Abstract
A human Recombinant T-cell receptor Ligand (RTL1000) consisting of DR2 α1 and β1 domains linked covalently to MOG-35-55 peptide can reverse clinical and histological signs of experimental autoimmune encephalomyelitis (EAE), and was evaluated for safety in a Phase 1 randomized, placebo-controlled, escalating dose study in 34 subjects with multiple sclerosis (MS). RTL1000 was safe and well tolerated at a dose of ≤60mg that is well within the effective dose range for EAE and did not cause worsening of MS disease at doses ≤200mg. RTL1000 represents a novel approach for the treatment of MS that promises potent immunoregulation and CNS repair without global immunosuppression.
Keywords: Experimental Autoimmune Encephalomyelitis (EAE), Multiple Sclerosis (MS), Recombinant T cell receptor Ligand (RTL), neuroprotection, clinical trial
INTRODUCTION
Multiple sclerosis (MS) is a devastating disease of the central nervous system that causes loss of neurological function of motor and cognitive pathways. The disease is marked by progressive demyelination of white matter tissue leading to axonal loss and severe functional impairment. One of the most important issues facing researchers today is how to reverse the MS disease process and promote CNS repair in order to have a meaningful impact on the quality of life for affected MS subjects. Recent findings suggest that reducing inflammation in the brain can slow disease progression and promote natural repair processes. Additionally, new drugs are being sought to attract myelin producing cells into areas of damage and induce active production of myelin that wraps axons to restore neurological function.
We designed a novel therapeutic drug for MS called Recombinant T cell receptor Ligand (RTL) 1000 that was evaluated recently in a Phase 1 safety study. RTL1000 as well as other RTL constructs can inhibit the inflammatory activity of T cells that attack and destroy myelin and can reverse clinical paralysis in mice developing experimental autoimmune encephalomyelitis (EAE), an MS-like disease. To our surprise, we also discovered that successfully treated mice had evidence of remyelination and axonal regeneration, raising the possibility that RTLs could promote CNS repair. The potent therapeutic effects of RTLs in reversing clinical and histological signs of EAE and their possible remyelinating and neuroprotective effects on damaged CNS tissue provide a strong rationale for testing RTL therapy in subjects with multiple sclerosis.
PRECLINICAL STUDIES USING RTLS IN EAE
Rational design of Recombinant TCR ligands (RTLs)
To develop a simple and effective agent that could bind selectively to the TCR, we designed molecules consisting of the α1 and β1 domains of MHC class II molecules genetically linked to autoantigenic peptides and expressed as a single polypeptide chain. Molecules with this design would be useful for studying binding specificity in vitro, for exploring primary TCR signaling events independent of co-stimulatory input associated with the MHC II α2 and β2 domains or with other molecules expressed by antigen presenting cells, and for treating CD4+ T cell-mediated autoimmune disease in an MHC II/epitope-specific manner.
The single chain RTLs derived from the α1 and β1 domains of MHC class II molecules refold in a manner that allows binding of allele-specific epitopes that associate either as free peptides or as genetically engineered N-terminal extensions. The folded molecules can be recovered in high yield from inclusion bodies with a final yield of approximately 15–30 mg/liter culture. Conformational integrity of the molecules was inferred from the presence of a disulfide bond between cysteines β15 and β79 and biological activity by direct antigen-specific binding to TCR on T cell hybridomas (Burrows et al., 1998). Because of their biochemical stability, biological properties, and structural similarity with human class II homologues, RTLs represent a template for producing a novel class of unique therapeutic molecules that may be useful for treating CD4+ T cell-mediated autoimmune diseases in an epitope-specific manner. We have now constructed genes encoding RTLs derived from rat RT1.B, human HLA-D2, -DR3, -DR4, -DP2, -DP4, -DQ2, and -DQ8, and murine I-As, I-Ab and I-Ag7 class II molecules. In all three species the design involves genetically coupling the amino terminus of the alpha-1-domain to the carboxyl terminus of the beta-1-domain. These molecules have also been made with covalently coupled peptides attached by a linker to the N-terminus of the β-chain. The structure-based design of the RTLs is illustrated in Fig. 1 and is further described in detail in our previous publications (Burrows et al., 1998; Burrows et al., 1999; Chang et al., 2001; Huan et al., 2004).
Figure 1. Cartoon of the folded RTL molecule.
The RTL is a single exon comprised of the β1 (blue) and α1 (red) domains of a chosen class II molecule with a tethered target peptide (green). RTL1000 that was tested in our Phase 1 clinical trial in MS consists of HLA-DR2 (DRA:DRB1*1501) MHC domains linked to the myelin oligodendrocyte glycoprotein (MOG)-35-55 peptide. Figure reproduced from(Burrows et al., 1999).
Specificity of RTL Therapy in Different EAE Models
RTLs were shown to signal directly through the TCR as a partial agonist (Wang et al., 2003), and could prevent and treat active or passive myelin basic protein (MBP)-induced monophasic EAE in Lewis rats (RTL201) (Burrows et al., 2000), myelin oligodendrocyte glycoprotein (MOG)-35-55 peptide-induced chronic EAE in DR2 transgenic mice (RTL342) (Vandenbark et al., 2003), proteolipid protein (PLP)-139-151 peptide- and MBP-84-104 peptide-induced relapsing EAE in SJL/J mice (RTL401 and RTL403, respectively) (Huan et al., 2004) and MOG-35-55 peptide-induced chronic EAE in C57BL/6 mice (RTL551) (Sinha, 2007). RTL constructs derived from DR2 inhibited activation but induced IL-10 secretion in human DR2-restricted T cell clones specific for MBP-85–99 or cABL peptides (Burrows et al., 2001).
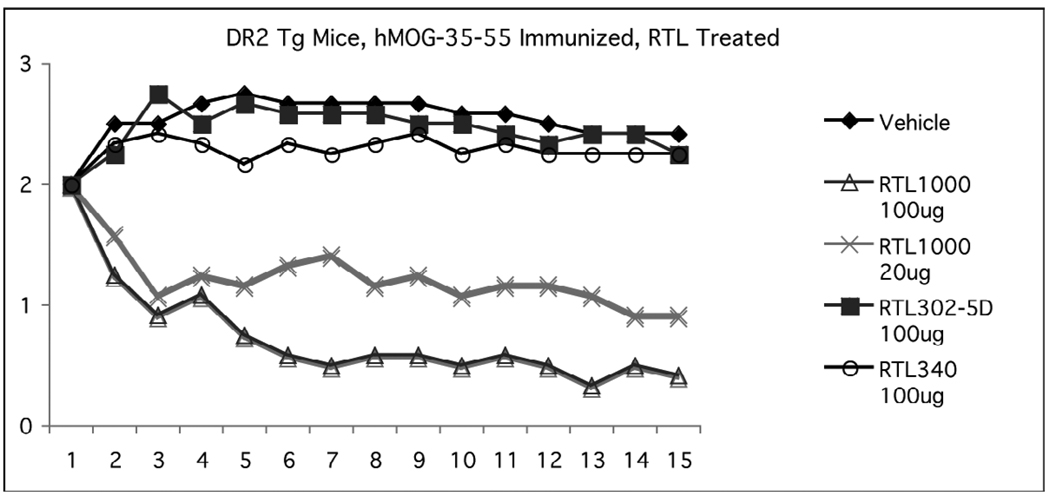
Therapeutic effects of our lead human drug for MS, RTL1000 (DR2 with attached MOG-35-55 peptide), could be tested directly in DR2 Tg mice that are susceptible to chronic EAE induced by human MOG-35-55 peptide (Chou et al., 2005). As shown in Figure 2, five daily doses of 100µg RTL1000 rapidly and significantly reversed established clinical signs of EAE nearly to baseline compared to mice treated with vehicle (TRIS-HCl buffer) in dextrose solution (D5W), with a lesser effect produced using five daily doses of 20µg RTL1000. Notably, 100µg doses of “empty” RTL302-5D without a tethered peptide and RTL340 containing the MBP-85-99 peptide had no therapeutic effect on EAE, establishing the peptide specificity of RTL1000 effects on EAE.
Figure 2. Specificity of RTL1000 treatment effects on EAE in DR2 Tg mice.
Chronic EAE was induced in DR2 Tg mice with human (h)MOG-35-55 peptide emulsified in CFA plus Pertussis toxin. The mice were treated at onset of EAE when clinical scores reached a severity of 2 with five daily i.v. injections of vehicle (control), RTL1000 (DR2/hMOG-35-55 peptide used in the Phase 1 clinical trial), “empty” RTL302-5D (DR2 without a tethered peptide) or RTL340 (DR2/MBP-85-99). The mice were scored daily for clinical signs of EAE. RTL1000 significantly inhibited daily clinical scores of EAE from Day 3 forward and cumulative Disease (sum of daily scores) compared to vehicle, RTL302-5D and RTL340.
Bystander suppression and cytokine switch
RTL treatment is effective in treating EAE induced with multiple encephalitogens
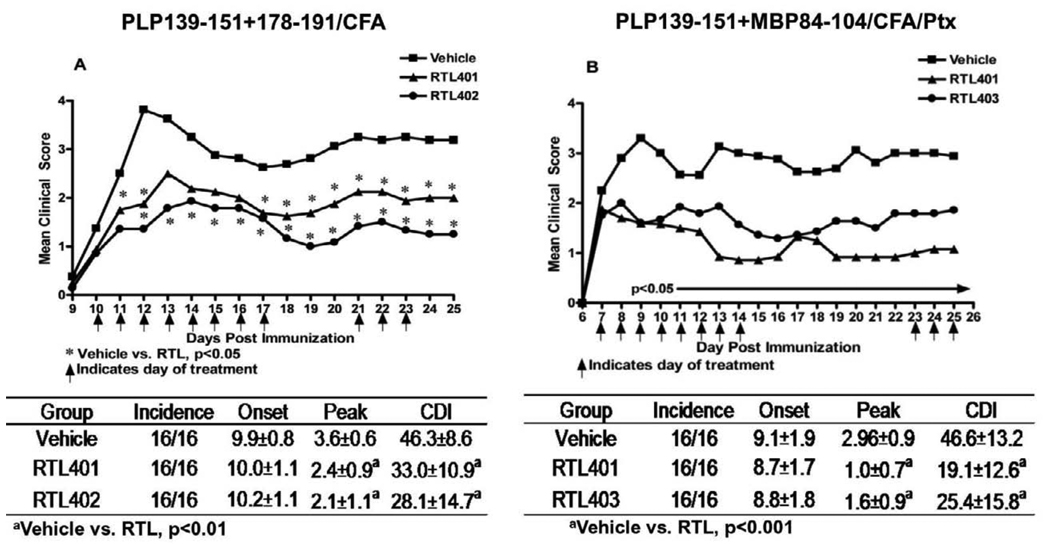
Effective RTL treatment in MS may depend on whether a single RTL can inhibit autoimmune responses involving multiple encephalitogenic T cell specificities. To test this, single or combinations of RTLs containing PLP-139-151 (RTL401), PLP-178-191 (RTL402) and MBP-84-104 (RTL403) peptides were used respectively to treat EAE induced by mixed peptides (PLP-139-151, PLP-178-191 and MBP-84-104) or syngeneic whole spinal cord homogenate (WSCH). Our results demonstrated that a single RTL was capable of reversing clinical and histological signs of EAE, given that one of the immunizing peptides was present in the RTL molecule used for the treatment. Thus’ RTL401 significantly reversed EAE induced by PLP-139-151 (cognate peptide) + PLP-178-191 or MBP-84-104 (Fig. 3). Moreover, RTL401 was very effective in treating WSCH induced EAE, potentially involving all myelin antigens (not shown). Similarly, RTL402 and RTL403 were each effective at treating EAE induced by the cognate plus another myelin peptide.
Figure 3. Treatment with single RTL is sufficient to treat EAE induced with two peptides provided one of the immunizing peptide is present in the RTL used for the treatment.
SJL/J mice were immunized with PLP-139-151+PLP-178-191/CFA (A) or PLP-139-151+MBP-84-104/CFA/Ptx (B). At disease onset mice were divided into three groups treated with vehicle or each cognate RTL for 8 days s.c. Data presented are combined from two separate experiments for each group, with 8 mice per group. Significant differences between groups were determined using the Mann-Whitney test (p< 0.05). Proliferative responses to both immunizing peptides were present in the LN 26 days post immunization, indicating induction of T cells of each specificity (data not shown). Figure reproduced from(Sinha et al., 2009).
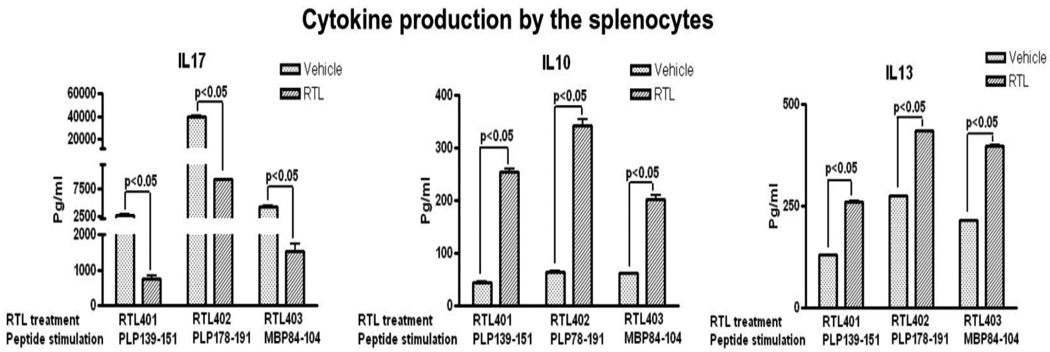
Cognate RTLs reduce the production of IL-17 and increase the production of IL-10 and IL-13 from splenocytes of RTL-treated vs. control mice
Mice from the above RTL treatment experiments were euthanized 26 days post-immunization and spleen cell supernatants were evaluated for pro-inflammatory vs. anti-inflammatory cytokines induced ex vivo by the immunizing myelin peptides. As is shown in Fig. 4, splenocytes from mice treated only with RTL401, RTL402 or RTL403 consistently had reduced IL-17 responses and increased IL-10 & IL-13 responses to cognate PLP-139-151, PLP-178-191 and MBP-84-104 peptides respectively compared to control mice. It is noteworthy that spleen cells from control mice with EAE had increased levels of IL-17, but relatively low levels of IFN-γ, corroborating previous reports that IL-17 is the dominant pro-inflammatory cytokine in EAE (Komiyama et al., 2006). These data demonstrate conclusively that RTL treatment induces a dramatic cytokine shift in mice with EAE.
Figure 4. Cognate RTL decreased splenocyte secretion of IL-17 and increased secretion of IL-10 & IL-13.
Mice were immunized with two peptides and were treated with individual RTLs at disease onset for 8 days. Seven days later, splenocytes from control and individual RTL-treated group were stimulated with respective myelin peptides for 48h. Supernatants were assayed for cytokine production using a Bio-Plex cytokine assay kit. Significant differences between control and experimental groups were determined using Student’s t test (*, p< 0.05). Data are presented as the mean ± SD of three replicate cultures from pooled cells, and are representative of two experiments. Figure reproduced from(Sinha et al., 2009).
RTL treatment reverses passive EAE and immune inflammation in the CNS
RTL treatment reduces encephalitogenicity of pathogenic cells
Although RTLs can reverse clinical signs of EAE, little is known about the fate and migration of encephalitogenic cells in mice treated with RTL. To address this issue, we used a passive transfer model in which GFP-Tg C57BL/6 mice served as donors of 50 million encephalitogenic cells specific for MOG-35-55 peptide that were transferred and tracked in recipient WT C57BL/6 mice. At disease onset (d7–12) mice were treated daily for 5 days with RTL551 (I-Ab/MOG-35-55 peptide) given intravenously. As with active induction, RTL treatment of passive EAE nearly abolished clinical signs, reducing CDI scores from 49.8±7.1 observed in control mice to 14.8±7.8 (p<0.05). The peak disease score was also significantly lower in the RTL551-treated mice (5±0 in controls vs. 2.1±0.4 in RTL-treated mice; p<0.01).
Spleens were harvested 19 days post immunization and the cells were sorted to recover GFP+ vs. GFP non-expressing populations. Four million sorted cells from each group were cultured in the presence of irradiated APC and 25µg MOG-35-55 peptide for 48 hours, followed by cytokine detection in the culture supernatants. The results from this experiment (Fig. 5) showed 1) that both IL-17 and TNF-α, known pathogenic cytokines, were contributed mainly by GFP+ encephalitogenic cells, and 2) that treatment of the mice with RTL551 strongly reduced production of these cytokines. RTL treatment thus selectively modified the pathogenic phenotype of the encephalitogenic cells.
Figure 5. RTL551 treatment inhibits inflammatory cytokine production by transferred GFP+ encephalitogenic T cells in WT C57BL/6 mice with EAE.
Fifty million GFP+ MOG-35-55 specific cells were transferred into WT C57BL/6 mice to induce EAE, and at onset, mice were treated daily for 5 days with buffer or RTL551. At onset and on Day 8 after starting treatment, GFP+ and GFP− cells from spleen were activated with MOG-35-55 peptide and 48h cultures assayed for IL-17 and TNF-α. A) Secreted protein in supernatants. B) Cytokine gene expression in cell pellets. Significant differences between control and RTL-treated groups were determined using Student’s t test (*, p<0.05). Results indicate the mean ± SD of three replicate cultures of pooled cells from 3 experiments. ND, Not detectable. Figure reproduced from(Sinha et al., 2007).
RTL551 treatment reverses autoimmune inflammation in the spinal cord (SC) of mice with EAE
We evaluated the effect of RTL treatment on the infiltration of GFP+ encephalitogenic cells in SC of mice with passive EAE (shown in Fig. 6). Mice were euthanized prior to and 8 days after the initiation of a 5 day course of treatment with RTL551 or buffer. GFP+ encephalitogenic cells that were present in SC when treatment was started were increased in number 8 days later in vehicle-treated mice, but were essentially undetectable in RTL treated mice, concomitant with reduced EAE scores. Note that RTL treatment not only reduced the number of already infiltrated cells, but was also capable of blocking the entry of new inflammatory cells into the CNS. In order to localize more precisely the transferred encephalitogenic cells in SC of treated mice, lumbar sections were stained with biotinylated anti-GFP and counter-stained with hematoxylin. We found a few GFP+ cells that were restricted to the sub-arachnoid space in SC of the RTL551-treated group (data not shown) and therefore, were unavailable to contribute to CNS damage. In contrast, GFP+ cells in control SC sections were distributed throughout the parenchyma of the white matter and along the perivascular space (not shown).
Figure 6. RTL551 treatment reverses autoimmune inflammation in the spinal cords of mice with passive EAE.
Fixed, frozen SC sections from mice were visualized under a fluorescence microscope at onset (top), or 8 days after initiation of a 5-day course of treatment with vehicle (middle) or RTL (bottom). Magnification top, middle and bottom×4; inset×20. Figure reproduced from(Sinha et al., 2007).
RTL551 reduces expression of ICAM, VCAM and many chemokines and chemokine receptors in the CNS
Treatment of EAE mice with RTL551 also inhibited expression of ICAM and VCAM on CNS vascular endothelial cells, possibly accounting for reduced cellular infiltration and the rapid disappearance of encephalitogenic MOG-35-55 specific T cells from CNS parenchyma shown above. Moreover, the CNS of RTL-treated mice had reduced levels of inflammatory cytokines, chemokines and chemokine receptors, including CXCL1 & CXCL2 and their specific receptor, CXCR2 (known to inhibit remyelination), that were profoundly reduced by 11-, 35- and 13-fold, respectively (Sinha et al., 2007).
RTL therapy promotes remyelination and neuroprotection
RTL treatment of EAE mice promotes neuroregeneration
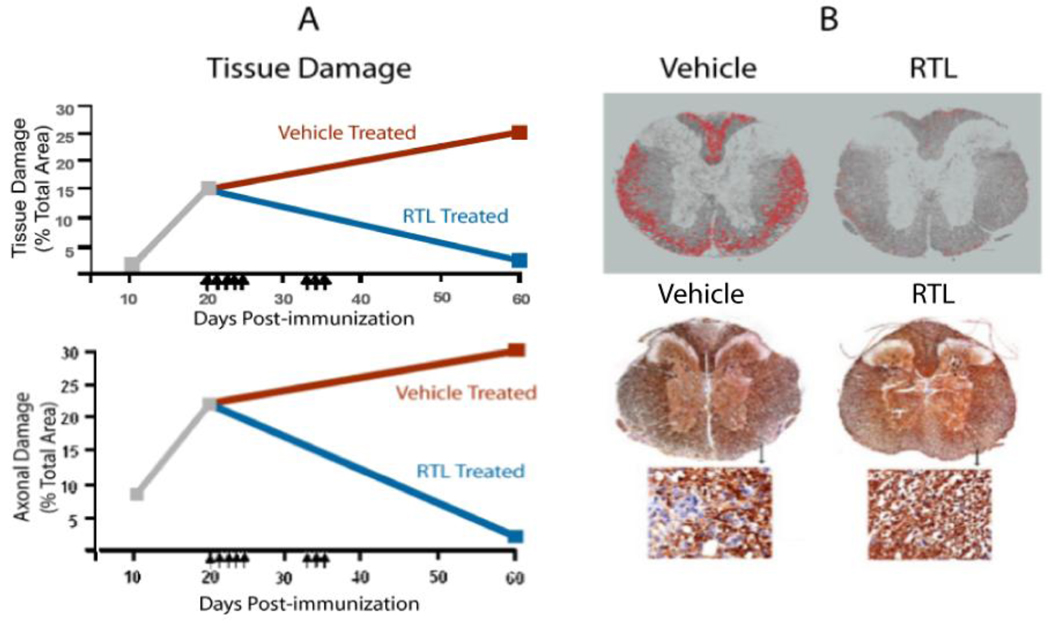
T cell mediated inflammation results in CNS damage in MS and EAE, and it is not known how much repair of injured myelin and axons can occur following highly selective anti-inflammatory therapy such as RTLs. To evaluate this question, SJL/J mice with established PLP-139-151 induced EAE were treated with RTL401 or buffer after the peak of EAE when demyelination and axonal damage were evident, and the mice were evaluated 40 days later for changes in clinical scores, neuroinflammation, and myelin and axonal damage in their spinal cords (Wang et al., 2006). As shown in Fig. 7, injection of RTL401 into mice with white matter damage (day 20) resulted in reduced inflammation, remyelination and axonal repair back to pretreatment levels, and improvement of clinical disease scores (Wang et al., 2006). In contrast, all of these measures had worsened in buffer-treated mice. These findings indicate that RTL therapy targeting encephalitogenic T cells may promote CNS neuroregenerative processes.
Figure 7. RTL401 promotes remyelination and axonal repair.
Damage to myelin (Top) and axons (Bottom) was significantly reduced on Day 60 (40 days after initiation of RTL treatment) in spinal cord sections of RTL401- vs. Vehicle-treated SJL/J mice with EAE (A). SC sections were stained and evaluated for damaged myelin (red circumscribed neurons) and axons (less brown staining) (B). Reduced pathology and increased axonal sprouting were observed by EM (not shown). Arrows indicate days of treatment with 100 µg RTL401 or vehicle. Portions of Figure reproduced from(Wang et al., 2006).
Working model of RTL mechanism of action
As designed, RTL constructs bind specifically to the complementary shape of cognate T-cell receptors (TCRs) with low avidity. However, lacking the full-length MHC molecule, in particular the β2 domain that contains the CD4 binding site, RTL ligation of TCR results in a unique pattern of downstream activation (Burrows et al., 2001; Huan et al., 2004; Wang et al., 2003). We propose that this signaling pathway reduces encephalitogenic activity of the T cells. On the other hand, RTLs bind with high affinity to as-yet uncharacterized RTL receptors on the surface of macrophages, dendritic cells (DC) and B cells in a peptide-non-specific manner (Fig. 8A&B) (Sinha et al., 2010), thus possibly accounting for the rapid disappearance of injected RTL from plasma (half life < 5min). Using RTL-specific Fab, we also detected strong binding of RTLs to endothelial cells and numerous parenchymal cells resembling astrocytes (Fig. 9, right panel). These findings suggest that cells other than T cells may also be directly affected by RTLs, including astrocytes as the chief producers of CXCL1 and CXCL2 chemokines in the CNS (Ambrosini and Aloisi, 2004; Huang et al., 2000). Oligodendrocyte lineage cells express a receptor, CXCR2, for these chemokines (Nguyen and Stangel, 2001), whose inhibition prevents oligodendrocyte degeneration and enhances remyelination (Carlson et al., 2008; Gorio et al., 2007; Kerstetter et al., 2009). These considerations suggest that RTL therapy of EAE might promote remyelination through two different pathways: (1) antigen-specific ligation of TCRs on pathogenic T cells resulting in reduced generation of CXCL1/2 that abrogates inflammatory damage (Quill, 1987), and (2) antigenic peptide-independent RTL binding to astrocytes or other CNS parenchymal cells that reduces CXCL1/2 production, and thus enhances repopulation of lesions by oligodendrocytes and ultimately remyelination. Based on these studies, we anticipate that treatment of MS subjects with appropriate doses of RTL1000 (α1β1 DR2/MOG-35-55) could similarly induce tolerance in MOG-35-55 specific T cells, regulate bystander T cells of other myelin antigen specificities and inhibit or reverse infiltration of inflammatory cells into the CNS. These effects could potentially lead to a reversal of clinical deficits and MRI lesions and possibly promote neuroregeneration in the CNS of subjects with MS.
Figure 8. RTL binds to immune cells.
RTL401 binds in an antigenic peptide non-specific manner to splenic CD19+ B cells, CD11b+ macrophages and CD11c+ DC from SJL/J mice by FACS (A) and on RTL-coated cover slips (B). Figure reproduced from(Sinha et al., 2010).
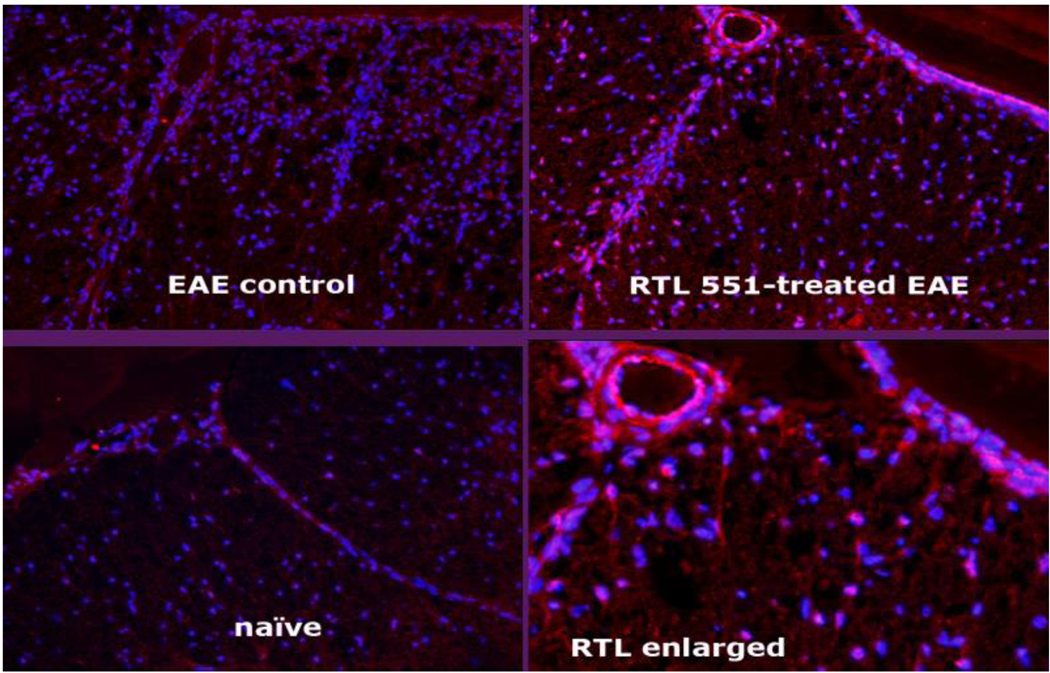
Figure 9. RTL binds to CNS cells.
RTL551 binds to CNS cells in C57BL/6 mice with EAE treated for 5 days with RTL551 (upper right panel and enlarged lower right panel). Spinal cord sections were stained with anti-RTL-specific biotin-Fab311 and detected with PE-labeled streptavidin (pink color). RTL551 was detected on CNS parenchymal cells, the internal lumen and tissue surface of the blood vessel and on the pial surface. No staining was detected in vehicle-treated mice with EAE (upper left panel) or naïve mice (lower left panel) that were not injected with RTL551.
PHASE 1 CLINICAL TRIAL IN MS USING RTL1000 (IND#100128 supported by Artielle ImmunoTherapeutics, Inc., Tigard, OR)
RTL1000 was recently evaluated for safety and tolerability in a multi-center, double-blind, placebo-controlled Phase I dose-escalation study in DR2+ subjects with relapsing remitting or secondary progressive MS (Yadav et al., 2010). Subjects were randomly assigned to receive a single IV infusion of RTL1000 or placebo at a ratio of 4:2, respectively, in consecutive cohorts of 6 subjects receiving 2mg, 6mg, 20mg, 60mg, 200mg and 100 mg. Subjects were monitored for toxicity based on clinical, laboratory and MRI findings over three months. Pharmacokinetic and immunological studies were assessed in a subset of subjects.
Thirty-four subjects were randomized (23 receiving RTL1000 and 11 receiving Placebo, Table 1), with all subjects completing the protocol and none withdrawing from the study. All subjects tolerated the 2–60 mg doses. Doses ≥100mg caused hypotension and diarrhea in 3 of 4 subjects, and further enrollment was discontinued. There was no evidence of disease worsening caused by infusion of any dose of RTL1000 as determined by clinical assessment (EDSS, 25 foot timed walk, 9-hole peg test) and brain MRIs assessed prior to and 28 days post-infusion. It is noteworthy that the fraction of subjects with gadolinium positive lesions did not increase among those receiving RTL 1000, and there was a suggestion of decreased activity with increasing dose of RTL1000.
Table 1.
Disposition of the Subjects
| Cohorts | RTL 1000 dose (in mg) |
No. of Subjects receiving RTL 1000 |
No. of Subjects receiving placebo |
Total subjects |
|---|---|---|---|---|
| I | 2 | 4 | 2 | 6 |
| II a | 6 | 4 | 2 | 6 |
| II b | 6 | 3 | 3 | 6 |
| III | 20 | 4 | 2 | 6 |
| IV | 60 | 4 | 2 | 6 |
| V | 200 | 3 | 0 | 3 |
| VI | 100 | 1 | 0 | 1 |
| Total subjects | 23 | 11 | 34 |
The half-life of RTL1000 in plasma was <5 minutes, possibly due to rapid partitioning to blood cells as described above. Moreover, small increases above baseline in non-neutralizing IgM and IgG antibodies specific for RTL1000 were observed in some placebo and RTL recipients. Drug dose-dependent cytokines were transiently increased in plasma and blood mononuclear cells similar to those observed in DR2 transgenic mice with EAE receiving RTL1000. These results demonstrate that IV infusion of RTL1000 at a dose of 60mg is well tolerated and induces similar immunologic effects in MS subjects as in successfully treated DR2 transgenic mice with EAE. Of note, the maximum tolerated single dose of 60mg of RTL1000 in subjects with MS is equivalent based on body surface area to a single dose of 250µg of RTL1000 in a mouse, which is at least 2.5-fold more than the 100µg dose needed to produce a dramatic reduction in EAE disease scores that are retained for at least 28 days without further boosting (Figure 10).
Figure 10. A single dose of 100µg RTL1000 is sufficient to treat DR2 mice with EAE for at least 28 days without further boosting.
EAE was induced in DR2 Tg mice with MOG-35-55 peptide/CFA/Ptx and the mice were treated at onset of clinical signs with a single dose of 100µg RTL1000 or vehicle and scored for 28 days. This highly effective dose of RTL1000 is equivalent to a human dose of ~25mg RTL1000 by a body surface area comparison, well below the maximum tolerated dose of 60mg in MS subjects.
Conclusion
We have carried out extensive pre-clinical studies with various RTLs that clearly demonstrate their ability to reverse clinical and histological signs of EAE, reduce pathogenic T cell responses and promote CNS repair processes. Our Phase 1 clinical trial using RTL1000 in subjects with MS demonstrated that the drug is safe and well tolerated at a dose of ≤60mg that is well within the effective dose range for treating mice with EAE. Based on these exciting results, we are now planning a Phase 2 study in a larger cohort of MS patients to evaluate clinical efficacy. RTL1000 clearly represents a novel approach for the treatment of MS that promises potent immunoregulation and CNS repair without global immunosuppression.
ACKNOWLEDGEMENTS
This work was supported by the National Multiple Sclerosis Society Grants RG3794B, RG3794A and RG3468A, NIH Grants NS47661, AI43960, NS41965, and NS46877, and the Biomedical Laboratory R&D Service, Department of Veterans Affairs. The authors wish to thank Ms. Eva Niehaus for assistance in preparing the manuscript and to acknowledge the contributions of Chunhe Wang, Sushmita Sinha, and Shayne Andrew. Dr. Offner, Dr. Burrows and Dr. Vandenbark have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the result of this research and technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
REFERENCES
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Adlard KL, B. F. Bebo J, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol. 2000;164:6366–6371. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161:5987–5996. [PubMed] [Google Scholar]
- Burrows GG, Chang JW, Bachinger H-P, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Prot Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Mechling DE, Bachinger H-P, Burrows GG. Design, engineering, and production of human recombinant T cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276:24170–24176. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- Chou YK, Edwards DM, Weinberg AD, Vandenbark AA, Kotzin BL, Fontenot AP, Burrows GG. Activation pathways implicate anti-HLA-DP and anti-LFA-1 antibodies as lead candidates for intervention in chronic berylliosis. J Immunol. 2005;174:4316–4324. doi: 10.4049/jimmunol.174.7.4316. [DOI] [PubMed] [Google Scholar]
- Gorio A, Madaschi L, Zadra G, Marfia G, Cavaleri B, Bertini R, Giulio AM. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharmacol Exp Ther. 2007;322:973–981. doi: 10.1124/jpet.107.123679. [DOI] [PubMed] [Google Scholar]
- Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol. 2004;172:4556–4566. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O'Bryan S, Bielecki B, Zhou ZL, Majumder S, Ransohoff RM. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunological Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- Kerstetter AE, Padovani-Claudio DA, Bai L, Miller RH. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp Neurol. 2009;220:44–56. doi: 10.1016/j.expneurol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Stangel M. Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells. Dev. Brain Res. 2001;128:77–81. doi: 10.1016/s0165-3806(01)00128-6. [DOI] [PubMed] [Google Scholar]
- Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planer lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3709. [PubMed] [Google Scholar]
- Sinha S, Miller L, Subramanain S, McCarty OJT, Proctor T, Meza-Romero R, Huan J, Burrows GG, Vandenbark AA, Offner H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.04.013. doi:10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H. Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T-cell receptor ligand. J Neurosci. 2009;29:3816–3823. doi: 10.1523/JNEUROSCI.5812-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H. A promising therapeutic approach for multiple sclerosis: Recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27:12531–12539. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to MOG-35-55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- Wang C, Gold BG, Kaler LJ, Yu X, Afentoulis ME, Burrows GG, Vandenbark AA, Bourdette DN, Offner H. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98:1817–1827. doi: 10.1111/j.1471-4159.2006.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- Yadav V, Bourdette D, Bowen JD, Lynch SG, Mattson D, Preinigerova J, Rose C, Stead RB, Ferro AJ, Goldstein AS, Burrows GG, Offner H, Vandenbark AA. Recombinant T cell receptor ligand (RTL) for the treatment of multiple sclerosis: Report of a Phase I clinical trial. Neurology. 2010;74(S2):A293–A294. [Google Scholar]