Abstract
Early epidemiologic and serologic studies have suggested preexisting immunity to the pandemic A (H1N1) 2009 influenza virus (H1N1pdm) may be altering its morbidity and mortality in humans. To determine the role that contemporary seasonal H1N1 virus infection or trivalent inactivated vaccine (TIV) might be playing in this immunity we conducted a vaccination-challenge study in ferrets. Vaccination with TIV was unable to alter subsequent morbidity or contact transmission in ferrets following challenge with H1N1pdm. Conversely, prior infection with the contemporary seasonal H1N1 strain altered morbidity, but not transmission, of H1N1pdm despite the detection of only minimal levels of cross reactive antibodies.
Introduction
The emergence of the H1N1pdm virus is a serious global public health concern [1]. Preliminary analysis of early virus isolates showed that they possess 6 gene segments phylogenetically related to those of triple-reassortant viruses known to circulate in swine in North America and Asia and 2 genes (neuraminidase and matrix) related to those of influenza A viruses circulating in the Eurasian swine population [2]. The virus has spread globally leading to the first influenza pandemic of the 21st century.
Although animal models suggest that H1N1pdm viruses are intrinsically more pathogenic than contemporary seasonal human H1N1 strains [3–5], clinical observations show that both strains have similar pathogenicities in humans in most age groups [6]. This disparity between animal models and humans could be explained by a degree of existing immunity in humans to the pandemic strain. Indeed, the hemagglutinin (HA) gene ancestral to the pandemic and seasonal H1 strains is that which entered humans and swine during the 1918 influenza pandemic. Consistent with an impact of preexisting immunity are serologic studies that have shown an age dependent level of H1N1pdm-neutralizing antibody in individuals not yet exposed to the pandemic strain [3, 7] and studies in guinea pigs that have shown prior H1N1 or H3N2 infection can reduce H1N1pdm transmission [8]. Moreover, structural analyses of various H1 HA's either from the 1918 and the 2009 pandemic viruses or humans seasonal viruses have demonstrated the antigenic relatedness of the HA's from the 2 pandemic viruses [9, 10]. Although the age dependency of the serologic data, showing that the elderly have increased titers, suggests that prior infection is linked to the presence of cross-reactive antibodies, the contribution of contemporary seasonal trivalent inactivated vaccine (TIV) remains unresolved.
An examination of human sera collected before and after vaccination with seasonal influenza vaccines showed that vaccination is neither able to induce a cross-reactive humoral immune response to the pandemic virus in children nor significantly boost a cross-reactive antibody response in sera from adults as measured by microneutralization assay [11]. Experience with H5N1 influenza models have, however, shown that in vivo protection can occur even in the absence of a detectable in vitro response [12–14]. The data from clinical studies remains ambiguous in terms of the impact of TIV on H1N1pdm in humans. Two such studies, a case-cohort study in the US and a case-control study in Australia have concluded that prior TIV administration had no protective effects on H1N1pdm [15, 16]. Conversely, a case-control and a retrospective-cohort study conducted in Mexico have both found a protective capacity for TIV, especially against the severe forms of H1N1pdm induced disease [17, 18]. Moreover, Del Giudice and colleagues showed that in ferrets TIV administration immunologically prime for a better antibody response against the H1N1pdm monovalent vaccine [19]. Finally, a recently published Canadian study showed that prior recipients of the 2008–09 TIV were approximately twice as susceptible to developing illness following H1N1pdm infection compared to those who had not received the vaccine [20]. Overall, and despite the threat to public health, the actual degree to which TIV or prior seasonal H1N1 infection influences the pathogenicity and transmission of the pandemic virus is poorly understood.
The focus of this study was therefore to determine whether prior vaccination against or exposure to seasonal H1N1 virus could alter subsequent replication of a H1N1pdm virus in ferrets.
Methods
Viruses and cells
The H1N1 viruses A/Brisbane/59/2007 (contemporary seasonal vaccine strain, passaged three times in eggs and twice in MDCK cells before being used), A/Tennessee/1-560/2009 (a representative H1N1pdm strain, passaged three times in eggs before being used), and the A/California/07/2009 vaccine strain (H1N1pdm virus, rescued in eggs) were obtained from the World Health Organization influenza-collaborating laboratories. MDCK cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in a humidified atmosphere of 5% CO2 at 37°C in 1× MEM (Invitrogen, Carlsbad, CA) supplemented with 5% fetal calf serum, 200 mM L-glutamine, 40 mg/mL gentamicin, 1× MEM vitamins solution (Sigma, St. Louis, MO), and 1× antibiotic-antimycotic solution (Sigma).
Ferrets
Young male and female ferrets 3–4 months of age and seronegative for currently circulating influenza A H1N1, H3N2, and influenza B viruses were obtained from Triple F farms (Sayre, PA) and from the breeding program at the St Jude Children's Research Hospital. All animal experiments were performed in a biosafety level 2 laboratory, using biosafety level 3 practices. All animal experiments were approved by the Animal Care and Use Committee of St. Jude and performed in compliance with relevant institutional policies of the National Institutes of Health regulation and the Animal Welfare Act. A subcutaneous implantable temperature transponder (Bio Medic Data Systems, Seaford, DE) was placed in each ferret for identification and to take temperature readings.
Immunization and challenge
To mimic several possible scenarios of how prior TIV administration or A/Brisbane/59/2007 (H1N1) infection might affect the pathogenicity and spread of A/Tennessee/1-560/2009 (H1N1), we included a number of experimental groups in our study. Animals were divided into 4 groups, with 4 ferrets per group. Group 1 received two 15 μg doses of the 2008–09 northern hemisphere seasonal TIV (Fluzone, Sanofi Pasteur, Swift water, PA) in the form of intramuscular injection, at a 3-week interval to mimic the likely pandemic dosing schedule in naive individuals. Group 2 was intranasally infected with 106 50% egg infectious doses (EID50) in a 1 ml inoculum volume of live A/Brisbane/59/2007 (H1N1) (the current H1N1 component of the vaccine) and vaccinated 3 weeks later with the seasonal vaccine to mimic the scenario of exposure followed by vaccination. Group 3 received a dose of PBS followed 3 weeks later by a single dose of TIV. Group 4 received 2 doses of phosphate-buffered saline (PBS) as a control. Three weeks after the second immunization, all animals were challenged intranasally with 106 EID50 of A/Tennessee/1-560/2009 (H1N1) in a 1 ml inoculum volume, a representative pandemic strain. As a benchmark control, two additional groups of 4 ferrets were included: Group 5 was vaccinated twice at a 3-weeks interval with the seasonal TIV and Group 6 mock-vaccinated twice with PBS. Both groups were subsequently challenged intranasally in a 1 ml inoculum volume with 106 EID50 of the contemporary seasonal strain A/Brisbane/59/2007 (H1N1). Challenged ferrets were then monitored daily for 10 days for weight change, temperature and clinical disease signs. Body temperature was measured via transponders subcutaneously implanted between the shoulder blades (BioMedic Data Systems, Inc., Seaford, DE).
Transmission studies
For each of the vaccine groups described above, two ferrets were used for evaluation of viral contact transmission. Twenty-four hours after challenge, a naïve ferret was introduced into the cage housing an inoculated ferret (two contact/infected animal pairs per group). Male and female ferrets were used in the transmission arm of the study but we did not observe any difference in the pattern of virus transmission among these ferrets
Nasal washes
On days 2, 4, 7, and 9 after virus inoculation, ferrets were anesthetized with ketamine (25 mg/kg), and 0.5 ml of PBS with antibiotics was slowly introduced into each nostril, recovered, measured, and brought to a volume of 1.0 ml with sterile PBS containing antibiotics. Bovine serum albumin 7.5% was added at a ratio of 1:20 (v/v) as a stabilizing agent. Virus was titrated in eggs and expressed as log10 EID50 per milliliter, as calculated by the method of Reed and Muench [21]. The limit of virus detection was <0.75 log10 EID50/ml. Virus titers were compared by an ANOVA test, and when appropriate, Tukey's multiple comparison test.
Serologic testing
Ferret sera were obtained before vaccination or infection, before vaccine boost, before challenge, and 10 days after challenge. Serum samples were treated with receptor□destroying enzyme (Accurate Chemicals and Scientific, New York) overnight at 37°C, heat-inactivated at 56°C for 30 min, and diluted 1:10 in PBS and tested by the hemagglutination inhibition (HI) assay with 0.5% packed turkey red blood cells. Virus-neutralizing antibody titers were determined in MDCK cells. Briefly, the 50% tissue culture infectious dose (TCID50) was determined for each virus used, and 2-fold serial dilutions of serum were incubated with 100 TCID50 of virus for 1 h at 37°C. The mixture was then added to MDCK cells and incubated for 72 h at 37°C in 5% CO2. After 72 h, HA activity of the supernatant was assessed by the HA assay with 0.5% packed turkey red blood cells. Neutralizing titers were expressed as the reciprocal of the serum dilution that inhibited 50% of the HA activity of 100 TCID50 of virus. Both HI and MN assays were performed with A/Brisbane/59/2007 and the A/California/07/2009 vaccine seed strain in a BSL2+ laboratory. For antigen-specific ELISA, microtiter plates (Corning, Lowell, MA) were coated overnight at 4°C with inactivated purified whole A/Brisbane/59/2007 or A/Tennessee/1-560/2009 viruses in PBS. After an overnight incubation with serial dilutions of ferrets' sera, influenza-specific IgG antibodies were detected with a goat anti-ferret IgG alkaline-phosphatase conjugate (Biotrend, Cologne, Germany) diluted 1:1000 in PBS with 1% bovine serum albumin (BSA). The substrate (p-nitrophenyl phosphate; Sigma-Aldrich, Atlanta, GA) was added, plates were incubated for 30 min at room temperature for color development, and OD values were determined at 405 nm in an ELISA reader (Biorad, Los Angeles, CA).
Results
Impact of TIV or prior infection on H1N1pdm replication in ferrets
To determine the effect of prior priming scenarios on subsequent A/Tennessee/1-560/2009 (H1N1) replication, a number of clinical and virologic features were measured post challenge. TIV vaccinated groups 1 and 3 and group 4 unvaccinated animals all displayed similar clinical signs (weight loss, fever, and sneezing) after A/Tennessee/1-560/2009 (H1N1) challenge, indicating that the seasonal vaccine does not alter H1N1pdm disease progression in ferrets. These results were consistent with the lack of detectable serum-neutralizing antibodies generated by the TIV that were able to inhibit the pandemic strain, as measured by hemagglutination inhibition (HI) or microneutralization (MN), although non-neutralizing antibodies were detected by ELISA (Table 1). A/Tennessee/1-560/2009 (H1N1) induced disease in ferrets that was characterized by fever, weight loss, sneezing, and viral clearance by day 7 post infection (Table 2) [3–5].
Table 1.
| Titers against A/Brisbane/59/2007 (H1N1)a | Titers against the 2009 pandemic H1N1a | |||||||
|---|---|---|---|---|---|---|---|---|
| Ferret group | Vaccine regimenb | Challenge virusc | HI, meand (range) | MN, meand (range) | ELISA (IgG)e, mean | HI, meand | MN, meand | ELISA (IgG)e, mean |
| 1 | Two doses of the 2008/09 TIVf | A/Tennessee/1-560/2009 | 22.5 (35) | 22.5 (15) | 560 | < | < | 180 |
| 2 | Infection then vaccinationg | A/Tennessee/1-560/2009 | > | 800 (960) | 4800 | < | < | 600 |
| 3 | Single dose of the 2008/09 TIV | A/Tennessee/1-560/2009 | 13.75 (35) | 10 (10) | 130 | < | < | 65 |
| 4 | PBS | A/Tennessee/1-560/2009 | < | < | < | < | < | < |
| 5 | Two doses of the 2008/09 TIV | A/Brisbane/59/2007 | 38.75 (75) | 18.75 (20) | NDh | < | < | ND |
| 6 | PBS | A/Brisbane/59/2007 | < | < | ND | < | < | ND |
These are the titers of the serum samples collected prior to challenge.
For ferrets that received 2 doses, the vaccinations were administered 3 weeks apart.
The challenge occurred 3 weeks after the vaccination boost, challenge dose of live virus: 106 EID50 for each ferret.
HI=hemagglutination inhibition, expressed as the reciprocal of the highest serum dilution that inhibited 4 hemagglutinating units of virus; MN=microneutralization, expressed as the reciprocal of the highest serum dilution that neutralized 100 TCID50 of virus; To calculate arithmetic mean values, a titer of <10 was assigned a value of 5, and a titer of ≥1280 was assigned a value of 1280; < indicates that all ferrets had HI or MN titers <10; > indicates that all ferrets had titers ≥1280. For measuring titers against the pandemic H1N1 virus, a reverse genetics A/California/7/2009 virus (on A/Puerto Rico/8/1934 background) was used.
ELISA titers are presented as the mean per group of the highest dilution that yielded an optical density (OD) value three times higher than that for a 1:100 dilution of pre-immune serum; < indicates that all ferrets had titers <50.
Each dose of the 2008/09 seasonal trivalent inactivated influenza vaccine contains15 μg HA of each of the following 3 strains: A/Brisbane/59/2007 (H1N1)-like virus, A/Brisbane/10/2007 (H3N2)-like virus, and B/Florida/4/2006-like virus.
3 weeks after Intra nasal infection with 106 EID50 of the live A/Brisbane/59/2007 (H1N1) virus, these ferrets received a single dose of the 2008/09 seasonal trivalent inactivated influenza vaccine.
ND: not determined.
Table 2.
Clinical signs of inoculated and direct contact ferrets
| Group | Challenge or contact virus | Inoculated animals | Direct contact animals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight loss (%)a | Feverb | Sneezingc | Lethality | Virus sheddingd | Last day of sheddinge | Sero-conversionf | Virus sheddingd | Sero-conversionf | ||
| 1 | A/Tennessee/1-560/2009 | 4/4 (8.7) | 4/4 | 3/4 | 0/4 | 4/4 (5.6) | <7 | 4/4 (>) | 4/4 (4.1) | 4/4 (>) |
| 2 | 4/4 (6.8) | 4/4 | 3/4 | 0/4 | 4/4 (3.4) | <4 | 4/4 (>) | 4/4 (3.6) | 4/4 (>) | |
| 3 | 4/4 (7.3) | 4/4 | 4/4 | 0/4 | 4/4 (5.5) | <7 | 4/4 (>) | 4/4 (3.4) | 4/4 (>) | |
| 4 | 4/4 (7.9) | 4/4 | 3/4 | 0/4 | 4/4 (4.9) | <7 | 4/4 (>) | 3/4 (2.6) | 4/4 (>) | |
| 5 | A/Brisbane/59/2007 | 4/4 (6.2) | 2/4 | 3/4 | 0/4 | 4/4 (5.7) | <9 | 4/4 (>) | 4/4 (7.5) | 4/4 (w) |
| 6 | 4/4 (5.0) | 3/4 | 3/4 | 0/4 | 4/4 (6.6) | <9 | 4/4 (w) | 4/4 (7.4) | 4/4 (w) | |
Mean maximum percentage weight loss post challenge (as compared to weight at challenge day).
A temperature increase of ≥0.7°C above day 0 temperature was considered as a fever.
Number of ferrets observed sneezing during the 10 days after challenge.
Virus titers were measured in nasal washes. The mean peak value per group is indicated in parentheses, expressed in log10 EID50/mL.
<x indicates that at day x the ferrets were not shedding virus any longer.
Seroconversion was observed against the challenge (or contact) virus both by HI and MN assays. The mean arithmetic MN titer per group is indicated in parentheses; w stands for weak titers (<10 in HI and <70 in MN assay) and > for high titers (≥1280 in HI and ≥130 in MN assays).
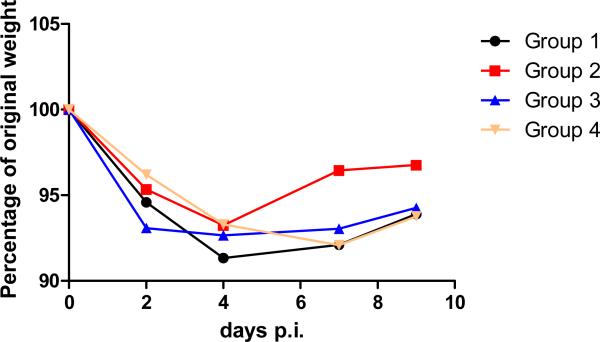
Although vaccination with TIV alone did not have a significant impact on A/Tennessee/1-560/2009–induced disease, prior infection with the seasonal strain followed by vaccination (group 2) significantly reduced titers (P ≤ 0.002 at day 2 post infection) and duration of viral shedding as compared with control and TIV-vaccinated animals, despite the lack of detectable cross-reacting antibodies prior to challenge (Figure 1 and Table 1). Correspondingly, group 2 ferrets pre-infected with A/Brisbane/59/2007 (H1N1) then vaccinated with TIV recovered faster than ferrets in other groups: ferrets in Group 2 started gaining weight as early as day 5 or 6 post infection whereas those in Groups 1, 3, and 4 did not recover until day 8 or 9 post infection (Figure 2).
Figure 1.
Shedding of influenza virus A/Tennessee/1-560/2009 (H1N1) in ferrets. Nasal washes were collected on days 2, 4, 7 and 9 post challenge for inoculated ferrets and on days 3, 6, and 8 post contact for naive contact animals. Virus titers were determined by end-point titration in embryonated chicken eggs. Arithmetic mean titers are given; error bars indicate standard error. The black, red, blue and yellow lines represent the titers of group 1, 2, 3, and 4 ferrets respectively. Titers of challenged ferrets are represented as solid lines and titers of their contacts are represented by dashed lines. *** refers to a statistically significant difference (Tukey's HSD: P≤0.002) between titers of group 2 inoculated ferrets and those of groups 1, 3, and 4 inoculated ferrets.
Figure 2.
Weight loss of A/Tennessee/1-560/2009 inoculated ferrets. The black, red, blue and yellow curves represent the titers of groups 1, 2, 3 and 4 respectively.
Impact of TIV on A/Brisbane/59/2007 (H1N1) replication in ferrets
To be able to put the effect of TIV administration on H1N1pdm replication into context, we also infected TIV dosed ferrets with A/Brisbane/59/2009 (H1N1), a component of the TIV. Somewhat surprisingly, the vaccine had only a minor protective capacity. Although TIV decreased subsequent A/Brisbane/59/2009 (H1N1) replication in the upper respiratory tract of vaccinated ferrets (group 5) at day 2 post infection, the reduction did not reach significance in comparison to control animals (Group 6); P=0.0528) (Figure 3). There were no other detectable differences between group 5 and 6 challenged animals in terms of weight loss or clinical signs.
Figure 3.
Shedding of influenza virus A/Brisbane/59/2007 (H1N1) in ferrets. Nasal washes were collected on days 2, 4, 7 and 9 post challenge for inoculated ferrets and on days 3, 6, and 8 post contact for naive contact animals. Virus titers were determined by end-point titration in embryonated chicken eggs. Arithmetic mean titers are given; error bars indicate standard error. The red and blue lines represent the titers of groups 5 and 6 respectively. Titers of challenged ferrets are represented as solid lines and titers of their contacts are represented by dashed lines.
Impact of TIV or prior infection on viral contact transmission in ferrets
Although TIV alone had little detectable impact on replication of the pandemic H1N1 virus, we also sought to determine if the transmission properties of the pandemic and seasonal strains were altered in vaccinated ferrets. Regardless of their previous vaccination and infection history, however, all infected ferrets transmitted virus to naive ferrets in direct contact with them (Figure 1). The partial protection acquired by group 2 ferrets after intranasal A/Brisbane/59/2007 (H1N1) infection followed by TIV administration did not prevent A/Tennessee/1-560/2009 (H1N1) transmission to, or reduce virus titers in, contact ferrets. Similarly in groups 5 and 6, TIV did not prevent transmission of the homologous A/Brisbane/59/2007 (H1N1) to the naïve contacts (Figure 3).
Discussion
The data presented here in ferrets provide direct in vivo evidence that seasonal H1N1 infection followed by TIV vaccination, but not TIV vaccination alone, can alter subsequent replication of the pandemic H1N1 strain in naive individuals. These data also supports suggestions that the protection against pandemic influenza seen in the elderly is primarily driven by prior exposure to H1N1 viruses and not by vaccination. Somewhat surprising is that protection from H1N1pdm, albeit marginal, was generated by prior infection with a contemporary H1N1 seasonal strain followed by TIV administration. This is mainly mediated by the infection because we did not observe any significant differences in the serologic titers against the pandemic virus in animals that were infected with the seasonal H1N1 viruses prior to and after receiving the seasonal vaccine. Although a specific vaccine is highly recommended for vaccine-induced protection, our data suggest that a higher proportion of the population will have basal levels of preexisting immunity to the novel strain than suggested by prior serologic studies [3, 7]. These studies demonstrated that the detectable antibody cross- reactivity's to H1N1pdm viruses is seen only in those individuals alive during the early stages of the 1918 influenza pandemic. The absence of detectable neutralizing antibodies against H1N1pdm after seasonal virus infection in our study is consistent with the results of these investigators; the subsequent protection seen in ferrets, however, is somewhat more encouraging from a public health standpoint and indicates that human serology underestimates the degree of existing H1N1pdm immunity. Although protection in the absence of detectable neutralizing antibody titers is not without precedent, it does raise questions as to the mechanisms involved. Three, by no means mutually exclusive, possible mechanisms are protection mediated by non-neutralizing antibodies (as detected by ELISA, Table 1), neutralizing antibodies below the assay detection thresholds, or cross-reactive memory CD8 T-cells. A recent computer search has indeed shown conservation of a number of known CD8+ T cell epitopes between seasonal and pandemic H1N1 strains [22, 23]. Data comparing the efficacy of live attenuated and TIV formulations of H1N1pdm may provide additional information as to the role of T cell mediated immune mechanism in the modulation of disease associated with the current pandemic.
Although the A/Brisbane/59/2007 driven cross immunity in the ferret model was not particularly potent, its effects at a population level in humans may be significant. It is also worth noting that we were only able to test transmission in a contact model and that the impact of previous exposures could be more substantial on droplet transmission. In terms of pandemic vaccination, it is encouraging to see that control and contact ferrets challenged with A/Tennessee/1-560/2009 were able to generate a faster and higher antibody response than their A/Brisbane/59/2007 counterparts (Table 2), suggesting that a human H1N1pdm vaccine may not have the poor immunogenicity seen in unadjuvanted H5N1 vaccines [24], a finding supported by recent clinical trial data [25, 26]. These data show that, unlike pre-trial predictions, a single 15ug dose of monovalent H1N1pdm vaccine was enough to elicit a seroprotective response (HI≥40) in over 90% of people in both trials. This result is in contrast to H5 and H9 vaccine trails which showed disappointing levels of immunogenicity with unadjuvanted vaccines [24, 27] and suggests that a majority of the population has been primed against the H1N1pdm vaccine to some degree. The ability of A/Brisbane/59/2007 to provide some protection against A/Tennessee/1-560/2009 in our experiments suggests that the seasonal H1N1 strains may be providing this priming. Future animal studies with the pandemic vaccine will help to confirm this.
A somewhat unexpected finding in this study was the relative ineffectiveness of TIV in protecting from the homologous A/Brisbane/59/2007 challenge. Although there was a slight reduction in nasal shedding in vaccinated versus control animals at some time points, there was no significant difference in weight loss or transmission between groups. The lack of efficacy was not due to an absence of a response to the vaccine as seroprotective titers were observed in 2 of 4 ferrets; the remaining 2 ferrets had HI titers of 30 and <10. This result raises the issue of the suitability of the ferret for modeling the human response to TIV. The clinical data of TIV in children, however, is somewhat equivocal in terms of vaccine efficacy (for recent discussion see Vasikari et al) [22]. Thus, it is quite possible that TIV administration in a naïve host has marginal efficacy against virus replication, particularly for virus with tropism for the upper airways, and that what we have observed in ferrets is a true representation of what is seen in humans.
Taken together our data provide evidence for a negligible role for seasonal TIV which is consistent with what Del Giudice et al. has shown [19], but not prior H1N1 infection, in altering H1N1pdm disease and transmission.
Acknowledgments
We gratefully acknowledge Heather Forrest, Patrick Seiler, Sharon Lokey, David Carey, Angela Ferguson, and Scott Krauss for their excellent technical assistance, Vani Shanker for editorial assistance. We thank the WHO Global Influenza Surveillance Network for providing the H1N1 viruses and the US National Institute of Allergy and Infectious Diseases (NIAID) for provision of TIV. This study was partially supported by the NIAID (Contract No. HHSN266200700005C), the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None declared. The corresponding author has had full access to all the data in the study and has had the final responsibility for the decision to submit this manuscript for publication.
References
- [1].Swine influenza A (H1N1) infection in two children--Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009 Apr 24;58(15):400–2. [PubMed] [Google Scholar]
- [2].Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009 Jun 25;459(7250):1122–5. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- [3].Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009 Jul 13; doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, et al. Transmission and Pathogenesis of Swine-Origin 2009 A(H1N1) Influenza Viruses in Ferrets and Mice. Science. 2009 Jul 2; doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, et al. Pathogenesis and Transmission of Swine-Origin 2009 A(H1N1) Influenza Virus in Ferrets. Science. 2009 Jul 2; doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009 Jun 18;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- [7].Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. 2009 Sep 10; doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- [8].Steel J, Staeheli P, Mubareka S, Garcia-Sastre A, Palese P, Lowen AC. Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. J Virol. 2009 Oct 14; doi: 10.1128/JVI.01732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wei CJ, Boyington JC, Dai K, Houser KV, Pearce MB, Kong WP, et al. Cross-neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci Transl Med. Mar 24;2(24):24ra1. doi: 10.1126/scitranslmed.3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr., Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. Apr 16;328(5976):357–60. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009 May 22;58(19):521–4. [PubMed] [Google Scholar]
- [12].Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006 Jul 15;194(2):159–67. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- [13].Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006 Oct 15;194(8):1040–3. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- [14].Middleton D, Rockman S, Pearse M, Barr I, Lowther S, Klippel J, et al. Evaluation of vaccines for H5N1 influenza virus in ferrets reveals the potential for protective single-shot immunization. J Virol. 2009 Aug;83(15):7770–8. doi: 10.1128/JVI.00241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Effectiveness of 2008–09 trivalent influenza vaccine against 2009 pandemic influenza A (H1N1) - United States, May–June 2009. MMWR Morb Mortal Wkly Rep. 2009 Nov 13;58(44):1241–5. [PubMed] [Google Scholar]
- [16].Kelly H, Grant K. Interim analysis of pandemic influenza (H1N1) 2009 in Australia: surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill. 2009 Aug 6;14(31) doi: 10.2807/ese.14.31.19288-en. [DOI] [PubMed] [Google Scholar]
- [17].Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, Grajales-Muniz C, Robles-Perez E, Gonzalez-Leon M, et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. 2009 Nov 11; doi: 10.1016/S0140-6736(09)61638-X. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Garcia L, Valdespino-Gomez JL, Lazcano-Ponce E, Jimenez-Corona A, Higuera-Iglesias A, Cruz-Hervert P, et al. Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-control study in Mexico City. BMJ. 2009;339:b3928. doi: 10.1136/bmj.b3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Del Giudice G, Stittelaar KJ, van Amerongen G, Simon J, Osterhaus AD, Stohr K, et al. Seasonal influenza vaccine provides priming for A/H1N1 immunization. Sci Transl Med. 2009 Dec 23;1(12):12re1. doi: 10.1126/scitranslmed.3000564. [DOI] [PubMed] [Google Scholar]
- [20].Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 7(4):e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reed LJ MH. A simple method of estimating fifty percent endpoints. American Journal of Hygiene American Journal of Hygiene. 27(3):493–7. [Google Scholar]
- [22].De Groot AS, Ardito M, McClaine EM, Moise L, Martin WD. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008–2009 conventional influenza vaccine. Vaccine. 2009 Sep 25;27(42):5740–7. doi: 10.1016/j.vaccine.2009.07.040. [DOI] [PubMed] [Google Scholar]
- [23].Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- [25].Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of Influenza A (H1N1) 2009 Monovalent MF59-Adjuvanted Vaccine -- Preliminary Report. N Engl J Med. 2009 Sep 10; doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- [26].Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response after One Dose of a Monovalent Influenza A (H1N1) 2009 Vaccine -- Preliminary Report. N Engl J Med. 2009 Sep 10; doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- [27].Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006 Nov 1;43(9):1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]