Abstract
Light chain receptor editing is an important mechanism that prevents B cell self-reactivity. We have previously shown that tonic signaling through the BCR represses RAG expression at the immature B cell stage, and that initiation of light chain rearrangements occurs in the absence of these tonic signals in an in vitro model of B cell development. To further test our hypothesis we studied the effect of itpkb deficiency (itpkb-/- mice) or Raf hyper-activation (Raf-CAAX transgenic mice), two mutations that enhance BCR signaling, on receptor editing in an in vivo model. This model relies on transferring bone marrow from wild-type or mutant mice into mice expressing an anti-kappa light chain transgene. The anti-kappa transgene induces receptor editing of all kappa light chain expressing B cells, leading to a high frequency of lambda light chain expressing B cells. Anti- κ transgenic recipients of bone marrow from itpkb-/- or Raf-CAAX mice showed lower levels of editing to λ light chain than did non-transgenic control recipients. These results provide evidence in an in vivo model that enhanced BCR signaling at the immature B cell stage of development suppresses light chain receptor editing.
Keywords: receptor editing, tonic BCR signaling
1. Introduction
Maintenance of B cell tolerance is critical for avoiding autoimmunity. The three processes known to tolerize B cells to self-proteins are clonal deletion, functional inactivation (anergy) and receptor editing. Evidence suggests that as many as 75% of the BCRs generated during B cell development are initially self-reactive [1] but these cells are usually tolerized thereby preventing autoimmunity. Importantly, up to 50% of B cells that make it out of the bone marrow have gone through receptor editing, which makes this process crucial for normal immune system function [2].
Receptor editing occurs at the immature B cell stage in the bone marrow, usually via excision of a rearranged light chain gene and rearrangement of a new light chain. The light chain loci contain variable (V) and joining (J) regions that are rearranged by the RAG protein complex at the immature B cell stage of development. The structure of the light chain loci makes sequential replacements possible. Specifically, when one light chain is part of a self-reactive BCR, it can undergo a secondary rearrangement in which an upstream V region is rearranged with a downstream J region, thereby excising the previously rearranged gene. If the secondary rearrangement is in-frame this generates a second BCR, which may have lost self-reactivity. Alternatively, if this rearrangement is not in-frame, or if rearrangement involves the κ-deleting element [3], then rearrangement on the other κ allele or at the λ light chain locus allows for a new light chain to be generated. This light chain then pairs with the existing heavy chain and goes through the process of selection again. Thus, receptor editing enhances the efficiency of B cell development by reducing the number of immature B cells that undergo apoptosis due to negative selection.
There are at least two ways in which receptor binding can alter BCR-dependent signaling. First, high affinity binding can clearly trigger BCR-dependent signal transduction pathways and this could potentially directly trigger receptor editing [4]. Alternatively, we have found that surface expression of IgM negatively correlates with RAG induction, suggesting that basal signals from the BCR inhibit receptor editing [5]. In this model, the BCR constantly induces low level basal signals that inhibit rag gene expression and subsequent antigen binding terminates these basal signals by promoting receptor internalization [6].
Nemazee and colleagues recently developed a new model that allows one to analyze receptor editing in a polyclonal system [7]. This system involves a pseudo-antibody transgene that is ubiquitously expressed and binds to the κ light chain, rendering all κ-expressing B cells self-reactive. This system induces both clonal deletion (B cell numbers are about half that in WT mice) and receptor editing (all remaining B cells express the λ light chain). By generating bone marrow chimeras in which donor bone marrow from mice defective in specific genes is injected into lethally irradiated anti-κ host mice, we can test the role of these specific genes on receptor editing. The original description of the anti-κ chimeras showed that editing in rag1+/- heterozygous bone chimeras was decreased to half the level observed in WT bone marrow chimeras [7]. These initial studies also tested the role of Bcl-2 (using bcl2 transgenic bone marrow) and found enhanced λ light chain expression due to the lengthened lifespan of the B cells with the anti-apoptotic bcl-2 transgene. Thus, the anti-kappa mice provide a useful model system for analyzing receptor editing in a polyclonal B cell population in vivo.
To test the hypothesis that BCR signaling inhibits receptor editing, we have used the anti-κ chimera system with bone marrow donors that should enhance basal signaling. The two mutants we used are itpkb-/- and Raf-CAAX transgenic mice. Itpkb-/- mice cannot convert IP3 into IP4; IP4 is required to inhibit store-operated calcium (SOC) channel activity [8]. Thus, immature B cells in the bone marrow of itpkb-/- mice exhibited increased calcium signaling when compared to WT immature B cells [9]. Raf-CCAX transgenic mice express a membrane targeted form of Raf, which leads to constitutive activation of the Raf/Mek/Erk signaling pathway [10,11]. If our hypothesis that receptor editing occurs due to reduced basal signaling following receptor internalization is correct, then we predict that receptor editing should be inhibited in both itpkb-/- and Raf-CAAX transgenic B cells. Such a result would complement our previous in vitro studies [5], and suggest that this model is operative in vivo when studied using a polyclonal population of B cells.
Using this system, we found that both itpkb-/- and Raf-CAAX gain-of-signaling mutant immature B cells had impaired editing responses when compared to WT immature B cells. Specifically, the mutant B cells had more residual κ-expressing cells and fewer λ-expressing cells in the bone marrow of anti-κ chimeras leading to a decreased λ/κ ratio. These results suggest that at the immature B cell stage, basal BCR signals involving Raf- and calcium-dependent pathways impair receptor editing, and that abrogation of such signals promotes receptor editing.
2. Materials & Methods
2.1 Mice
Itpkb-/-, Raf-CAAX and anti-κ transgenic mice and have been described previously [7,8,12]. CD45.1 mice were obtained from Jackson Laboratories (Stock number 002014). Mice used were between 6-12 weeks old and were maintained in specific pathogen-free conditions. All experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee.
2.2 Bone Marrow Chimeras
All recipient mice were congenically marked with the CD45.1 allele, while donors carried the CD45.2 allele. Recipients were either anti-κ transgenic or littermate controls. Recipients received 900 rads of γ irradiation from a cesium source on the morning of the transfer. Two hundred thousand to sixteen million cells were transferred i.v. per recipient. After 6 weeks the recipients were euthanized and bone marrow was analyzed by flow cytometry.
2.3 Flow cytometry
Single cell bone marrow suspensions were prepared by flushing BM from tibiae and fibulae, and mashing through a 40um filter with a 1cc syringe plunger, then rinsed with complete media (90% RPMI 1640 (Mediatech, Washington, DC), 10% heat-inactivated FBS (Atlas Biologicals, Fort Collins, CO), l-glutamine, penicillin, and streptomycin. Cell suspensions were depleted of RBC by lysis with 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA.
Cells were stained in FACS buffer (PBS with 2.5% FBS and 0.2% sodium azide) with APC-Igλ (BioLegend, San Diego, CA) or FITC-Igλ (BD Biosciences, San Jose, CA) and PE- Igκ (clone 187.1, SouthernBiotech, Birmingham, AL), Pacific blue-B220 (eBioscience, San Diego, CA), and Alexa Fluor® 700-B220 (eBioscience, San Diego, CA). Stained cells were analyzed on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using the FlowJo software (Treestar, Ashland, OR). Cells were gated on forward and side scatter to avoid contamination of dead cells or debris.
3. Results and Discussion
3.1 WT B cells edit efficiently in anti-κ chimeras
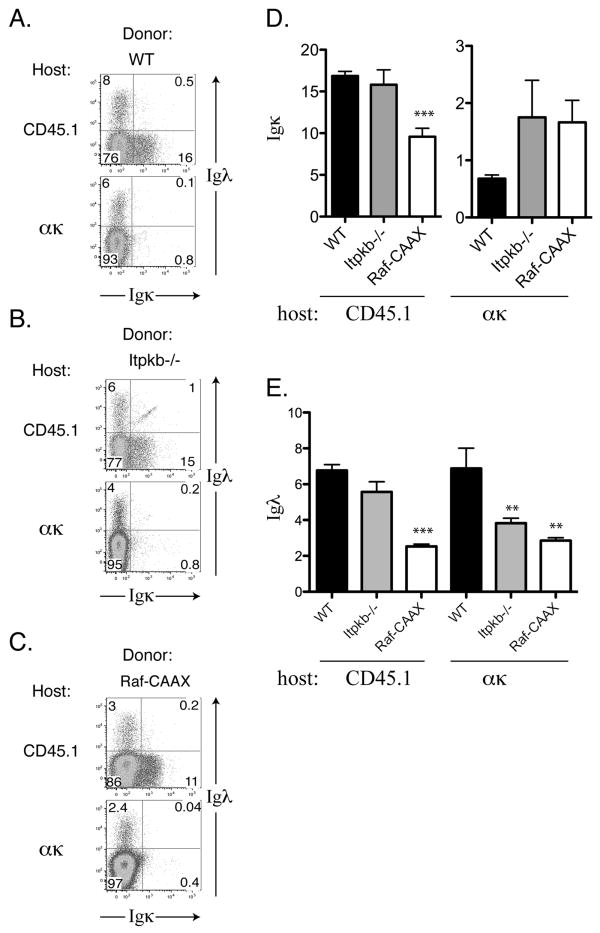
In order to test whether BCR signaling promotes or inhibits receptor editing, two BCR signaling mutants were tested as bone marrow donors in anti-κ chimeras. First, as a positive control we transferred 12 × 106 CD45.2+ C57Bl/6 cells (via i.v. injection) into lethally irradiated CD45.1 anti-κ transgenic mice or CD45.1 littermates. After six weeks the bone marrow from these chimeras was harvested and analyzed by flow cytometry. The immature B cells in the bone marrow of WT chimeras edited efficiently away from κ expression in anti-κ hosts (6.9% λ, 0.68% κ) while showing normal κ and λ expression in CD45.1 WT hosts (17% κ, 6.8% λ; Figure 1A). Thus, the presence of the anti-kappa transgene on host stromal cells effectively induces receptor editing.
Figure 1. Light chain expression of donor cells in BM chimeras.
Flow cytometry of the BM was performed, with a forward/side scatter gate for lymphocytes, then a CD45.2+ B220int gate was applied. Left, flow cytometry plots showing Igκ expression on the x-axis, while the y-axis shows Igλ expression. Right, the percentages of Igκ and Igλ-expressing cells are plotted in ακ hosts (left) and CD45.1 hosts (right) with standard error shown as error bars. A, WT donors, N=4 for ακ hosts and N=4 for CD45.1 hosts. B, Itpkb-/- donors, N=7 for for ακ hosts and N=4 for CD45.1 hosts. C, Raf-CAAX donors, N=6 for ακ hosts and N=7 for CD45.1 hosts. D, Percentage of immature Igκ+ B cells in CD45.1 hosts (left) and ακ hosts (right). E, Percentage of immature Igλ+ B cells in CD45.1 hosts (left) and ακ hosts (right). Error bars represent standard error. Asterisks indicate two-tailed student's t-test values, * p<0.05, ** p<0.01, *** p<0.005.
3.2 Itpkb-deficient cells have defective receptor editing in anti-κ chimeras
Inositol 1,4,5-triphosphate kinase B (Itpkb) converts inositol 1,4,5-triphosphate (IP3) into inositol 1,3,4,5-tetrakisphosphate (IP4) upon BCR stimulation. IP3 activates calcium signaling, while the conversion of IP3 to IP4 initiates a negative feedback loop that dampens calcium signaling after BCR stimulation. In the absence of Itpkb, calcium signaling is enhanced and lasts longer due to greater SOC channel activity [8]. Mice lacking Itpkb have a defect in T cell development in the thymus but normal B cell development in the bone marrow. However, peripheral subsets of B cells are reduced due to enhanced negative selection in the spleen.
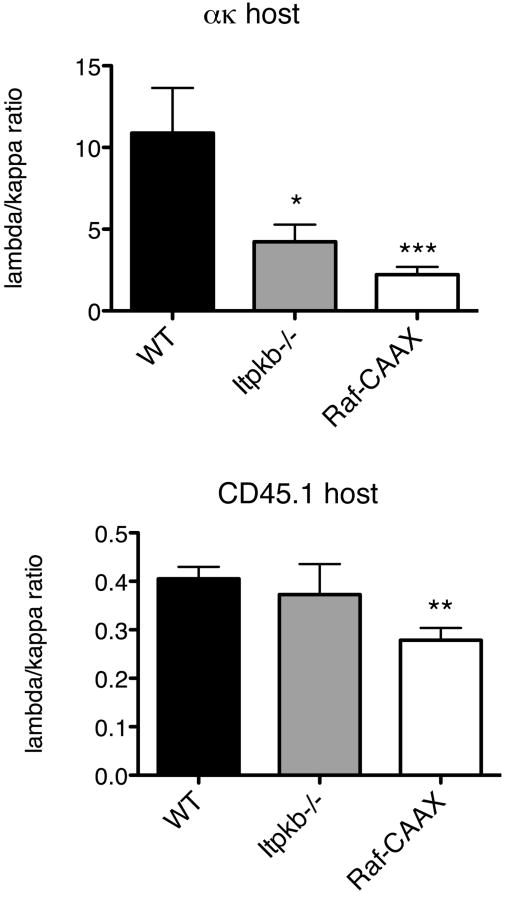
When itpkb-/- bone marrow was used to make bone marrow chimeras with anti-κ hosts, we observed a defect in receptor editing. At the immature B cell stage there were nearly twice as many residual κ-expressing cells and significantly fewer λ-expressing cells (44% of WT, p = 0.008) (Figure 1B,D,E). This can be more easily visualized by calculating the ratio of λ to κ expressing cells, which we refer to as the editing ratio; lower λ/κ editing ratios indicate less effective receptor editing. The λ/κ editing ratio in bone marrow chimeras generated with itpkb-/- bone marrow was significantly lower than the λ/κ editing ratio observed when using WT bone marrow (39% of WT, p = 0.02; Figure 2). In contrast, when itpkb-/- bone marrow was used to make bone marrow chimeras with CD45.1 WT hosts, there was no difference in the percentage of κ- or λ-expressing cells (p = 0.60 and p = 0.12, respectively) (Figure 1B,D,E), nor was there a difference in the editing ratio in the immature B cells in the bone marrow (p = 0.65 and p = 0.62, respectively; Figure 2). Thus, itpkb-/- cells show defects in editing only in the presence of an overwhelming stimulus to edit (the anti-κ Tg), not in a normal environment, presumably because the defect in BCR signaling is only evident after BCR stimulation, which will occur for all developing B cells in the anti-κ hosts but only in a fraction of such cells in CD45.1 WT hosts.
Figure 2. Editing Ratio in BM Chimeras.
Flow cytometry of BM, with a forward/side scatter gate for lymphocytes, then CD45.2 and B220 gates applied. The percentage of λ-expressing cells was divided by the percentage of κ-expressing cells to obtain the editing ratio, plotted with standard error shown as error bars. Asterisks indicate two-tailed student's t-test values, * p<0.05, ** p<0.01, ***p<0.005.
3.3 The Ras/Raf/MEK/ERK pathway inhibits receptor editing
Bone marrow from Raf-CAAX mice was used in the anti-κ system in order to test the affect of altering the Raf signaling pathway downstream of the BCR. Raf-CAAX mice have an expansion of pro-B cells in the bone marrow [12]. When the bone marrow from Raf-CAAX mice was transferred into lethally irradiated anti-κ transgenic host mice, there was a defect in receptor editing similar to that seen in itpkb-/- chimeras. Specifically, we observed a 2.5-fold increase in the percentage of κ-expressing cells and a 2.4-fold reduction in λ-expressing cells (p = 0.0023) in the immature BM compartment (Figure 1C-E). This resulted in a λ/κ editing ratio that was 4.9-fold lower at the immature B cell stage (p = 0.005; Figure 2). When Raf-CAAX bone marrow was transferred into lethally irradiated WT CD45.1 hosts, we observed lower percentages of both κ- and λ-expressing cells in the BM (57% and 37% of WT, respectively), due to the relative expansion of pro-B cells (Figure 1C). However, the λ/κ editing ratio in these chimeras was also 69% lower than that observed when WT bone marrow was transferred into WT CD45.1+ mice (p = 0.0095) (Figure 2). Since the Raf pathway is constitutively active in all of these cells, even in the absence of antigen triggered BCR signaling, these results support a role for continuous BCR signals inhibiting receptor editing at the immature B cell stage in the bone marrow.
Our data show that an increase in BCR signaling impairs receptor editing at the immature B cell stage. In the presence of the anti-κ transgene there is a strong stimulus to edit away from κ and to initiate λ gene rearrangement. Immature B cells with increased BCR signaling do not edit as well, as shown by a lower editing ratio in both the itpkb-/- and the Raf-CAAX transgenic cells, when these cells develop in anti-κ host mice. These results fit our model well, showing that an increase in BCR signaling inhibits receptor editing, rather than inducing receptor editing.
Our model postulates that BCR signals inhibit receptor editing through the Raf/MEK/ERK and Ca2+ signaling pathways. Both calcium signaling and Erk activation have been shown to be downstream of PI3K activation in mature B cells [13,14], consistent with our previous observation that PI3K inhibitors block RAG2-GFP expression (and hence receptor editing) in vitro [15]. Ca2+ signaling is impaired in B cells lacking PI3K subunits p85α or p110δ, which also show increased RAG expression and light chain editing [16,17]. ERK phosphorylation is also blocked by PI3K inhibitors LY294002 and wortmannin in B cells, indicating that PI3K affects downstream Raf targets [13,18]. This most likely reflects the fact that B cells can activate Raf via the classical Shc/Grb2/SOS/Ras pathway, which is unaffected by PI3K inhibitors [13,18], or via an alternative pathway that involves Btk and PLCγ dependent activation of RasGrp (and hence Ras), which is inhibited by PI3K inhibitors (presumably due to an affect on Btk and PLCγ [13].
4. Conclusions
B cell receptor editing is a complex process that remains poorly understood. Distinct studies have supported a role for both increased and decreased BCR signaling in initiating receptor editing. Herein we provide evidence supporting a role for Raf- and calcium-dependent signaling pathways in suppressing receptor editing. These results extend our previous in vitro work and further support a model in which basal BCR signaling actively inhibits light chain receptor editing.
Acknowledgments
We thank J. Bednar, C. Andersen, and R. Agneberg for assistance with animal husbandry, M. Weigert for providing the ακ mice, and B. Schram and T. Behrens for discussion and suggestions. This work was funded by a NIH grant (R01 AR043805) and a Leukemia and Lymphoma society Scholar award to M.A.F.
List of abbreviations
- ITPKB
Inositol 1,4,5-triphosphate kinase B
- IP3
inositol 1,4,5-triphosphate
- IP4
inositol 1,3,4,5-tetrakisphosphate
- PLCγ2
phospholipase Cγ2
- BCR
B cell receptor
Biographies
Laura Ramsey completed her Ph.D at the University of Minnesota in Molecular, Cellular, Developmental Biology & Genetics in July 2009. She is now a post-doctoral fellow at St. Jude Children's Research Hospital in the Pharmaceutical Sciences Department.
Amanda Vegoe was a research technician for Dr. Farrar until November 2009. She is currently raising her family in Apple Valley, MN.
Andrew Miller is a research investigator at The Genomics Institute of the Novartis Research Foundation.
Michael Cooke completed his Ph.D. in Immunology with Dr. Roger Perlmutter at the University of Washington. He carried out postdoctoral studies with Dr. Chris Goodnow at Stanford University. He is currently the Director of Immunology at The Genomics Institute of the Novartis Research Foundation in San Diego, CA.
Michael Farrar completed his Ph.D. in Immunology with Dr. Robert Schreiber at Washington University in St. Louis in 1993. He carried out postdoctoral studies with Dr. Roger Perlmutter at the University of Washington and at Merck Research Labs. He is currently an Associate Professor in the Center for Immunology at the University of Minnesota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura B. Ramsey, Email: robin444@umn.edu.
Amanda L. Vegoe, Email: baker112@umn.edu.
Andrew T. Miller, Email: amiller@gnf.org.
Michael P. Cooke, Email: mcooke@gnf.org.
References
- 1.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J Exp Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durdik J, Moore MW, Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984;307:749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- 4.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD- bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 5.Schram BR, Tze LE, Ramsey LB, Liu J, Najera L, Vegoe AL, Hardy RR, Hippen KL, Farrar MA, Behrens TW. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. J Immunol. 2008;180:4728–4741. doi: 10.4049/jimmunol.180.7.4728. [DOI] [PubMed] [Google Scholar]
- 6.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4:e200. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait-Azzouzene D, Verkoczy L, Peters J, Gavin A, Skog P, Vela JL, Nemazee D. An immunoglobulin C kappa-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J Exp Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AT, Sandberg M, Huang YH, Young M, Sutton S, Sauer K, Cooke MP. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol. 2007;8:514–521. doi: 10.1038/ni1458. [DOI] [PubMed] [Google Scholar]
- 9.Miller AT, Beisner DR, Liu D, Cooke MP. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J Immunol. 2009;182:4696–4704. doi: 10.4049/jimmunol.0802850. [DOI] [PubMed] [Google Scholar]
- 10.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 11.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 12.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob A, Cooney D, Pradhan M, Coggeshall KM. Convergence of signaling pathways on the activation of ERK in B cells. J Biol Chem. 2002;277:23420–23426. doi: 10.1074/jbc.M202485200. [DOI] [PubMed] [Google Scholar]
- 14.Kiener PA, Lioubin MN, Rohrschneider LR, Ledbetter JA, Nadler SG, Diegel ML. Co-ligation of the antigen and Fc receptors gives rise to the selective modulation of intracellular signaling in B cells Regulation of the association of phosphatidylinositol 3-kinase and inositol 5′-phosphatase with the antigen receptor complex. J Biol Chem. 1997;272:3838–3844. doi: 10.1074/jbc.272.6.3838. [DOI] [PubMed] [Google Scholar]
- 15.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, Kraus M, Hardy RR, Schlissel MS, Rajewsky K, Behrens TW. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B Cell Receptor-Directed Phosphatidylinositol 3-Kinase Signaling Turns Off RAGs and Promotes B Cell-Positive Selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llorian M, Stamataki Z, Hill S, Turner M, Martensson IL. The PI3K p110delta is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 18.Sakata N, Kawasome H, Terada N, Gerwins P, Johnson GL, Gelfand EW. Differential activation and regulation of mitogen-activated protein kinases through the antigen receptor and CD40 in human B cells. Eur J Immunol. 1999;29:2999–3008. doi: 10.1002/(SICI)1521-4141(199909)29:09<2999::AID-IMMU2999>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]