Abstract
Background and Purpose
Stroke survivors suffer from disproportionate muscle atrophy and other detrimental tissue composition changes on the paretic side. The purpose was to determine whether myostatin levels are higher in paretic versus non-paretic muscle and the effects of resistive training (RT) on paretic and non-paretic mid-thigh muscle composition and myostatin mRNA expression in stroke survivors.
Methods
Fifteen stroke survivors (50–76 years)underwent bilateral multi-slice thigh CT scanning from the knee to the hip, bilateral vastus lateralis skeletal muscle tissue biopsies, a total body scan by DXA, one-repetition (1-RM) strength tests before and after a 12- week, (3×/week) RT intervention.
Results
Total body fat mass and fat-free mass did not change. Bilateral leg press and leg extension 1-RM strength increased 31–56% with RT (P<0.001). Paretic and non-paretic muscle area of the mid-thigh increased 13% (P<0.01) and 9% (P<0.05), respectively after RT. Muscle attenuation of the mid-thigh increased 15% and 8% (both P<0.01) in the paretic and non-paretic thigh, respectively representing reduced intra-muscular fat. Muscle volume increased 14% (P<0.001) in the paretic thigh and 16% (P<0.05) in the non-paretic thigh after RT. Myostatin mRNA expression levels were 40% higher in the paretic than non-paretic muscle (P=0.001) at baseline and decreased 49% in the paretic muscle (P<0.005) and 27% in the non-paretic muscle (P=0.06) after RT.
Conclusions
Progressive RT stimulates significant muscle hypertrophy and intramuscular fat reductions in disabled stroke survivors. The increased myostatin mRNA in the paretic thigh and reduction with RT, imply an important regulatory role for myostatin after stroke.
Keywords: Exercise, Skeletal Muscle, Stroke, Myostatin
Introduction
Sarcopenia or the loss of fat-free mass with age increases the risk for subsequent injury and disability 1. The progression and consequences of sarcopenia may be especially severe after a stroke due to the relative inactivity and reduced strength and fitness levels in stroke survivors. In an earlier study, we demonstrated that reduced muscle mass and greater severity of hemiparetic gait deficits were independent determinants of lower peak VO2 in chronically disabled hemiparetic stroke survivors 2 illustrating a close relationship between sarcopenia and reduced fitness and frailty in stroke. Furthermore, the paretic thigh of stroke survivors had 20% lower muscle area and 25% higher intramuscular fat than the non-paretic thigh 3 demonstrating substantial atrophy and muscle composition change.
Although resistive training (RT) has proven effective for altering muscle mass in healthy elderly 4–6, no evidence currently supports the use of RT for reversing muscle atrophy and increased intramuscular fat after stroke. It remains unclear whether disabled stroke survivors can perform RT at an intensity level sufficient to produce meaningful changes in tissue composition. Progressive RT after stroke results in significant increases in both strength, power and function 7, 8. To our knowledge, there are no studies that have characterized body composition changes in hemiparetic stroke patients after RT.
It is also important consider the molecular regulators of stroke-induced atrophy and adaptation with RT after stroke. Myostatin is a member of the transforming growth factor beta family of secreted growth factors and thus, is a significant regulator of skeletal muscle development and size 9. Mutations in the myostatin (Mstn) gene which knock-out myostatin expression have led to dramatic increases in muscle mass in animals 10, 11 and were documented in a child 12. Both acute and chronic resistive exercise reduce basal myostatin levels in healthy individuals 13–16 providing evidence to support our hypothesis that RT may have the same effect in chronic stroke. Because of the muscle atrophy in the paretic thigh 3, we also hypothesized that myostatin levels would be elevated in paretic muscle in stroke survivors. Thus, the purpose of this study was to determine whether myostatin levels are higher in paretic versus non-paretic muscle and to determine the effects of relatively intense 3-month RT program on paretic and non-paretic mid-thigh muscle composition and myostatin mRNA expression in stroke survivors.
Methods
Subject Selection
Twenty-one individuals with a history of ischemic stroke (>six months latency) enrolled. Six individuals either did not complete the study due to time constraints or medical issues unrelated to study participation. The fifteen individuals (10 men, 5 women) who completed the study were between 50–76 years and had BMIs between 23–39 kg/m2. All stroke survivors had mild to moderate hemiparetic gait deficits and had completed conventional rehabilitation therapy. Evaluations included medical history, physical examination, fasting blood profile, and screening for dementia 17 and depression 18 to ensure adequate informed consent. Stroke participants were excluded if they had unstable angina, congestive heart failure (NYHA II), severe peripheral arterial disease, major post-stroke depression, dementia, severe receptive aphasia, and orthopedic or chronic pain conditions.
All tests were performed before and after the three-month training intervention. All methods and procedures were approved by the Institutional Review Board of the University of Maryland and the VA R&D committee. Each participant provided written informed consent.
Body Composition
Height and weight were measured. Fat mass, lean tissue mass and %body fat were determined by DXA (Prodigy LUNAR GE version 7.53.002). Thigh CT scans were performed every 4 cm starting at the patella and ending at the femoral head (Siemens Somatom Sensation 64 Scanner) to quantify skeletal muscle area, total fat area, low density lean tissue area 3, and muscle attenuation of both the paretic and non-paretic thighs. Scans were analyzed using MIPAV (Medical Image Processing, Analysis and Visualization, v.7.0, NIH). The cross-sectional area of each axial slice was multiplied by the distance between slices (4 cm) and summed across slices representing volume expressed in cm3.
Exercise and Functional Tests
Exercise testing with open circuit spirometry was conducted to measure VO2peak using a graded submaximal treadmill test 19. A standardized six minute walk test recorded the distance traveled, with stroke participants walking at their comfortable self-selected walking speed using their usual assistive devices. This walking test was chosen as a functional measure of gait deficit severity. A 1 repetition maximum (1-RM) strength test was conducted on the leg press and leg extension of each leg. Two familiarization sessions were included prior to baseline 1-RM testing to avoid the confounding effects of learning on baseline strength measures. Strength in the paretic and non-paretic legs was tested separately using pneumatic RT equipment that was built for single leg movement (Keiser, Fresno, CA).
Skeletal Muscle Biopsies and Analysis
Vastus lateralis biopsies of the paretic and non-paretic muscle were performed under local anesthesia from nine subjects after a 12-hour fast for the measurement of gene expression for myostatin and insulin-like growth factor 1 (IGF-1). Muscle biopsies of the paretic and non-paretic muscle were also obtained after the 3-month intervention, 24–36 hours after the last bout of RT in these nine individuals. Muscle was immediately freeze-clamped and stored at −80°C. Approximately 50–80 mg of muscle was used for RNA isolation.
RNA extraction and Reverse transcription for Real-time RT-PCR
Total RNA was extracted from skeletal muscle by the guanidinium isothiocyanate/phenol/chloroform method developed by Chomcynski and Sacchi 20. The RNA pellet was resuspended in RNAsecure Resuspension solution (Cat. #7010, Ambion Inc.) and RNA concentrations were measured in a spectrophotometer.
One μg of total RNA for each sample was reverse transcripted (RT) into first strand cDNA using Transcriptor First Strand cDNA Synthesis Kit (Cat# 04 896 866 001, Roche Applied Science) according to the detailed manufacturer’s protocol. The random primer was used as the primer and the RT reaction was carried out at 10 min at 25°C and then 55°C for 30 min in 20μl volume. The reaction was inactivated by incubating at 85°C for 10 min and stopped by placing the tube on ice. An RT control (master mix without RT enzyme) was performed.
Quantitative Real-time PCR (qPCR) and data analysis for myostatin and IGF-1 were performed in a LightCycler 480 Real-Time PCR System with LightCyclerR 480 software (Roche Applied Science). LightCycler 480 Multiwell plate 384 (Cat# 04 729 748 001), LightCycler 480 Probes Master kit (Cat# 04 887 301 001,) and Taqman gene expression primer/probe set (Applied Biosystems, Foster City, CA.) were used. Each qPCR reaction was carried out in a final volume of 10μl, consisting of 2μl 1:4 diluted template cDNA, 5μl LightCycler 480 Probes Master. 0.5μl Taqman gene expression primer and probe mix, and 2.5μl nuclease-free water. Water instead of cDNA served as the no template control. According to the manufacturer’s instruction, the qPCR protocol was adopted for all samples: after incubation at 95°C for 10 min to activate the DNA polymerase, 45 cycles of 95°C for 10s and 60°C for 30s each were performed to facilitate the PCR reaction. 36B4 served as an internal control for normalization. Data acquisition occurred at real time during the annealing/elongation incubation at 60°C. All samples were amplified in triplicate from the same RNA preparation. Gene expression data were analyzed by Roche LightCycle 480 system Software version 1.5 advanced relative quantification program. The average of three determinations for each sample and the normalized ratio of Target/Reference was used in statistical analyses.
Resistive Training Protocol
The training protocol was designed to provide a high volume, high intensity training stimulus for maximal adaptation in skeletal muscle mass across a 3 month period. Subjects trained 3×/week for 12 weeks, performing two sets of 20 unilateral repetitions on the leg press, leg extension and leg curl machines (Keiser, pneumatic resistance, Fresno, CA) at every session. Generally, resistance was set at a level that would cause muscle failure somewhere between the 10th and 15th repetition. Resistance would then be gradually reduced to allow completion of the full 20 repetition set. Participants trained each leg separately to account for differences in strength and progression requirements between limbs. Resistance was gradually increased every two to three weeks to account for strength gains and to maximize the intensity of the training.
Statistical Analyses
Baseline myostatin levels between paretic and non-paretic thigh muscle was determined using paired Student t-tests. Changes in the paretic and non-paretic leg were assessed using repeated measures ANOVA. All data were analyzed using SPSS 12.0. Data are presented as means ± SEM. P values <0.05 are statistically significant.
Results
Physical Characteristics (Table 1)
Table 1.
Characteristics of stroke survivors before and after RT.
| Variable | Pre | Post |
|---|---|---|
| Age (years) | 65 ± 2 | ------- |
| Latency since stroke (years) | 8 ± 2 | ------- |
| BMI (kg/m2) | 27.7 ± 1.2 | ------- |
| Weight (kg) | 83.1 ± 3.9 | 83.6 ± 4.3 |
| Fat mass (kg) | 29.6 ± 2.6 | 29.0 ± 2.7 |
| Fat-free mass (kg) | 53.5 ± 2.6 | 53.7 ± 2.5 |
| Percent body fat | 35.2 ± 2.1 | 34.8 ± 2.2 |
| VO2peak (ml·kg.min−1) | 20.3 ± 1.7 | 20.9 ± 1.7 |
| Self-selected walking speed (mph) | 1.58 ± 0.16 | 1.54 0.19 |
| Fastest walking speed (mph) | 2.11 ± 0.27 | 2.18 ± 0.31 |
| Paretic 1RM Leg Extension (lbs) | 53 ± 8 | 82 ± 11† |
| Non-paretic 1RM Leg Extension (lbs) | 105 ± 8 | 138 ± 8† |
| Paretic 1RM Leg Press (lbs) | 282 ± 36 | 375 ± 42† |
| Non-paretic 1RM Leg Press (lbs) | 422 ± 33 | 555 ± 33† |
Note lbs for strength levels are derived from pneumatic resistance equipment. BMI = body mass index; VO2peak = peak oxygen consumption. Values are means ± SEM.
P<0.001 pre vs. post.
Stroke survivors were mostly male (66%) but were racially mixed with 47% Caucasian (n=7) and 53% African-American (n=8). Body weight, whole body fat mass, fat-free mass, and %fat by DXA did not change significantly with RT. Muscle leg press improved 32% and 33% in non-paretic and paretic legs, respectively (P<0.001) with a group*time interaction (P<0.05). Leg extension increased 31% in non-paretic and 56% in paretic legs, respectively (P<0.001) with a greater increase in the paretic leg (P<0.001). VO2peak, self-selected walking speed and fastest walking speed did not significantly change with RT.
Muscle Hypertrophy (Table 2)
Table 2.
Mid-thigh muscle composition before and after RT in stroke survivors.
| Variable | Paretic | Non-paretic | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Muscle Area (cm2) | 68.7 ± 5.0 | 77.7 ± 3.9† | 88.1 ± 6.8 | 96.3 ± 6.0* |
| Subcutaneous fat area (cm2) | 78.8 ± 10.4 | 77.3 ± 10.9 | 73.8 ± 10.3 | 70.4 ± 9.8 |
| Low density lean tissue area (cm2) | 25.0 ± 2.6 | 24.0 ± 2.9 | 23.5 ± 2.4 | 22.5 ± 2.6 |
| Muscle Attenuation (HU) | 29.8 ± 1.5 | 34.2 ± 1.9† | 36.4 ± 1.5 | 39.4 ± 1.7† |
Values are means ± SEM.
P<0.05 and
P<0.01 pre vs. post RT.
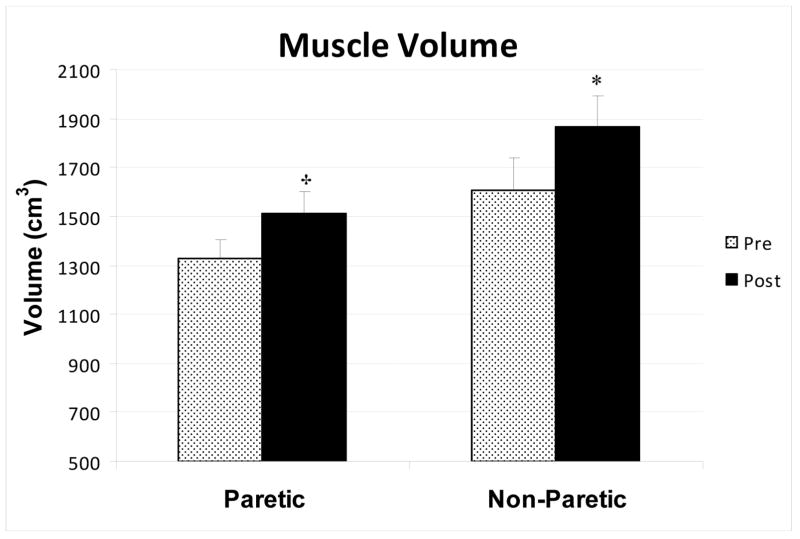
Paretic muscle area of a single mid-thigh cross-section slice increased 13% after RT (P<0.01). Likewise, non-paretic mid-thigh muscle area increased 9% after RT (P<0.05). There were no significant changes in single-slice subcutaneous fat area and low density lean tissue. Muscle attenuation of the mid-thigh cross-section increased after RT in the paretic thigh by 15% (P<0.01) and non-paretic thigh by 8% (P < 0.01), representing a decrease in intra-muscular fat. Using multi-slice measurements of the thigh, we observed a 14% (P<0.001) increase in muscle volume in the paretic thigh and 16% (P<0.05) increase in the non-paretic thigh after RT (Figure 1). Subcutaneous fat volume of the paretic (1829 ± 223 vs. 1688 ± 250 cm3) and non-paretic (1757 ± 218 vs. 1713 ± 230 cm3) as well as low density lean tissue volume of the paretic (460 ± 44 vs. 463 ± 53 cm3) and non-paretic (454 ± 37 vs. 433 ± 45 cm3) thighs did not change with RT.
Figure 1.
Muscle tissue volumes in the non-paretic and paretic thighs of stroke subjects before and after resistive training (n=15). * P< 0.05, † P < 0.001
Skeletal Muscle Myostatin and IGF-1 Levels
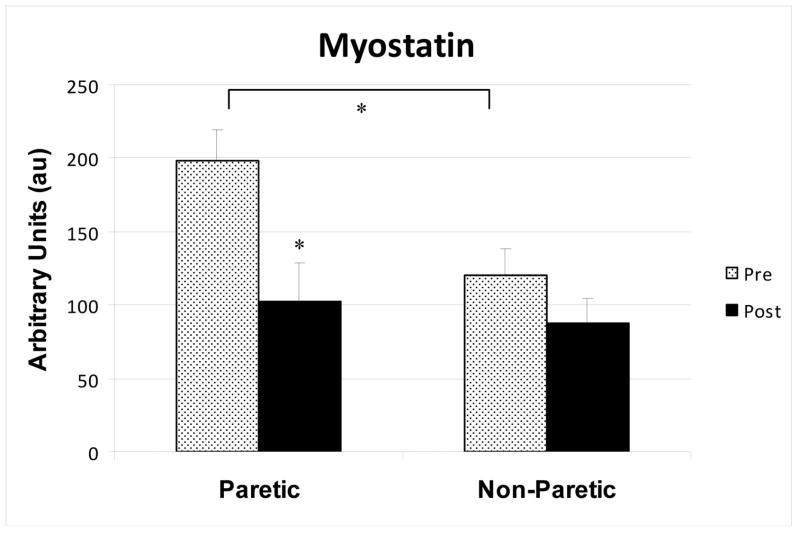
Myostatin levels were 40% higher in the paretic than the non-paretic muscle (P=0.001, Figure 2). After RT, myostatin mRNA levels decreased 49% in the paretic muscle (P<0.005, Figure 2). In the non-paretic muscle, myostatin expression tended to decrease by 27% (P=0.06). The reduction in myostatin was greater in the paretic than non-paretic muscle (P<0.001) by repeated measures ANOVA. IGF-1 mRNA was not different between paretic and non-paretic muscle prior to RT (3.57 ± 0.48 vs. 4.11 ± 0.66 AU). IGF-1 levels did not change significantly before vs. after RT in paretic (3.57 ± 0.48 vs. 3.37 ± 0.47 AU) and non-paretic (4.11 ± 0.66 vs. 2.93 ± 0.16 AU) muscle.
Figure 2.
Skeletal muscle myostatin mRNA levels in the paretic and non-paretic thighs of stroke survivors before and after resistive training (n=9) * P< 0.01.
Discussion
The present study is the first to show that resistive training results in lower extremity muscle hypertrophy and loss of intramuscular fat in stroke survivors. We utilized multi-slice CT imaging to make muscle volume determinations, representing the most comprehensive approach for assessing regional skeletal muscle hypertrophy. In addition, we provide the first evidence that myostatin mRNA expression are higher in the paretic muscle than the non-paretic muscle and that RT can reduce myostatin expression in stroke survivors. This suggests that myostatin is a key molecular regulator for paretic side muscle atrophy that can respond favorably to aggressive RT treatment interventions.
Numerous studies indicate that progressive resistance training is well tolerated and increases muscle strength in stroke survivors 21–25. Our results confirm both the nature and magnitude of these RT-induced strength gains after stroke. Interestingly, we had similar relative improvements in leg press strength between the paretic and non-paretic legs, but the leg extension gains were almost a two-fold higher on the paretic side. This may have been attributed to the greater difference in strength between the two legs at baseline for the leg extension strength test. Alternatively, it may be indicative that factors unrelated to muscle mass (i.e. muscle quality) play a greater relative role in the strength adaptations related to open chain kinetic movements on the paretic side. Although we did not measure muscle power, other stroke studies have shown increases in lower body power after RT 24. According to a recent meta-analysis of thirteen randomized controlled trials 26, RT can also improve upper-limb strength and function.
Our functional outcome results are consistent with randomized and non-randomized trials that fail to show improvements in walking distance or gait velocity with RT 23, 25, 27. Specifically, we did not see any changes in fastest or usual pace walking speed with RT. In contrast, circuit weight training that includes exercises specifically designed to improve gait and balance resulted in a greater distance walked in stroke subjects compared to control 28. Yang et al. 21 also reported increased gait speed, stride length and six-minute walk distance after RT in stroke survivors compared to controls. Similarly, when progressive RT is combined with aerobic training, there are improvements in gait performance (6 min-walking speed) 29, 30. Lastly, there is some evidence that progressive functional strength training in the sub-acute stroke recovery period involving weight bearing exercises may improve walking ability (speed) 31 and muscle strength of knee flexors 32. Collectively, these studies suggest that RT alone may not change function but that hybrid interventions are effective and may work best to maximize the functional impact of RT after stroke.
The magnitude of hypertrophy with RT in stroke survivors is consistent with muscle hypertrophy after RT in healthy older individuals 4, 6. Longer duration RT studies are needed to answer whether RT can further diminish the differences in paretic and non-paretic muscle area. We did not see significant changes in whole body fat-free mass by DXA after RT although others have reported increased FFM with RT in healthy elderly 5, 6, 33, 34. This is likely because our RT protocol was limited to training the lower extremities, making whole body DXA not sufficiently sensitive enough to register the regional compositional change taking place in the thighs. Using multi-slice CT measurements of the thigh, we found significant comparable muscle hypertrophy across the thigh in the paretic and non-paretic thighs, demonstrating that the unilateral training was an effective stimulus for both legs. The atrophy in the paretic limb at baseline with subsequent hypertrophy of the muscle after the RT is encouraging and suggests that this type of exercise may be especially important in stroke survivors. Moreover, we observed an increase in muscle attenuation after RT in both paretic and non-paretic thighs indicating a loss of adipose tissue interspersedaround muscle. Muscle attenuation is associated with greater specific force production and muscular strength in elderly individuals of the Health ABC study 35. In addition, there is augmented fat infiltration within muscle (reduced muscle attenuation) in obesity 36, 37. RThas been shown to increase the attenuation of muscle and strength in elderly women 38 but this is the first account of larger and leaner muscle in stroke survivors.
Our results suggest that the myostatin cascade is a signaling pathway involved in post-stroke muscle atrophy. Myostatin is a negative key regulator of muscle mass as indicated in case studies of humans and animals 10–12. Myostatin knockout mice have increased muscle mass accounted for by both increased muscle fiber size and number 9. The hypertrophic effect of myostatin inhibition may be partly due to increased activity of satellite cells 39. Our findings in the stroke model of atrophy in the paretic thigh compared to the non-paretic thigh coincide nicely with the significantly higher myostatin expression in the paretic limb than the non-paretic limb and provide indirect evidence that myostatin contributes to human muscle atrophy. Our RT intervention resulted in a significant decrease in myostatin mRNA levels in the paretic limb and approached significance in the non-paretic thigh. These results corroborate investigations in healthy adults where myostatin mRNA expression has been reported to decrease after resistance training 16. We did not see changes in IGF-1 expression indicating that this is a less important regulator in the context of RT-related hypertrophy after stroke although other studies have shown it is important in healthy adults 40. Future studies could examine additional growth factors and satellite cell proliferation in paretic and non-paretic muscle in regulating muscle growth with RT in chronic stroke.
Summary
We are the first to report significant hypertrophy and improved skeletal muscle composition of the thigh with a 3-month RT program in older stroke survivors. Moreover, there is higher myostatin mRNA expression in the paretic skeletal muscle prior to RT which decreases with the intervention. Future investigations are necessary to elucidate the role of additional downstream factors involved in stroke-related muscle atrophy and to identify targeted exercise rehabilitation strategies that best mitigate these muscle composition and gene expression effects.
Acknowledgments
Our appreciation is extended to those stroke survivors who participated in this study. We are grateful to the nurses and laboratory staff in the Geriatrics Services at the Baltimore Veterans Administration (VA) Medical Center, for technical assistance.
Sources of Funding
This study was supported by funds from: VA Research Scientist Award, NIH grants R01-AG19310, Claude D. Pepper Older Americans Independence Center (P60AG12583), K01AG19242, T32AG00219, the Baltimore VA Geriatric Research, Education, and Clinical Center (GRECC), and VA Rehabilitation Research & Development Maryland Exercise and Robotics Center of Excellence (B3688R; RR &D MERCE).
Footnotes
Disclosures
None
Reference List
- 1.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12:493–498. doi: 10.1007/BF02982711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan AS, Dobrovolny CL, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: role of muscle mass and gait deficit severity. J Stroke Cerebro Disease. 2000;9:185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–7. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 4.Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey ME, Fleg JL, Hurley BF. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000;55:M641–M648. doi: 10.1093/gerona/55.11.m641. [DOI] [PubMed] [Google Scholar]
- 5.Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol. 1995;79:818–23. doi: 10.1152/jappl.1995.79.3.818. [DOI] [PubMed] [Google Scholar]
- 6.Treuth MS, Ryan AS, Pratley RE, Rubin MA, Miller JP, Nicklas BJ, Sorkin J, Harman SM, Goldberg AP, Hurley BF. Effects of strength training on total and regional body composition in older men. J Appl Physiol. 1994;77:614–20. doi: 10.1152/jappl.1994.77.2.614. [DOI] [PubMed] [Google Scholar]
- 7.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil. 2004;18:27–39. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 8.Badics E, Wittmann A, Rupp M, Stabauer B, Zifko UA. Systematic muscle building exercises in the rehabilitation of stroke patients. NeuroRehabilitation. 2002;17:211–4. [PubMed] [Google Scholar]
- 9.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;1:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 10.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci. 1997;N;11:12457–61. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–4. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 12.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–8. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 13.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104:371–8. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- 14.Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, Peterson CA. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics. 2008;19;32:393–400. doi: 10.1152/physiolgenomics.00191.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288:E1110–E1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 16.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med. 2003;228:706–9. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979;88:174–81. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 19.Macko RF, DeSouza CA, Tretter LD, Silver KH, Smith GV, Anderson PA, Tomoyasu N, Gorman P, Dengel DR. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–30. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006;20:860–70. doi: 10.1177/0269215506070701. [DOI] [PubMed] [Google Scholar]
- 22.Kim CM, Eng JJ, MacIntyre DL, Dawson AS. Effects of isokinetic strength training on walking in persons with stroke: a double-blind controlled pilot study. J Stroke Cerebrovasc Dis. 2001;10:265–73. doi: 10.1053/jscd.2001.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MJ, Kilbreath SL, Singh MF, Zeman B, Lord SR, Raymond J, Davis GM. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise-controlled study. J Am Geriatr Soc. 2008;56:976–85. doi: 10.1111/j.1532-5415.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee MJ, Kilbreath SL, Singh MF, Zeman B, Davis GM. Effect of progressive resistance training on muscle performance after chronic stroke. Med Sci Sports Exerc. 2010;42:23–34. doi: 10.1249/MSS.0b013e3181b07a31. [DOI] [PubMed] [Google Scholar]
- 25.Ouellette MM, LeBrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, Fielding RA. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35:1404–9. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 26.Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke: a meta-analysis. Stroke. 2010;41:136–40. doi: 10.1161/STROKEAHA.109.567438. [DOI] [PubMed] [Google Scholar]
- 27.Weiss A, Suzuki T, Bean J, Fielding RA. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79:369–76. doi: 10.1097/00002060-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Mudge S, Barber PA, Stott NS. Circuit-based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1989–96. doi: 10.1016/j.apmr.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen JR, Bech-Pedersen DT, Zeeman P, Sorensen J, Andersen LL, Schonberger M. Effect of intensive outpatient physical training on gait performance and cardiovascular health in people with hemiparesis after stroke. Phys Ther. 2010;90:527–37. doi: 10.2522/ptj.20080404. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira-Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999;80:1211–8. doi: 10.1016/s0003-9993(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 31.Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial. Neurorehabil Neural Repair. 2010;24:88–96. doi: 10.1177/1545968309343216. [DOI] [PubMed] [Google Scholar]
- 32.Bale M, Strand LI. Does functional strength training of the leg in subacute stroke improve physical performance? A pilot randomized controlled trial. Clin Rehabil. 2008;22:911–21. doi: 10.1177/0269215508090092. [DOI] [PubMed] [Google Scholar]
- 33.Treuth MS, Hunter GR, Kekes-Szabo T, Weinsier RL, Goran MI, Berland L. Reduction in intra-abdominal adipose tissue after strength training in older women. J Appl Physiol. 1995;78:1425–31. doi: 10.1152/jappl.1995.78.4.1425. [DOI] [PubMed] [Google Scholar]
- 34.Lemmer JT, Ivey FM, Ryan AS, Martel GF, Hurlbut DE, Metter JE, Fozard JL, Fleg JL, Hurley BF. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001;33:532–41. doi: 10.1097/00005768-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 37.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–32. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 38.Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–40. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 39.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 40.Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fiber area and expression of IGF-I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res. 2010;20:134–40. doi: 10.1016/j.ghir.2009.11.005. [DOI] [PubMed] [Google Scholar]