with gastrointestinal GVHD which impair the inhibitory function of Tregs, regardless of their specificity, source, or number. Indeed, ample numbers of intramucosal Tregs also exist in inflammatory bowel disease49, 50, and have intact intrinsic inhibitory function in vitro51, yet fail to control mucosal inflammation in vivo. Perhaps there are conditions within the intestinal microenvironment that impair their inhibitory function in IBD, which may also be relevant in gastrointestinal GVHD.
Acknowledgments
We thank Harmony Allison, Janelle Brown-Chang, Janet King, Andrew Scanga, and Lei Yu for subject identification, Greg Levine for flow cytometry, and Karine Valliant-Saunders for assistance with image analysis software. James Lord was supported by grants from the NIH/NIDDK (1K08 DK081659, T32 DK07742) and the NIH/NIAID (T32 AI-007411) while preparing this manuscript. Steve Ziegler was supported by a grant from the NIH/NIAID (1R21 AI069788-01). Robert Hackman and George McDonald were supported by grants from the NIH/NCI (NIH/NCI CA15704 and 18029).
Footnotes
Financial Disclosure Statement: The following authors have no financial relationships to disclose that would be pertinent to this manuscript:
James D. Lord, M.D., Ph.D.
Robert C. Hackman, M.D.
Ted A. Gooley, Ph.D.
Brent L. Wood, M.D., Ph.D.
Amanda C. Moklebust
David M. Hockenbery, M.D.
Gideon Steinbach, M.D., Ph.D.
Steven F. Ziegler, Ph.D.
George B. McDonald, M.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Iqbal N, Salzman D, Lazenby AJ, Wilcox CM. Diagnosis of gastrointestinal graft-versus-host disease. Am J Gastroenterol. 2000;95(11):3034–3038. doi: 10.1111/j.1572-0241.2000.03250.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 4.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170(8):3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196(3):401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105(5):2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson BD, Konkol MC, Truitt RL. CD25+ immunoregulatory T-cells of donor origin suppress alloreactivity after BMT. Biol Blood Marrow Transplant. 2002;8(10):525–535. doi: 10.1053/bbmt.2002.v8.pm12434947. [DOI] [PubMed] [Google Scholar]
- 9.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104(12):3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 11.Trenado A, Sudres M, Tang Q, et al. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol. 2006;176(2):1266–1273. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- 12.Xia G, Truitt RL, Johnson BD. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol Blood Marrow Transplant. 2006;12(4):397–407. doi: 10.1016/j.bbmt.2005.11.519. [DOI] [PubMed] [Google Scholar]
- 13.Mielke S, Rezvani K, Savani BN, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110(5):1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 15.Pabst C, Schirutschke H, Ehninger G, Bornhauser M, Platzbecker U. The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res. 2007;13(10):2916–2922. doi: 10.1158/1078-0432.CCR-06-2602. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf D, Wolf AM, Fong D, et al. Regulatory T-cells in the graft and the risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2007;83(8):1107–1113. doi: 10.1097/01.tp.0000260140.04815.77. [DOI] [PubMed] [Google Scholar]
- 18.Zhai Z, Sun Z, Li Q, et al. Correlation of the CD4+CD25high T-regulatory cells in recipients and their corresponding donors to acute GVHD. Transpl Int. 2007;20(5):440–446. doi: 10.1111/j.1432-2277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 21.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 22.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31(4):1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17(2):167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 25.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15(4):430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 27.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9(4):243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 28.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112(11):1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meignin V, Peffault dL, Zuber J, et al. Numbers of Foxp3-expressing CD4+CD25high T cells do not correlate with the establishment of long-term tolerance after allogeneic stem cell transplantation. Exp Hematol. 2005;33(8):894–900. doi: 10.1016/j.exphem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez J, Casano J, Alvarez MA, et al. Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2004;126(5):697–703. doi: 10.1111/j.1365-2141.2004.05108.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark FJ, Gregg R, Piper K, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103(6):2410–2416. doi: 10.1182/blood-2003-06-2073. [DOI] [PubMed] [Google Scholar]
- 32.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177(7):4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 33.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17(5):349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6(4):367–371. [PubMed] [Google Scholar]
- 35.Shulman HM, Hackman RC, Sale GE. Pathology of hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Chicester, UK: Wiley-Blackwell; 10 A.D. pp. 390–405. [Google Scholar]
- 36.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 37.Schneider M, Munder M, Karakhanova S, Ho AD, Goerner M. The initial phase of graft-versus-host disease is associated with a decrease of CD4+CD25+ regulatory T cells in the peripheral blood of patients after allogeneic stem cell transplantation. Clin Lab Haematol. 2006;28(6):382–390. doi: 10.1111/j.1365-2257.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 38.Stanzani M, Martins SL, Saliba RM, et al. CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk for graft-versus-host disease after HLA-identical stem cell transplantation in humans. Blood. 2004;103(3):1140–1146. doi: 10.1182/blood-2003-06-2085. [DOI] [PubMed] [Google Scholar]
- 39.Seidel MG, Ernst U, Printz D, et al. Expression of the putatively regulatory T-cell marker FOXP3 by CD4(+)CD25+ T cells after pediatric hematopoietic stem cell transplantation. Haematologica. 2006;91(4):566–569. [PubMed] [Google Scholar]
- 40.Noel G, Bruniquel D, Birebent B, et al. Patients suffering from acute graft-versus-host disease after bone-marrow transplantation have functional CD4+CD25hiFoxp3+ regulatory T cells. Clin Immunol. 2008;129(2):241–248. doi: 10.1016/j.clim.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 42.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic cell transplantation. N Engl J Med. 2010 doi: 10.1056/NEJMoa1004383. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox GJ, Matsui SM, Lo RS, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107(5):1398–1407. doi: 10.1016/0016-5085(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 44.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 45.Long SA, Buckner JH. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun. 2008;30(4):293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J Clin Invest. 2003;112(9):1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+ Proc Natl Acad Sci U S A. 2005;102(11):4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+C. Clin Immunol. 2009;133(1):22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128(7):1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177(9):5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makita S, Kanai T, Oshima S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173(5):3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]