Abstract
Lead (Pb) is an environmental factor suspected of contributing to neurodegenerative diseases such as Alzheimer’s disease (AD). In AD, it has been postulated that increased production and/or decreased metabolism/clearance of beta-amyloid (Aβ) may lead to amyloid plaque deposition as well as a cascade of other neuropathological changes. It has been suggested that Pb exposure may be associated with AD-like pathology and severe memory deficits in humans. Therefore, we investigated whether Pb exposure could induce Aβ accumulation in the brain. In this study, we demonstrated that acute Pb treatments lead to increased levels of Aβ in the cerebrospinal fluid (CSF) and brain tissues. Interestingly, Pb treatments did not affect Aβ production in brain neurons. Furthermore, Pb treatments significantly decreased LRP1 protein expression in the choroid plexus (CP). Our results suggest disrupted LRP1-mediated transport of Aβ in this region may be responsible for the Aβ accumulation in brain.
Keywords: Lead, Alzheimer’s disease, beta amyloid, LRP1, choroid plexus, APP transgenic mice
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. It is characterized by the progressive loss of memory and impairment of cognitive function. Accumulation of beta-amyloid (Aβ) within extracellular spaces of the brain is believed to be an initial feature of AD pathogenesis [19]. Additionally, it has been reported that Aβ can stimulate hyperphosphorylation of tau that leads to formation of neurofibrillary tangles in the brain, another hallmark of Alzheimer disease (AD) [10].
Several processes may increase Aβ levels in the brains of AD patients including increased production of Aβ, decreased clearance within neurons, and disrupted influx or efflux transport of Aβ through brain barrier systems. The blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier (BCB) are two brain barriers that separate the systemic blood circulation from the brain. The choroid plexus (CP), located within brain ventricles, is the major BCB site. CSF is produced in the brain by the choroid plexus (CP) and absorbed by arachnoid granulations. Several studies had shown the presence of Aβ and its transport across the BCB [3, 18, 20–22]. Since the CP is in direct continuity with the cerebral interstitial fluid (ISF) and CSF, Aβ in the brain extracellular space can freely enter into the CSF [2]. The concentration ratio of Aβ1-42 and Aβ1-40 in CSF has been considered a potential biomarker for AD diagnosis [25]. Aβ has been detected in the CP of AD patients [13]. This tissue has been demonstrated to be immunoreactive to antibodies against Aβ and its precursor protein, APP [4, 20], suggesting that the CP may be involved in Aβ brain clearance [1, 3]. Thus, it is interesting to explore the role of the BCB in Aβ transport and metabolism.
The sporadic nature of most AD cases strongly suggests that environmental factors may play significant roles in AD pathogenesis. Lead (Pb) is an environmental neurotoxin known to produce detrimental effects in the nervous system [17]. Pb exposure is associated with both peripheral and central neurological effects including memory deficits and AD-like pathology [24, 26]. Animal studies and human autopsy data have established a clear relationship between Pb exposure and the accumulation of Pb in the CP [16, 30]. Accumulation of Pb in the CP reduces the tightness of BCB [23]. Recent evidence suggests that Aβ1-40 is actively transported from the CSF to the blood via the CP [3] and another recent study showed an increased intracellular accumulation of Aβ in the CP of rats that had been pretreated with Pb for 24 hours [1]. However, it remains unclear how brain Aβ levels are affected by Pb treatment.
Low-density lipoprotein receptor-related protein-1 (LRP1) is a member of the LDL receptor family that is involved in the clearance pathway of Aβ [11]. Evidence suggests that LRP-1 in the BBB may play a role in Aβ efflux from the brain to the blood [9]. Recently, it was reported that reduced LRP1 expression in the CP following Pb treatments may contribute to Pb-induced intracellular accumulation of Aβ, indicating this protein may be involved in Pb-inhibited Aβ efflux through the CP [1]. However, it remains to be determined whether and how the LRP1 expression in different types of brain cells is affected by Pb in transgenic AD mice.
The current study design uses a amyloid precursor protein (APP) transgenic mouse model overexpressing human APP with a mutation (V717F) that causes an autosomal dominant form of familial AD [6, 8] in order to test whether acute Pb exposure increases brain levels of Aβ and disrupts its clearance by the CP. Studies were designed to investigate (1) the levels of Aβ in the CSF, blood and selected brain regions following acute exposure to Pb and (2) the expression of LRP1 in the CP and brain regions after Pb exposure. The outcomes of this study should help explore the mechanism whereby Pb exposure disturbs brain homeostasis of Aβ and its relationship to LRP1 in the BCB.
APP transgenic mice (APP V717F) and their wild type littermates (all on a C57BL/6 genetic background) were bred in the Animal Center at Indiana University School of Medicine. The mice were 2 months of age at the time of the experiment. The mice (n=9 per group) were injected, i.p., with 50 mg Pb-acetate/kg (i.e., 27 mg Pb/kg) or with an equivalent molar concentration of Na-acetate, and were sacrificed 24 h later. At 24 h post injection, mice were euthanized. Whole blood, CSF and brain tissues were collected from each animal and the blood was centrifuged for 10 min (2000g) to separate plasma and red blood cells (RBCs). Elemental profiling via inductively coupled plasma mass spectrometry (ICP-MS) was performed for Pb. Samples were transferred to a Teflon 96-well plate and digested with 0.15 mL of concentrated HNO3 (Mallinckrodt, AR Select grade, Hazelwood, MO, USA) at 110˚ C for 4 h. Each sample was diluted to 1.45 mL with 18 MΩ water and analyzed on a PerkinElmer Elan DRCe ICP-MS. Indium (EM Science, Gibbstown, NJ, USA) was used as an internal standard[14]. Mice cortical and hippocampal neurons were generated from the forebrains of 1-day old pups of APP transgenic mice as previously described [7]. After three days in culture, neurons were treated with 1, 5, or 10 μM Pb for 24 h. Media were then collected to determine levels of Aβtotal. Levels of Aβ were assayed by sandwich ELISA as previously described [12]. Briefly, the tissue or cell samples were assayed using 96-well ELISA plates that were coated with antibodies, 2G3, 21F12, and 266.2 (generous gifts from Eli Lilly, Indianapolis, IN, USA) to determine Aβ1-40, Aβ1-42, and Aβtotal, respectively. Biotin-3D6 (another generous gift from Eli Lilly, Indianapolis, IN, USA) was used to detect Aβ. Total protein concentrations in the brain were determined using the Bradford protein assay, and the concentration of Aβ in the tissues was reported as ng/mg of total protein. Western blot analyses were performed using an antibody directly against LRP1 (1:250, Aviva Systems Biology, San Diego, CA, USA) on the mice CP, cortex, hippocampus and cerebellum. β-actin was also assayed as a loading control. Band intensities were quantified and results were reported as the ratio of LRP1 to β-actin in CP [27]. Following Pb exposure, brains from APP transgenic mice were fixed and made into paraffin sections. The sections were stained with rabbit anti-mouse LRP1 (1:350, Aviva Systems Biology, San Diego, CA, USA), followed by a biotin goat-anti rabbit secondary antibody. After washing, the sections were incubated with fluorescein avidin DCS (1:500, Vector, Burlingame, CA, USA) for 30min and visualized under the microscope [15]. The fluorescence intensity was quantified using Image J software and reported in arbitrary units (a.u.). The data were analyzed using SPSS 16.0 for Windows (SPSS, Inc, Chicago, IL, USA). Statistical analyses of the differences between groups were done using Mann-Whitney nonparametric tests. All data are expressed as mean±SD. Quartiles were used to measure the spread of data distribution on APP mice brain Aβ levels. Differences between two means were considered significant when p was equal or less than 0.05. All statistical tests applied were 2-sided.
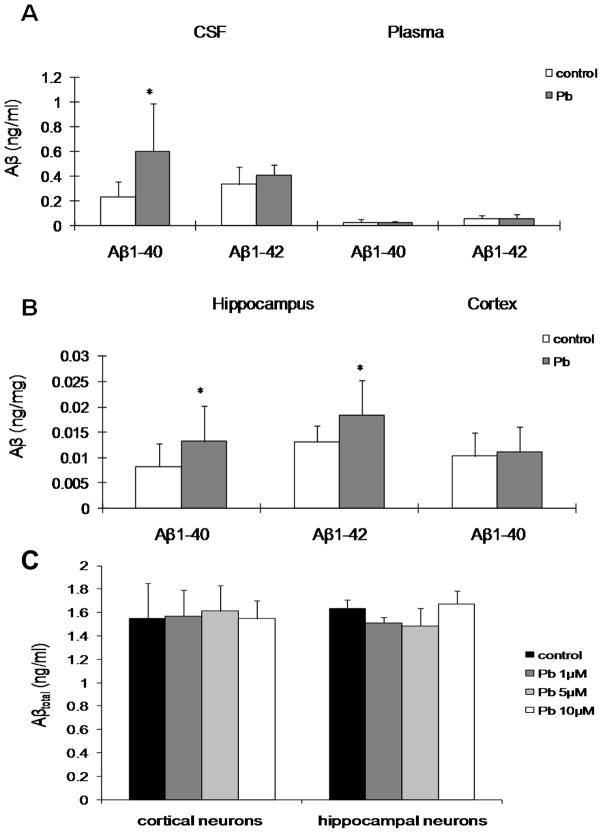
In order to examine whether Pb exposure affected brain levels of Aβ, we treated APP transgenic mice with a single injection of 50 mg/kg of Pb acetate (27mg Pb/kg) or an equivalent molar concentration of Na-acetate, intraperitoneally (ip). This dose regimen has been shown to produce a significant accumulation of Pb in the CP during a short period of time [30]. After 24 h exposure, the levels of Pb in brain tissues and blood were measured by ICP-MS. The amount of Pb in control mice CP was 0.029±0.018 μg/g tissue; cortex, 0.001±0.000 μg/g; hippocampus, 0.001±0.000 μg/g; cerebellum, 0.001±0.000 μg/g and blood, 0.452±0.291 μg/dL. In addition, The amount of Pb in Pb-treated mice was found to be about 10–200 fold greater than control mice, the CP was 0.2458±0.1106 μg/g; cortex, 0.007±0.002 μg/g; hippocampus, 0.014±0.008 μg/g; cerebellum, 0.011±0.004 μg/g and blood, 63.22±15.70 μg/dL (p<0.05, n=3–6). Pb accumulated extensively in the CP after acute exposure. Levels of Aβ1-40 and Aβ1-42 in plasma, CSF and brain tissues were determined by ELISA. The CSF levels of Aβ1-40 in Pb-treated mice and control mice were 0.590±0.300 ng/ml (median, 0.571 [range, 0.280–0.919]) and 0.255±0.120 ng/ml (median, 0.245 [range, 0.133–0.359]). The data showed a significant increase (131.4%, p<0.05) in Pb-treated mice as compared to controls, whereas there was no significant difference in levels of Aβ1-42 in the CSF (Fig. 1A). Additionally, hippocampal levels of Aβ1-40 and Aβ1-42 in Pb-treated mice were 0.013±0.007 ng/mg (median, 0.013 [range, 0.008–0.015]) and 0.018±0.007 (median, 0.019 [range, 0.014–0.021]) ng/mg; in control mice were 0.008±0.004 (median, 0.013 [range, 0.008–0.015]) ng/mg and 0.013±0.003 (median, 0.013 [range, 0.011–0.015]) ng/mg. Pb exposure significantly increased hippocampal levels of Aβ1-40 and Aβ1-42 (62.5% in Aβ1-40, and 38.5% in Aβ1-42, p<0.05) (Fig. 1B). Furthermore, cortical levels of Aβ1-40 in Pb-treated mice were also higher than the control group, although the difference did not reach a statistical significance (Fig. 1B). There were no significant differences in the cortical level of Aβ1-42 and cerebellar levels of Aβ1-40 and Aβ1-42 between Pb-treated and control groups (data not shown). The plasma levels of Aβ in both treated and untreated mice were also not significantly different (Fig. 1A).
Fig. 1.
A & B: Alteration of Aβ levels in the CSF, plasma and brain tissues of APP transgenic mice following acute Pb exposure. APP transgenic mice received a single ip injection of 50 mg/kg of Pb acetate (27 mg Pb/kg) or with an equivalent molar concentration of Na-acetate as control. The levels of Aβ1-40 and Aβ1-42 were determined by ELISA. A: Aβ1-40 and Aβ1-42 in the CSF and plasma. B: Aβ1-40 and Aβ1-42 in hippocampus and cortex. C: Effect of Pb exposure on the production of Aβtotal in primary culture of cortical and hippocampal neurons from APP transgenic mice. Cultured cells were treated with 1, 5, or 10 μM Pb for 24 h, followed by assessment of Aβtotal level. Level of Aβtotal in brain neuron conditional medium was measured by ELISA.
The white bars represent controls and the grey bars represent Pb exposed mice. Data represent mean ± SD, n = 8 per group in Fig.1.A, n=9 per group in Fig.1.B, n=6 per group in Fig.1.C; *: p <0.05.
The increase of Aβ in the brain following Pb exposure may be the result of overproduction of Aβ in the brain or disrupted transport of Aβ out of brain by the brain barrier system. To test whether Pb exposure affected Aβ production in brain, we treated primary cultures of cortical and hippocampal neurons that were isolated from the cortices and hippocampi of 1-day old APP transgenic mice pups with Pb. Following incubation of cortical and hippocampal neurons with 1, 5, or 10 μM Pb for 24 h, the levels of Aβtotal in culture media were not changed (Fig. 1C). The toxicity of Pb to neurons was also tested; the results indicated that Pb at these concentrations were not toxic to primary neurons.
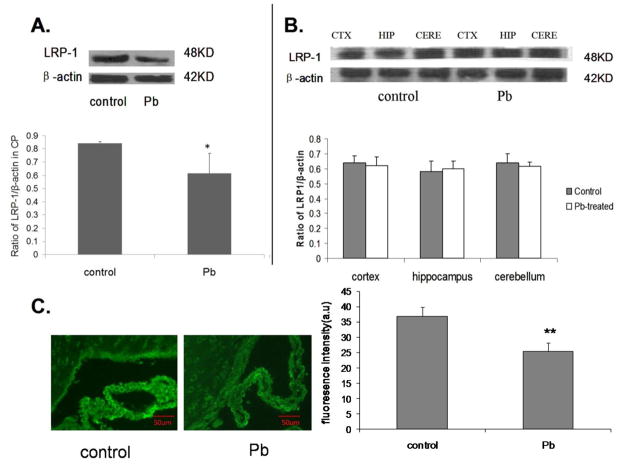
Since it appeared that acute exposure of Pb did not affect Aβ production in brain cells, we hypothesized that the increases of brain Aβ levels following Pb exposure may result from disrupted Aβ transport at the BCB system. APP transgenic mice received acute Pb exposure (27 mg Pb/kg, ip, 24 h) and the protein levels of LRP1 in the CP were determined by Western blot. Our results demonstrated that the CP levels of LRP1 were significantly lower in APP transgenic mice receiving acute Pb exposure than those without Pb treatment (27.1%, p<0.05, Fig. 2A, B). To confirm this observation, the frozen brain sections of mice with or without Pb treatments were stained with immunofluorescence-labeled LRP1 antibody. Quantification of the fluorescence intensity using Image J software revealed a significant difference between control mice (36.9±3.0) and Pb-treated mice (25.4±2.7) (p<0.001). Fluorescence intensity was expressed in arbitrary units (a.u.).The levels of LRP1 were markedly lower in Pb-treated mice than control mice (Fig. 2C). Protein levels of LRP1 in the brain regions were also determined by Western blot. There was no difference in LRP1 expression in the brains of control mice and Pb-treated mice, further suggesting LRP1 in CP may be involved in Pb-induced brain levels of Aβ.
Fig. 2.
Alteration of LRP1 protein expressions in the CP, cortex, hippocampus and cerebellum of APP transgenic mice following acute Pb exposure. LRP1 protein expression was determined by Western blot; β-actin was used as the protein loading control. Amounts of LRP1 were estimated from the corresponding band densities and normalized to those of β-actin. A: LRP1 protein expression in the CP. B: LRP1 protein expression in cortex, hippocampus and cerebellum. C: Immunofluorescence staining of LRP1 in the CP of APP transgenic mice. Data represent mean ± SD, n=3 per group; *: p <0.05, **: p<0.001.
Exposure to Pb has caused symptoms and features similar to AD including memory deficits and neurodegeneration [24, 28]. However, it remains unclear whether Pb-associated memory deficits are due to Aβ accumulation in brain. The concentration of Pb in human blood at which results in Pb-induced neurotoxicity is 25 μg/dL or higher (>70 μg/dL) (also called an elevated blood lead level or EBLL). The CDC’s National Surveillance Data (1997–2007) showed that some children have EBLL >=70 μg/dL. In the present study, the blood Pb concentration in Pb-treated mice is about 60 μg/dL. Thus, compared to human exposure, the Pb dose used in this study is causes symptoms similar to those found in children. Animal studies and human autopsy data have shown that the CP is one of the brain targets of Pb accumulation [16, 30]. Our data also demonstrate that after acute Pb exposure, Pb in the CP is increased compared to other brain regions. Our data clearly demonstrate that acute exposure to Pb increases levels of Aβ in the CSF and hippocampus in APP transgenic mice, which genetically over-express Aβ. This is the first study showing that Pb acute exposure increases brain Aβ levels in APP transgenic mice. In the present study, the CSF level of Aβ1-40 showed a substantial elevation in Pb-treated mice as compared to controls. Additionally, both Aβ1-40 and Aβ1-42 were significantly increased in the hippocampus, a region with the most abundant Aβ software production. Interestingly, this phenomenon between Pb and Aβ was not found in primary cortical and hippocampal neuronal cultures. The results suggest that the increased brain levels of Aβ may be not a direct result of overproduction of Aβ in the brain. Similar to CSF, cortical levels of Aβ1-40 in Pb-treated mice were also higher than the control group, albeit not significantly. Additionally, there was no significant difference in the level of Aβ1-42 in CSF and cortex. Aβ1-40 is soluble and easily cleared by the brain barrier system, while Aβ1-42 is more hydrophobic, rendering its transport through the brain barrier system to the plasma more difficult. Therefore, Pb exposure appears to inhibit primarily Aβ1-40 not Aβ1-42 transport in the brain barrier systems. In other words, this phenomenon suggests that Pb exposure may decrease or inhibit Aβ clearance systems. Noticeably, the plasma levels of Aβ in Pb exposed mice were not changed. Since the levels of Aβ in plasma were very low, it is possible that the subtle changes of eluted Aβ in the relatively large volume of blood following Pb treatments may be diluted below the detection limit in the current experiment. In order to further elucidate the relationship between Pb exposure and brain accumulation of Aβ, a chronic lead exposure experiment is currently underway.
LRP1 is considered to be an important protein that mediates the transport of Aβ through cellular membranes in the brain [11]. A previous study with rats showed that Pb pretreatment inhibits LRP-1 expression in the CP and this inhibition may cause an intra-cellular accumulation of infused synthetic Aβ [1]. However, it remains unclear whether acute exposure of Pb in transgenic APP animals with genetically over-expressed Aβ could also affect CP levels of LRP-1. Data presented in this study clearly indicate that exposure to Pb in APP transgenic mice results in a substantial decrease in the CP level of LRP1, which does not occur in other brain regions tested. This suggests that increased levels of Aβ after Pb treatment in transgenic APP mice is mediated, at least in part, by the inhibition of LRP1 in the CP, but not in the brain parenchyma. Conceivably, a decreased level of LRP1 may result in a functional deficit in CP transport of Aβ from the CSF to the blood stream as observed in our study with increased Aβ in mice CSF and brain tissue. Thus, it becomes logical that LRP1 plays an important role in transporting Aβ out of the CSF, and this process could be altered by Pb accumulation in the CP tissue. Interestingly, the promoter region of the LRP1 gene contains a Sp1-rich domain [5] and Pb has been shown to alter the binding of Sp1 to its targeted DNA sequences [29]. Thus, it is possible that Pb, by interfering with the Sp1 binding capacity, may interfere with gene expression of LRP1. Certainly, it remains to be determined why acute exposure of Pb only affects CP levels of LRP1. Furthermore, since CSF is reabsorbed at the granulationes arachnoideales, it is reasonable to investigate whether Aβ levels in that area are affected by Pb exposures in future.
In summary, acute exposure to Pb leads to increased CSF and hippocampal levels of Aβ in APP transgenic mice. This effect may be due to its inhibition of LRP1, a key Aβ transport protein in the CP. Our study suggests that Pb is a potential environmental factor in the dysregulation of Aβ homeostasis and may subsequently contribute to the pathogenesis of AD.
Research Highlights.
Pb significantly increased Aβ levels in CSF and hippocampus.
Accumulation of Pb decreased LRP1 protein expression in the choroid plexus.
Pb didn’t affect Aβ production of cortical and hippocampal neurons.
Acknowledgments
This work was supported in part by NIH/National Institute of Environmental Health Sciences Grants Numbers ES017055 (YD, WZ) and ES008146 (WZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol Appl Pharmacol. 2009;240:245–254. doi: 10.1016/j.taap.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230:771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossgrove JS, Smith EL, Zheng W. Macromolecules involved in production and metabolism of beta-amyloid at the brain barriers. Brain Res. 2007;1138:187–195. doi: 10.1016/j.brainres.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson PA, Hofmann SL, van der Westhuyzen DR, Sudhof TC, Brown MS, Goldstein JL. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988;263:3372–3379. [PubMed] [Google Scholar]
- 6.Dodart JC, Bales KR, Johnstone EM, Little SP, Paul SM. Apolipoprotein E alters the processing of the beta-amyloid precursor protein in APP(V717F) transgenic mice. Brain Res. 2002;955:191–199. doi: 10.1016/s0006-8993(02)03437-6. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Wei X, Dodel R, Sommer N, Hampel H, Gao F, Ma Z, Zhao L, Oertel WH, Farlow M. Human anti-beta-amyloid antibodies block beta-amyloid fibril formation and prevent beta-amyloid-induced neurotoxicity. Brain. 2003;126:1935–1939. doi: 10.1093/brain/awg191. [DOI] [PubMed] [Google Scholar]
- 8.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 9.Goto JJ, Tanzi RE. The role of the low-density lipoprotein receptor-related protein (LRP1) in Alzheimer's A beta generation: development of a cell-based model system. J Mol Neurosci. 2002;19:37–41. doi: 10.1007/s12031-002-0008-4. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 22:22–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyslop PA, Bender MH. Methods for sample preparation for direct immunoassay measurement of analytes in tissue homogenates: ELISA assay of amyloid beta-peptides. Curr Protoc Neurosci. 2002;Chapter 7(Unit 7):20. doi: 10.1002/0471142301.ns0720s18. [DOI] [PubMed] [Google Scholar]
- 13.Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer's disease. Brain Res Mol Brain Res. 1996;35:58–68. doi: 10.1016/0169-328x(95)00180-z. [DOI] [PubMed] [Google Scholar]
- 14.Kambe T, Geiser J, Lahner B, Salt DE, Andrews GK. Slc39a1 to 3 (subfamily II) Zip genes in mice have unique cell-specific functions during adaptation to zinc deficiency. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1474–1481. doi: 10.1152/ajpregu.00130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z, Wei X, Fontanilla C, Noelker C, Dodel R, Hampel H, Du Y. Caffeic acid phenethyl ester blocks free radical generation and 6-hydroxydopamine-induced neurotoxicity. Life Sci. 2006;79:1307–1311. doi: 10.1016/j.lfs.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Manton WI, Kirkpatrick JB, Cook JD. Does the choroid plexus really protect the brain from lead? Lancet. 1984;2:351. doi: 10.1016/s0140-6736(84)92719-3. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti C. Molecular targets of lead in brain neurotoxicity. Neurotox Res. 2003;5:221–236. doi: 10.1007/BF03033142. [DOI] [PubMed] [Google Scholar]
- 18.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 19.Ogomori K, Kitamoto T, Tateishi J, Sato Y, Suetsugu M, Abe M. Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol. 1989;134:243–251. [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki A, Iijima M, Yokoo H, Shoji M, Nakazato Y. Human choroid plexus is an uniquely involved area of the brain in amyloidosis: a histochemical, immunohistochemical and ultrastructural study. Brain Res. 1997;755:193–201. doi: 10.1016/s0006-8993(97)00097-8. [DOI] [PubMed] [Google Scholar]
- 21.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer's disease. Front Biosci. 2003;8:s515–521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- 22.Serot JM, Foliguet B, Bene MC, Faure GC. Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci. 2001;14:794–798. doi: 10.1046/j.0953-816x.2001.01693.x. [DOI] [PubMed] [Google Scholar]
- 23.Shi LZ, Zheng W. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum Exp Toxicol. 2007;26:159–167. doi: 10.1177/0960327107070560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115:483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spies PE, Slats D, Sjogren JM, Kremer BP, Verhey FR, Rikkert MG, Verbeek MM. The Cerebrospinal Fluid Amyloid beta(42/40) Ratio in the Differentiation of Alzheimer's Disease from Non-Alzheimer's Dementia. Curr Alzheimer Res. 2009 doi: 10.2174/156720510791383796. [DOI] [PubMed] [Google Scholar]
- 26.Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S, Youssem D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- 27.Tan J, Ma Z, Han L, Du R, Zhao L, Wei X, Hou D, Johnstone BH, Farlow MR, Du Y. Caffeic acid phenethyl ester possesses potent cardioprotective effects in a rabbit model of acute myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;289:H2265–2271. doi: 10.1152/ajpheart.01106.2004. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Luo W, Zheng W, Liu Y, Xu H, Zheng G, Dai Z, Zhang W, Chen Y, Chen J. Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol Appl Pharmacol. 2007;219:33–41. doi: 10.1016/j.taap.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zawia NH. Transcriptional involvement in neurotoxicity. Toxicol Appl Pharmacol. 2003;190:177–188. doi: 10.1016/s0041-008x(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Perry DF, Nelson DL, Aposhian HV. Choroid plexus protects cerebrospinal fluid against toxic metals. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]