Abstract
This review of literature and our data suggests that up-regulated production of interferon-gamma (IFNG) in periphery and brain triggers a merger of tryptophan (TRY)–kynurenine (KYN) and guanine–tetrahydrobiopterin (BH4) metabolic pathways into inflammation cascade involved in aging and aging-associated medical and psychiatric disorders (AAMPD) (metabolic syndrome, depression, vascular cognitive impairment). IFNG-inducible KYN/pteridines inflammation cascade is characterized by up-regulation of nitric oxide synthase (NOS) activity (induced by KYN) and decreased formation of NOS cofactor, BH4, that results in uncoupling of NOS that shifting arginine from NO to superoxide anion production. Superoxide anion and free radicals among KYN derivatives trigger phospholipase A2-arachidonic acid cascade associated with AAMPD. IFNG-induced up-regulation of indoleamine 2,3-dioxygenase (IDO), rate-limiting enzyme of TRY–KYN pathway, decreases TRY conversion into serotonin (substrate of antidepressant effect) and increases production of KYN associated with diabetes [xanthurenic acid (XA)], anxiety (KYN), psychoses and cognitive impairment (kynurenic acid). IFNG-inducible KYN/pteridines inflammation cascade is impacted by IFNG (+874) T/A genotypes, encoding cytokine production. In addition to literature data on KYN/TRY ratio (IDO activity index), we observe neopterin levels (index of activity of rate-limiting enzyme of guanine–BH4 pathway) to be higher in carriers of high (T) than of low (A) producers alleles; and to correlate with AAMPD markers (e.g., insulin resistance, body mass index, mortality risk), and with IFN-alpha-induced depression in hepatitis C patients. IFNG-inducible cascade is influenced by environmental factors (e.g., vitamin B6 deficiency increases XA formation) and by pharmacological agents; and might offer new approaches for anti-aging and anti-AAMPD interventions.
Keywords: Interferon-gamma, Neopterin, Kynurenines, Metabolic syndrome, Aging, Aging-associated disorders, Major depression
Inflammation and aging-associated disorders
Low grade, T-helper (Th)-1 type, chronic inflammation in the periphery (macrophages) and brain (glia) is a common feature of aging and aging-associated medical (e.g., central obesity, dyslipidemia, hypertension, insulin resistance, menopause, immunosuppression, increased production of cortisol, increased incidents of some types of cancer) (Dilman 1971, 1994) and psychiatric (e.g., late-onset depression, Dilman et al. 1979; vascular cognitive impairment, Oxenkrug 2007, 2010a, b) disorders. The cluster of the aging-associated disorders (central obesity, dyslipidemia, hypertension, insulin resistance) has been known as a metabolic syndrome (MetS) (Fulop et al. 2006; Holvoet 2008) (Table 1).
Table 1.
Aging-associated medical and psychiatric disorders and metabolic syndrome
| Metabolic syndrome | Age-associated diseases (Dilman 1971) |
| Dyslipidemia | Atherosclerosis |
| Obesity | Obesity |
| Hypertension | Hypertension |
| Insulin resistance | Pre-diabetes (type 2 diabetes) |
| Immunosuppression (metabolic and autoimmune) | |
| Climacteric (menopause) | |
| Hypercortisolism (increased cortisol) | |
| Cancrophilia (increased probability of cancer development) | |
| Major depressive disorder (late-onset depression) (Dilman et al. 1979) | |
| Vascular cognitive impairment (Oxenkrug 2007) |
The impact of chronic, Th-1 type inflammation on aging and aging-associated medical and psychiatric disorders (AAMPD) is mediated by the unique ability of pro-inflammatory, Th-1 type, cytokine, interferon-gamma (IFNG), to transcriptionally induce the rate-limiting enzymes of guanine–tetrahydrobiopterin (BH4), and tryptophan (TRY)–kynurenine (KYN) metabolic pathways (TNF-alpha acts synergistically with IFNG) (Robinson et al. 2005; Peterson and Katusic 2005). Some of the KYN derivatives [e.g., quinolinic (QUIN) and picolinic (PICA) acids] transcriptionally activate nitric oxide synthase (NOS) (Melillo et al. 1994; Perez-Severiano et al. 1998) while BH4 serves as an essential NOS cofactor. Therefore, it might be hypothesized that during chronic, Th-1 type inflammation (and up-regulated IFNG production), the two pathways form the KYN/pteridines cascade affecting biosynthesis of nitric oxide (NO) (Fig. 1).
Fig. 1.
Interferon-gamma (+874) and pteridines and kynurenine metabolic pathways. IFNG interferon-gamma, GTP guanosine triphosphate, GTPCH GTP cyclohydrolase, NEO neopterin, BH4 tetrahydrobiopterin, ARG arginine, NO nitric oxide, NOS nitric oxide synthase, TRY tryptophan, IDO indoleamine 2,3-dioxygenase, superoxide anion, PA picolinic acid, QA quinolinic acid
IFNG and TRY–KYN pathway
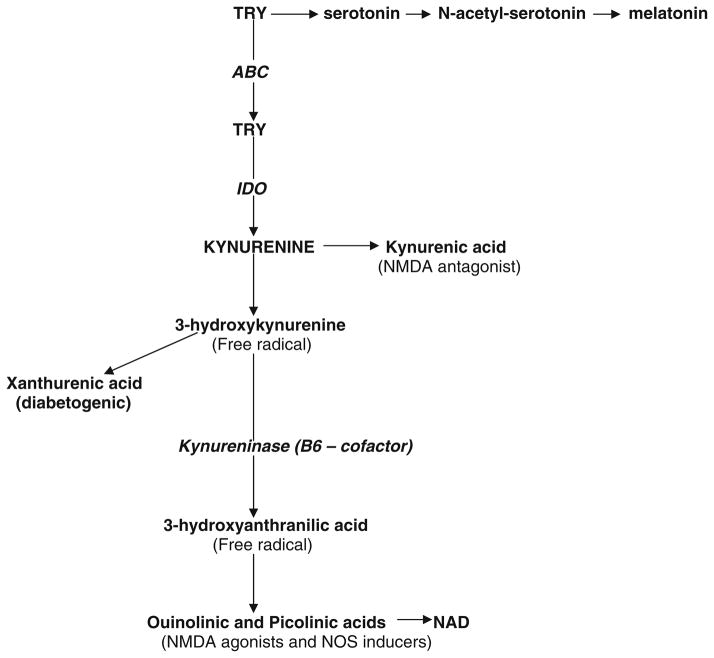
TRY is an essential (for humans) amino acid. Approximately 99% of the dietary TRY, not used in protein synthesis, is metabolized along the KYN pathway to produce nicotinamide adenine dinucleotide (NAD) (Gál and Sherman 1980; Han et al. 2010). KYN is formed in the mammalian brain (40%), and is taken up from the periphery (60%) (Németh et al. 2005). The rest of TRY is utilized as a substrate for methoxyindoles (serotonin, N-acetylserotonin, melatonin) biosynthesis (Oxenkrug 2007) (Fig. 2). The shift of TRY metabolism from methoxyindoles to KYN pathway was suggested as a trigger of depressive disease (Lapin and Oxenkrug 1969; Oxenkrug and Lapin 1974; Oxenkrug 2010b).
Fig. 2.
Major derivatives of tryptophan–kynurenine metabolism. TRY tryptophan, ABC ATP-binding cassette drug transporter, NAS N-acetylserotonin, IDO indoleamine 2,3-dioxygenase, NAD nicotinamide adenine dinucleotide
Availability of TRY as an initial substrate for methoxyindoles and KYN formation is regulated by ATP-binding cassette (ABC) transporter (Sullivan et al. 1980) and nutritional factors (Oxenkrug 2007). Deficiency of TRY results in pellagra (dermatitis, diarrhea and depression) first described in 1735 by Gaspar Casal (Pitche 2005).
Indoleamine 2,3-dioxygenase (IDO) is a flavin-dependent enzyme which uses superoxide anion as a co-substrate to catalyze the first and rate-limiting step of TRY–KYN pathway: the cleavage of the indole ring of TRY followed by KYN formation (Fig. 2) (Hayaishi 1976).
KYN is further metabolized along the two distinct routes (post-KYN metabolism) competing for KYN as a substrate:
KYN: kynurenic acid (KYNA) pathway that produces KYNA, an endogenous broad-spectrum antagonist at all subtypes of ionotropic glutamate receptors that preferentially active at the strychnine-insensitive glycine allosteric site of the N-methyl-D-aspartate (NMDA) receptor, and a non-competitive antagonist at the alpha7 nicotinic receptor that may implicate increased KYNA production with cognitive impairment and psychosis (Schwarcz and Pellicciari 2002; Han et al. 2010).
KYN: NAD pathway that produces NMDA agonists, QUIN and PICA acids, exerting apoptotic and anxiogenic effects (Lapin 1996, 1998, 2003). QUIN and PICA transcriptionally (and robustly: by 327%) induce NOS activity in in vivo and in vitro experiments (Melillo et al. 1994; Perez-Severiano et al. 1998). It was suggested that QUIN and PICA mediate IFNG-induced activation of NOS since IDO inhibitors (norharmane and l-TRY) decreased IFNG-induced formation of not only KYN metabolites but also of NO (Chiarugi et al. 2000). Among KYN derivatives of KYN–NAD pathway there are free radicals generators, 3-hydroxyanthranilic acid and 3-hydroxykynurenine. Cortical 3-hydroxykynurenine concentrations were substantially increased in patients with hepatic encephalopathy suggesting a dysfunction of TRY metabolism in this disease (Riederer and Reynolds 1981; Pearson and Reynolds 1991). Another KYN derivate is xanthurenic acid (XA), possible diabetogenic agent (Kotaki et al. 1975).
Nutritional (environmental) impact on post-KYN metabolism might be mediated by availability of vitamin B6, the cofactor of kynureninase, the enzyme catalyzing formation of 3-hydroxyanthranilic acid from 3-hydroxykynurenine: vitamin B6 deficiency interrupts KYN–NAD pathway, and, consequently, leads to increased formation of XA (Bender et al. 1990). KYN–NAD is involved in Alzheimer’s disease since A beta 1–42, a cleavage product of amyloid precursor protein, induces production of QUIN, in neurotoxic concentrations, by macrophages and microglia (Kincses et al. 2010; Guillemin and Brew 2002).
IFNG and guanine–BH4 pathway
Availability of guanine as an initial substrate for BH4 biosynthesis is regulated by ABC transporter implicated in eye pigmentation (Mackenzie et al. 1999) and male-to-male courtship in Drosophila (Zhang and Odenwald 1995). Human homolog of ABC transporter is similar to multidrug resistance protein 1 (Beedholm-Ebsen et al. 2010), and is implicated in cholesterol metabolism (Voelker 2009), and bipolar male disorder (Kirov et al. 2001), and depression and schizophrenia (Knight et al. 2009). The ABC transporter was identified as the defect in Tangier disease (Fredrickson 1964).
Phosphorylated guanine nucleoside, guanosine triphosphate (GTP) is catalyzed by GTP cyclohydrolase I (GTPCH), the first and rate-limiting step of pteridines biosynthesis, resulting in formation of 7,8-dihydroneopterin triphosphate (BH2). The subsequent step is catalyzed by pyruvoyl tetrahydropterin synthase (PTPS) (Schoedon et al. 1986) (Fig. 3).
Fig. 3.
Interferon-gamma and guanine–BH4 pathway. ABC ATP-binding cassette transporter, IFNG interferon-gamma, TNF-alpha tumor necrosis factor-alpha, GTP guanosine triphosphate, GTPCH GTPcyclohydrolase 1, BH2 7,8-dihydroneopterin, PTPS pyruvoyl tetrahydropterin synthase, BH4 tetrahydrobiopterin, NOS nitric oxide synthase
In humans, IFNG-induced stimulation of GTPCH in monocyte-derived macrophages, dendritic cells and astrocytes does not result in correspondent up-regulation of PTPS that becomes the rate-limiting step with consequent accumulation of BH2 and its stable metabolite, neopterin (Schoedon et al. 1986). Therefore, the enhanced production of neopterin occurs at the expense of BH4 formation (Fuchs et al. 2009). Demand for BH4 might be increased under condition of KYN-induced activation of NOS triggered by IFNG-induced up-regulation of KYN pathway of TRY metabolism (Oxenkrug 2007, 2010a). Deficiency of BH4 results in uncoupling of NOS and shifting arginine metabolism to production of superoxide anion rather than NO (Pou et al. 1992) (Fig. 1).
In addition to their ability to induce NOS, QUIN and PICA together with 3-hydroxykynurenine and 3-hydroxyanthranilic acids (and neopterin and other pteridines) increase lipid peroxidation, and trigger arachidonic acid metabolism resulting in the increased production of inflammatory factors: prostaglandins, via activation of cyclooxygenase (COX) and leucotrienes, via activation of arachidonate 5-lipoxygenase (5-LO) (Stewart et al. 2007) (Fig. 4).
Fig. 4.
IDO/GTPCH upregulation and AAMPD. TRY tryptophan, IDO indoleamine 2,3-dioxygenase, BBB blood brain barrier, GTP guanosine triphosphate, KYN kynurenine, 3HK 3-hydroxyKYN, KYNA kynurenic acid, QA quinolinic acid, PA picolinic acid, NO nitric oxide, NOS NO synthase, PLA phospholipase 2, AA arachidonic acid, COX cyclooxygenase, 5-LO arachidonate 5-lipoxygenase, PGE prostaglandins
COX-2 is of particular interest since its inhibitors blocked KYNA production (Schwieler et al. 2006) and exerted anti-depressant and antipsychotics effects (Müller et al. 2009) while 5-LO was suggested as a link between depression and atherosclerosis (Manev and Manev 2007). It is noteworthy, that prostaglandins F2 alpha and thromboxane B2, additional products of the COX pathway of arachidonic acid metabolism, but leukotriene C4, a product of the lipoxygenase pathway were elevated in dementia (Griffin et al. 1994).
IFNG (+874) gene polymorphisms and regulation of kynurenines/pteridines inflammation cascade
Production of IFNG is encoded by polymorphic gene, IFNG (874) with T (high) and A (low) producers alleles (Pravica et al. 2000). T allele (TA and TT genotypes) are associated with higher that AA genotypes blood IFNG cytokine levels (Anuradha et al. 2008); higher cytokine concentration released by stimulated peripheral blood mononuclear cells (Pravica et al. 2000); and higher IFNG mRNA in white blood cells (Biolo et al. 2006). Increased IDO activity (elevated plasma KYN/TRY ratio) is associated with high producer T allele of (+874) IFNG gene in healthy female subjects in Finland (Raitala et al. 2005). Our recent study of 174 American Caucasian subjects revealed an association of increased GTPCH activity (evaluated by plasma neopterin levels) in TT genotypes IFNG (+874) in comparison with AA genotypes (Perianayagam and Oxenkrug 2010, submitted data).
Therefore, literature and our data indicate that IFNG (+874) T/A genotypes impact the activity of both IDO and GTPCH, and suggest that IFNG (+874) T/A genotypes regulate the activity of IFNG-inducible KYN/pteridines inflammation cascade and translate genetic predisposition into development of chronic inflammation-related conditions (such as AAMPD) (Fig. 5).
Fig. 5.
IFNG-inducible KYNs/pteridines inflammation cascade. ABC ATP-binding cassette transporter, IFNG interferon-gamma, TNF-alpha tumor necrosis factor-alpha, TRY tryptophan, IDO indoleamine 2,3-dioxygenase, KYN kynurenine, XA xanthurenic acid, QA quinolinic acid, PA picolinic acid, GTP guanosine triphosphate, GTPCH GTPcyclohydrolase, BH4 tetrahydrobiopterin, NO nitric oxide, NOS NO synthase, superoxide anion
Considering that TNF-alpha enhances IFNG-induced activation of IDO/GTPCH (Robinson et al. 2005; Peterson and Katusic 2005), the presence of TNF-alpha (−308) high promoter (A) might strength the association between IFNG (+874) high promoter (T) and IDO/GTPCH up-regulation (Oxenkrug 2007, 2010a).
Clinical evaluation of kynurenines/pteridines inflammation cascade
Neopterin as an index of GTPCH activity
As it was mentioned above, neopterin is used in clinical settings as an index of IFNG-induced GTPCH activity (Fuchs et al. 2009). In humans the increased level of neopterin reflects IFNG-induced activation of GTPCH induced by IFNG but not by other pro-inflammatory factors (e.g., interleukin-1-beta) (Sucher et al. 2010; Dinarello 2007).
In addition to its role as a marker of GTPCH activation and IFNG activity, neopterin (as some other pteridines), might modulate oxidative stress (Oettl and Reibnegger 2005).
KYN/TRY ratio as an index of IDO activity
Blood KYN/TRY ratio is used as an index of IDO activity (Raitala et al. 2005). However, this ratio might be affected by the activity of the other enzyme, TRY 2,3-dioxygenase (TDO), catalyzing KYN formation from TRY but whose location (in liver) and regulation (by stress hormones and TRY) are different from IDO (Oxenkrug and Lapin 1974; Dang et al. 2000; Oxenkrug 2007). Therefore, human plasma KYN/TRY ratio might reflect the activity of both IDO and TDO while in humans neopterin levels reflect only IFNG activity (Fuchs et al. 2009).
Clinical studies of IFNG-inducible kynurenines/pteridines inflammation cascade and AAMPD
Available clinical data dedicated to this subject was reported elsewhere (Oxenkrug 2010a, b). Current paper will review most recent data and some specific topics dedicated to aging, obesity, insulin resistance and depression.
Aging and IFNG-induced kynurenines/pteridines inflammation cascade
Interferon-related genes were identified among six pathways (the cell cycle pRB/p53, cytoskeletal, insulin growth factor-related, MAP kinase and oxidative stress) regulating senescence/immortalization (Fridman and Tainsky 2008). Prolonged treatment with IFNG induces cellular senescence in human vascular endothelial cells via up-regulation of senescence-associated genes (Kim et al. 2009). Age-dependent increase in IFNG production was reported in in vitro (Poynter and Daynes 1999) and in vivo studies with minor changes in the remaining evaluated cytokines in senescence-accelerated mice (Rodríguez et al. 2007). Age-associated increase of NO levels of senescence-accelerated mice might depend on IFNG-induced activation of iNOS mediated by KYN (Melillo et al. 1994).
Animal and human data point to aging-associated activation of IDO and GTPCH. Increased formation of KYN derivative, KYNA, was observed in aged rat brain (Moroni et al. 1998; Gramsbergen et al. 1992) and in human serum (Urbańska et al. 2006). KYN/TRY ratio positively correlated with increased aging in humans when comparing three age groups (34–60, 61–71, and 72–93 years) (Frick et al. 2004) and nonagenarians with 45 years old subjects (Pertovaara et al. 2006). The higher rate of TRY conversion into KYN at the entry into the study was predictive of higher mortality in 10-year prospective study of nonagenarians (Pertovaara et al. 2007).
Increased plasma levels of neopterin (but not other 33 independent immune parameters) separated the aged and a healthy younger group (Fahey et al. 2000). Neopterin levels increased with age (Allegra et al. 1992) and were higher among white than blacks with no gender differences (Diamondstone et al. 1994; Schennach et al. 2002) while age-associated increase of IDO was more prominent in women than in men (Raitala et al. 2005).
The presence of T allele of IFNG (+874) gene (Lio et al. 2002), elevated IFNG level (Rodríguez et al. 2007) and high IDO activity (Pertovaara et al. 2006) are associated with high mortality while IFNG (+874) A (low producer) allele, low level of IFNG, and low IDO activity promote longer life span (Lio et al. 2002). In the same vein, Drosophila melanogaster mutants with impaired ABC transporter that impacts the availability of TRY and guanine as the initial substrates of TRY–KYN and guanine–BH4 pathways have twofold longer life span than wild types flies (Oxenkrug 2010c).
Menopause is a core component of aging-associated conditions (Dilman 1971, 1994; Janssen et al. 2008). Development of experimental menopause is associated with inflammation (Nicole et al. 2008). Production of IFNG in women in their 40s and in postmenopausal women was significantly higher compared with that of younger women (Deguchi et al. 2001).
Down’s syndrome, a condition representing an accelerated aging, was associated with higher percentages of IFNG-producing cell in comparison with mentally retarded and healthy controls consistent with observation of an increased KYNA levels in Down’s syndrome patients (Baran et al. 1996).
KYN/TRY ratio and neopterin levels in AAMPD
Literature data indicate significant correlation between aging, major components of AAMPD [such as body mass index (BMI), truncal and morbid obesity, blood lipids, insulin resistance], total and disease-specific mortality with plasma TRY/KYN ratio (Pertovaara et al. 2007; Wirleitner et al. 2003; Niinisalo et al. 2008) and/or with serum neopterin levels (Ray et al. 2007; Grammer et al. 2009; Solichova et al. 2001) in population of European ancestry. Considering that genetic composition of studied population might impact the activity of IFNG-induced KYN/pteridines inflammation cascade, we analyzed plasma neopterin levels in the Puerto Ricans population that characterized by the unique mixture of various genetic backgrounds (Mattei et al. 2009), and by increased prevalence of AAMPD (Shen et al. 2010; Tucker et al. 2010). Plasma neopterin levels in Puerto Ricans community dwellers resided in Boston correlate with some markers of AAMPD [e.g., waist, high density lipoprotein and insulin resistance evaluated by the homoeostasis model assessment (HOMA)]. Neopterin level of >16 nmol/L at the entry of the study was associated with the increased risk of mortality in a subgroup of 112 subjects followed up for 6 years (Oxenkrug and Tucker, submitted data).
Insulin resistance and IFNG-induced inflammation cascade
Insulin resistance underlies the major features of the AAMPD and is a precursor of type 2 diabetes but it causes remain uncertain.
XA and insulin resistance
IFNG up-regulates IDO protein expression and increased intracellular IDO enzyme activity in pancreatic islets (Sarkar et al. 2007). IFNG-induced super-activation of IDO might lead to increased formation of XA. Recent meta-bolomic study found changes in mice model of insulin resistance compatible with increased formation of XA (Li et al. 2010). Injection of XA to rats-induced experimental diabetes (Kotaki et al. 1975). The increased concentration of XA was found in urine of non-insulin-dependent diabetes mellitus patients in comparison to normal healthy subjects (Hattori et al. 1984).
Increased XA might contribute to the development of insulin resistance by formation of chelate complexes with insulin (XA–In). XA–In complex is antigenetically indistinguishable from insulin but it activity was 49% lower than activity of pure insulin (Kotaki et al. 1975). In addition, XA might exert toxic effect in isolated pancreatic islets because of formation of complexes with Zn2+-ions in β-cells (Meyramov et al. 1984).
Since enzymatic formation of QUIN and PICA from 3HK requires vitamin B6 as a cofactor, deficiency of vitamin B6 results in increased formation of XA in expense of QUIN and PICA formation (Bender et al. 1990). Inflammation-associated increased formation of KYN from TRY might lead to excessive production of XA in B6 deficient population (e.g., elderly) and, considering diabetogenic effect of XA, might cause and/or contribute to the development of insulin resistance (Fig. 6). Combination of B6 deficiency in chronic inflammation with increased production of KYN and 3-hydroxyKYN in depression (Lapin and Oxenkrug 1969; Oxenkrug 2010b) might contribute to the increased incidence of diabetes in depressed patients (Campayo et al. 2010). This hypothesis might be further supported by reported correlation between B6 and depression (Merete et al. 2008), B6 and inflammation (Morris et al. 2010; Shen et al. 2010) and by our findings of correlation between neopterin and insulin resistance (Oxenkrug and Tucker, submitted data) and neopterin and B6 (Tucker and Oxenkrug, submitted data) (Fig. 7).
Fig. 6.
Xanthurenic acid and insulin resistance. IFNG interferon-gamma, TNF-alpha tumor necrosis factor-alpha, XA xanthurenic acid, In insulin, Zn zink
Fig. 7.
Shift of post-KYN metabolism toward formation of XA in vitamin B6 deficiency. IDO indoleamine 2,3,-dioxygenase, KYN kynurenine, 3OHKYN 3-hydroxyKYN, QUIN quinolinic acid, PICA picolinic acid, XA xanthurenic acid, NOS nitric oxide synthase
Obesity and IFNG-inducible kynurenines/pteridines inflammation cascade
White adipose tissue secretes IFNG-inducible protein 10, and TNF-alpha (Meier and Thalmann 2007). Obese mice produced more IFNG than controls and had deficient IFNG receptor (Rocha et al. 2008).
Secretion of IFNG was significantly higher in the obese than in the control subjects that might be partly depend on action of leptin, an adipocyte-secreted hormone, that shifts Th cells toward a Th1 phenotype (You et al. 2008). In obese children, a shift to Th1-cytokine profile is dominated by the production of IFNG and is related to insulin resistance (Pacifico et al. 2006). Proinflammatory cytokines exacerbate insulin resistance, impair insulin action, and, thus contributes to the development of type 2 diabetes (Lann and LeRoith 2007).
Free TRY was decreased in the plasma of obese rats. (Finkelstein 1982) Plasma TRY concentrations were decreased in obese subjects independently of weight reduction or dietary intake (Breum et al. 2003; Brandacher et al. 2006). Preoperative KYN/TRY ratio and neopterin levels in morbidly obese patients were significantly increased compared to the control group, and post-operative weight reduction did not normalize KYN/TRY ratio and neopterin levels. In addition, these TRY metabolic changes may subsequently reduce 5-HT production and cause mood disturbances, depression, and impaired satiety ultimately leading to increased caloric uptake and obesity (Brandacher 2007).
Significantly higher serum neopterin levels were reported in subjects with increased waist-to-hip ratio displayed (Bozdemir et al. 2006) and increased BMI (Ledochowski et al. 1999; Ursavaş et al. 2008).
Major depressive disorder and IFNG-inducible kynurenines/pteridines inflammation cascade
The shift of TRY metabolism from methoxyindoles to KYN pathway was suggested as a trigger of depressive disease in 1969 (Lapin and Oxenkrug 1969). Most recent up-date of “serotonin hypothesis” suggested that the presence of high producers (T) alleles of IFNG (+874) gene might identify subject-at-risk for the development of depression (Oxenkrug 2010b). This suggestion might be further supported by our recent finding of 80% more of T and 60% less A carriers among depressed than among not depressed hepatitis C patients treated with interferon-alpha (Oxenkrug et al., submitted data).
Inhibition of TRY–KYN/guanine–pteridines metabolism as a new target for prevention and treatment of metabolic syndrome and related aging-associated disorders
The present paper suggests that IFNG-induced combined up-regulation of KYN formation from TRY and BH4 formation from guanine (IFNG-induced KYN/pteridines inflammation cascade) triggers and/or contribute to mechanisms of aging and AAMPD (including MetS). This hypothesis implies that inhibition of TRY–KYN and guanine–pteridines pathways might slow down aging processes and development of AAMPD. Our recent data on increased lifespan in Drosophila mutants with impaired transmembrane transport of guanine and TRY (white) or deficient conversion of TRY into KYN (vermilion) (almost twofold in comparison with wild type Oregon strain) support this suggestion (Oxenkrug 2010c).
Potential pharmacological interventions in at risk subjects (e.g., carriers of high producer alleles (T) of IFNG (+874) [and, possibly, and A alleles of TNF-alpha (−308) gene] may include:
inhibition of IDO activity by 1-methyl-L-TRY (Cady and Sono 1991), minocycline (Ryu et al. 2006) and MAO inhibitors (Samsonova and Oxenkrug 1972; Sono and Cady 1989; Oxenkrug 1991, 2005). Minocycline deserved a special consideration since it a drug that had been on a market (acne treatment) for more than 50 years. Minocycline inhibits microglial inflammation (Filipovic and Zecevic 2008; Hashimoto 2008), IDO (Ryu et al. 2006), and revealed antipsychotic and cognitive enhancing effect in patients suffering from schizophrenia (Levkovitz et al. 2010), and exerted anti-depressant activity in animal experiments (Molina-Hernandez et al. 2008), and was suggested for clinical antidepressants trials (Pae et al. 2008).
Pharmacological agents based on discovery that worm infection down-regulates Th1 pathways (Weinstock and Elliott 2009);
inhibition of cytokine production by IFNG inhibiting protein (Koga et al. 2007), antibodies to TNF-alpha and IFNG (Skurkovich and Skurkovich 2006) and by antidepressants, e.g., selective 5-HT inhibitor, fluoxetine (Kenis and Maes 2002) and dopamine enhancer, wellbutrin (Brustolim et al. 2006)
administration of methoxyindoles (melatonin and N-acetylserotonin) that inhibit KYN formation from TRY (Walsh and Daya 1997) and modulate the TRY–KYN pathway due to their inhibitory effect on production of cortisol (s1s0s5) and proinflammatory cytokines (Bachurin et al. 1999; Perianayagam et al. 2005; Requintina and Oxenkrug 2003). Methoxyindoles (melatonin, in particular) might attenuate excitatory, glutamate-mediated responses triggered by KYN pathway metabolites (Lapin et al. 1998; Prakhie and Oxenkrug 1998)
administration of melatonin agonists, e.g., Ramelteon that attenuates age-associated hypertension and body weigh gain (Oxenkrug and Summergrad 2010).
Acknowledgments
This paper was supported by NIH MH083225.
References
- Allegra A, Corica F, Di Cesare E, et al. The influence of hypercholesterolemia on some aspects of immune pattern in the elderly. Arch Gerontol Geriatr. 1992;15:13–19. doi: 10.1016/0167-4943(92)90035-3. [DOI] [PubMed] [Google Scholar]
- Anuradha B, Rakh SS, Ishaq M, et al. Interferon-gamma low producer genotype +874 overrepresented in Bacillus Calmette-Guerin nonresponding children. Pediatr Infect Dis J. 2008;27:325–329. doi: 10.1097/INF.0b013e31816099e6. [DOI] [PubMed] [Google Scholar]
- Bachurin S, et al. N-acetylserotonin, melatonin and their derivatives improve cognition and protect against beta-amyloid-induced neurotoxicity. Ann N Y Acad Sci. 1999;890:155–166. doi: 10.1111/j.1749-6632.1999.tb07990.x. [DOI] [PubMed] [Google Scholar]
- Baran H, et al. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 1996;58:1891–1899. doi: 10.1016/0024-3205(96)00173-7. [DOI] [PubMed] [Google Scholar]
- Beedholm-Ebsen R, van de Wetering K, Hardlei T, et al. Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood. 2010;115:1632–1639. doi: 10.1182/blood-2009-07-232587. [DOI] [PubMed] [Google Scholar]
- Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63:27–36. doi: 10.1079/bjn19900089. [DOI] [PubMed] [Google Scholar]
- Biolo G, Amoroso A, Savoldi S, et al. Association of interferon-gamma +874A polymorphism with reduced long-term inflammatory response in haemodialysis patients. Nephrol Dial Transplant. 2006;21:1317–1322. doi: 10.1093/ndt/gfk033. [DOI] [PubMed] [Google Scholar]
- Bozdemir AE, Barutcuoglu B, Dereli D, et al. C-reactive protein and neopterin levels in healthy non-obese adults. Clin Chem Lab Med. 2006;44:317–321. doi: 10.1515/CCLM.2006.055. [DOI] [PubMed] [Google Scholar]
- Brandacher G. Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab. 2007;8:289–295. doi: 10.2174/138920007780362590. [DOI] [PubMed] [Google Scholar]
- Brandacher G, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- Breum L, Rasmussen MH, Hilsted J, Fernstrom JD. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. Am J Clin Nutr. 2003;77:1112–1118. doi: 10.1093/ajcn/77.5.1112. [DOI] [PubMed] [Google Scholar]
- Brustolim D, et al. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–990. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Campayo A, de Jonge P, Roy JF, Saz P, de la Camara C, Quintamilla MA, Marcos G, Santabárbara J, Lobo A. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Dello Sbarba P, Paccagnini A, et al. Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-gamma-activated macrophages. J Leukoc Biol. 2000;68:260–266. [PubMed] [Google Scholar]
- Dang Y, et al. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic Biol Med. 2000;28:615–624. doi: 10.1016/s0891-5849(99)00272-5. [DOI] [PubMed] [Google Scholar]
- Deguchi K, et al. Postmenopausal changes in production of type 1 and type 2 cytokines and the effects of hormone replacement therapy. Menopause. 2001;8:266–273. doi: 10.1097/00042192-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Diamondstone LS, Tollerud DJ, Fuchs D, et al. Factors influencing serum neopterin and beta 2-microglobulin levels in a healthy diverse population. J Clin Immunol. 1994;14:368–374. doi: 10.1007/BF01546321. [DOI] [PubMed] [Google Scholar]
- Dilman VM. Age-associated elevation of hypothalamic threshold to feedback control, and its role in development, ageing, and disease. Lancet. 1971;1(7711):1211–1219. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- Dilman VM. Development, aging and disease: a new rationale for an intervention strategy. Harvard Acad. Publ; Zurich: 1994. [Google Scholar]
- Dilman VM, Lapin IP, Oxenkrug GF. Serotonin and aging. In: Essman W, editor. Serotonin in health and disease. Vol. 5. Spectrum Press; London: 1979. pp. 111–123. [Google Scholar]
- Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Fahey JL, Schnelle JF, et al. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;115:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- Filipovic R, Zecevic N. Neuroprotective role of minocycline in co-cultures of human fetal neurons and microglia. Exp Neurol. 2008;211:41–51. doi: 10.1016/j.expneurol.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Finkelstein JA. Brain serotonergic activity and plasma amino acid levels in genetically obese Zucker rats. Pharmacol Biochem Behav. 1982;17:939–944. doi: 10.1016/0091-3057(82)90476-2. [DOI] [PubMed] [Google Scholar]
- Fredrickson DS. The inheritance of high density lipoprotein deficiency (Tangier disease) J Clin Invest. 1964;43:228–236. doi: 10.1172/JCI104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Avanzas P, et al. The role of neopterin in atherosclerosis and cardiovascular risk assessment. Curr Med Chem. 2009;16:4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- Fulop T. The metabolic syndrome. Pathol Biol. 2006;54:375–386. doi: 10.1016/j.patbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Gál EM, Sherman AD. L-Kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- Grammer TB, Fuchs D, Boehm BO, et al. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2009;55:1135–1146. doi: 10.1373/clinchem.2008.118844. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- Han Q, Tao C, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Microglial activation in schizophrenia and minocycline treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1758–1759. doi: 10.1016/j.pnpbp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Hattori M, Kotake Y, Kotake Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitaminol Enzymol. 1984;6:221–228. [PubMed] [Google Scholar]
- Hayaishi O. Properties and function of indoleamine 2,3-dioxygenase. Properties and function of indoleamine 2,3-dioxygenase. J Biochem (Tokyo) 1976;79:13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- Janssen I, et al. Menopause and metabolic syndrome: the studies of women’s health across the nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kim KS, Kang KW, Seu YB, et al. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130:179–188. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Kincses ZT, Toldi J, Vécsei L. Kynurenines, neurodegeneration and Alzheimer’s disease. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Lowry CA, Stephens M, Oldfield S, O’Donovan MC, Lightman SL, Owen MJ. Screening ABCG1, the human homologue of the Drosophila white gene, for polymorphisms and association with bipolar affective disorder. Mol Psychiatry. 2001;6(6):671–677. doi: 10.1038/sj.mp.4000899. [DOI] [PubMed] [Google Scholar]
- Knight H, et al. A cytogenic abnormality and rare coding variants identify ABCA13 as a candidate gene in schizophrenia, bipolar disorder, and depression. Am J Hum Genet. 2009;85:833–846. doi: 10.1016/j.ajhg.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, et al. Postnatal blocking of interferon-gamma function prevented atherosclerotic plaque formation in apolipoprotein E-knockout mice. Hypertens Res. 2007;30:259–267. doi: 10.1291/hypres.30.259. [DOI] [PubMed] [Google Scholar]
- Kotaki Y, Ueda T, Mori T, Igaki S, Hattori M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA) Acta Vitaminol Enzymol. 1975;29:236–239. [PubMed] [Google Scholar]
- Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin N Am. 2007;91:1063–1077. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Kynurenines and anxiety. Adv Exp Med Biol. 1996;398:191–194. doi: 10.1007/978-1-4613-0381-7_31. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Antagonism of kynurenic acid to anxiogens in mice. Life Sci. 1998;63:231–236. doi: 10.1016/s0024-3205(98)00404-4. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. Adv Exp Med Biol. 2003;527:121–125. doi: 10.1007/978-1-4615-0135-0_14. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1:32–39. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- Lapin IP, et al. Anticonvulsant activity of melatonin against seizures induced by quinolinate, kainate, glutamate, NMDA and pentylenetetrazole in mice. J Pineal Res. 1998;24:215–218. doi: 10.1111/j.1600-079x.1998.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta. 1999;282(1–2):115–123. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Mendlovich S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA. Early hepatic insulin resistance in mice: a metabolomics analysis. Mol Endocrinol. 2010;24:657–666. doi: 10.1210/me.2009-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio D, et al. Allele frequencies of +874T-A single nucleotide polymorphism at the first intron of interferon-gamma gene in a group Italian centenarians. Exp Gerontol. 2002;37:315–319. doi: 10.1016/s0531-5565(01)00198-x. [DOI] [PubMed] [Google Scholar]
- Mackenzie SM, Brooker MR, Gill TR, Cox GB, Howells AJ, Ewart GD. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colouration. Biochim Biophys Acta. 1999;1419:173–185. doi: 10.1016/s0005-2736(99)00064-4. [DOI] [PubMed] [Google Scholar]
- Manev G, Manev R. 5-lipoxygenase as a possible biological link between depressive symptoms and atherosclerosis. Arch Gen Psychiatry. 2007;64:1333. doi: 10.1001/archpsyc.64.11.1333. [DOI] [PubMed] [Google Scholar]
- Mattei J, Parnell LD, et al. Disparities in allele frequencies and population differentiation for 101 disease-associated single nucleotide polymorphisms between Puerto Ricans and non-Hispanic whites. BMC Genet. 2009;10:45. doi: 10.1186/1471-2156-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier CA, Thalmann S. White adipose tissue, inflammation and atherosclerosis. Bull Acad Natl Med. 2007;191:897–908. [PubMed] [Google Scholar]
- Melillo G, Cox DW, Biragyn A, Sheffler LA. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem. 1994;269:8128–8813. [PubMed] [Google Scholar]
- Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27:421–427. doi: 10.1080/07315724.2008.10719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyramov G, Korchin V, Kocheryzkina N. Diabetogenic activity of xanthurenic acid determined by its chelating properties? Acta Vitaminol Enzymol. 1984;6:221–228. doi: 10.1016/s0041-1345(98)00788-x. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, et al. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopoharmacol Biol Psychiatry. 2008;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Moroni F, Russi P, Carlá V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1998;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–110. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Myint AM, Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders—relation to drug treatment. Dialogues Clin Neurosci. 2009;11:319–332. doi: 10.31887/DCNS.2009.11.3/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh H, Toldi J, Vécsei L. Role of kynurenines in the central and peripheral nervous systems. Curr Neurovasc Res. 2005;2:249–260. doi: 10.2174/1567202054368326. [DOI] [PubMed] [Google Scholar]
- Nicole NH, Perfield JW, et al. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2008;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinisalo P, et al. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest. 2008;2008(68):767–770. doi: 10.1080/00365510802245685. [DOI] [PubMed] [Google Scholar]
- Oettl K, Reibnegger G. Pteridine derivatives as modulators of oxidative stress. Clin Biochem. 2005;38:916–919. doi: 10.2174/1389200024605127. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. The acute effect of monoamine oxidase inhibitors on serotonin conversion to melatonin. In: Sandler M, Coppen A, Harnett S, editors. 5-hydroxytryptamine in psychiatry: a spectrum of ideas. Oxford University Press; New York: 1991. pp. 98–109. [Google Scholar]
- Oxenkrug GF. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann N Y Acad Sci. 2005;1053:334–347. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: new target for clinical intervention in vascular dementia, depression and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders and dysregulation of tryptophan–kynurenine pathway metabolism. Ann N Y Acad Sci. 2010a;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Tryptophan–kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: “the serotonin hypothesis” revisited 40 years later. Israel J Psychiatry. 2010b;47(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010c;117:23–26. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF, Lapin IP. Kynurenine pathway of the metabolism of tryptophan and its possible neuropharmacologic role. In: Yakovelev V, editor. Chemistry and pharmacology of indole compounds. Stinza, Kishinev; 1974. pp. 5–18. [Google Scholar]
- Oxenkrug GF, Summergrad P. Ramelteon attenuates age-associated hypertension and weight gain in spontaneously hypertension rats. Ann N Y Acad Sci. 2010;1199:114–120. doi: 10.1111/j.1749-6632.2009.05355.x. [DOI] [PubMed] [Google Scholar]
- Oxenkrug G, Perianaygam M, et al. Interferon-gamma (+874) T/A genotypes and risk of IFN-alpha-induced depression. 2010 doi: 10.1007/s00702-010-0525-1. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF, Tucker KJ, et al. Neopterin, an interferon-related marker of inflammation, correlates with metabolic syndrome markers and mortality risk in adult Boston community dwellers of Puerto Rican origin. Ann N Y Acad Sci. 2010 doi: 10.1166/ajnn.2011.1024. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico L, et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- Pae CU, et al. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008;62:308–311. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Reynolds GP. Determination of 3-hydroxykynurenine in human brain and plasma by high-performance liquid chromatography with electrochemical detection. Increased concentrations in hepatic encephalopathy. J Chromatogr. 1991;565:436–440. doi: 10.1016/0378-4347(91)80406-3. [DOI] [PubMed] [Google Scholar]
- Perez-Severiano F, Escalante B, Rios C. Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem Res. 1998;23:1297–1302. doi: 10.1023/a:1020700401678. [DOI] [PubMed] [Google Scholar]
- Perianayagam MC, et al. Immune modulating effects of melatonin, N-acetylserotonin and N-acetyl dopamine. Ann N Y Acad Sci. 2005;1053:386–393. doi: 10.1111/j.1749-6632.2005.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Perianayagam M, Oxenkrug G. Interferon-gamma (+874) single nucleotide polymorphism and human plasma neopterin levels. 2010 (submitted) [Google Scholar]
- Pertovaara M, Raitala A, Lehtimäki T. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Pertovaara M, et al. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2007;148:106–111. doi: 10.1111/j.1365-2249.2007.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TE, Katusic ZS. Transcribing the cross-talk of cytokine-induced tetrahydrobiopterin synthesis in endothelial cells. Circ Res. 2005;96:141–143. doi: 10.1161/01.RES.0000156078.12390.44. [DOI] [PubMed] [Google Scholar]
- Pitche P. [Pellagra] Sante. 2005;15:205–208. [PubMed] [Google Scholar]
- Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- Poynter ME, Daynes RA. Age-associated alterations in splenic iNOS regulation: influence of constitutively expressed IFN-gamma and correction following supplementation with PPAR-alpha activators or vitamin E. Cell Immunol. 1999;195:127–136. doi: 10.1006/cimm.1999.1525. [DOI] [PubMed] [Google Scholar]
- Prakhie IV, Oxenkrug GF. The effect of nifedipine, Ca++ antagonist, on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. Int J Neuropsychopharmacol. 1998;1:35–40. doi: 10.1017/S1461145798001096. [DOI] [PubMed] [Google Scholar]
- Pravica V, Perrey C, Stevens A, et al. A single nucleotide polymorphism in the first intron of the human INFG gene: absolute correlation with a polymorphic CA micro marker of high INFG gene. Hum Immunol. 2000;61:8333–8366. doi: 10.1016/s0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- Raitala A, Pertovaara M, Karjalainen J, et al. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol. 2005;61:387–390. doi: 10.1111/j.1365-3083.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, Braunwald E. Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation. 2007;115:3071–3078. doi: 10.1161/CIRCULATIONAHA.106.666511. [DOI] [PubMed] [Google Scholar]
- Requintina PJ, Oxenkrug GF. Differential effects of lipopolysaccharide on lipid peroxidation in F344N, SHR rats and BALB/C mice and protection of melatonin and NAS against its toxicity. Ann N Y Acad Sci. 2003;993:325–333. doi: 10.1111/j.1749-6632.2003.tb07540.x. [DOI] [PubMed] [Google Scholar]
- Riederer P, Reynolds GP. Determination of a wide range of urinary amine metabolites using a simple high-performance liquid chromatographic technique. J Chromatogr. 1981;225:179–184. doi: 10.1016/s0378-4347(00)80257-1. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez MI, Escames G, López LC, et al. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, McLarnon JG. Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington’s disease. Neuroscience. 2006;141:1835–1848. doi: 10.1016/j.neuroscience.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Samsonova ML, Oxenkrug GF. Inhibition of substrate induction of TRY-pyrrolase in liver and increase in the content in 5-HT in brain under action of inhibitors of MAO. Vopr Med Khim. 1972;17:198–201. [PubMed] [Google Scholar]
- Sarkar SA, Wong R, Hackl SI, et al. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes. 2007;56:72–79. doi: 10.2337/db06-0617. [DOI] [PubMed] [Google Scholar]
- Schennach H, Murr C, Gächter E, et al. Factors influencing serum neopterin concentrations in a population of blood donors. Clin Chem. 2002;48:643–645. [PubMed] [Google Scholar]
- Schoedon G, Troppmair J, Adolf G, et al. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986;6:697–703. doi: 10.1089/jir.1986.6.697. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Nilsson L, et al. Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons—possible involvement of endogenous kynurenic acid. Synapse. 2006;59:290–298. doi: 10.1002/syn.20241. [DOI] [PubMed] [Google Scholar]
- Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–342. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurkovich B, Skurkovich S. Inhibition of IFN-gamma as a method of treatment of various autoimmune diseases, including skin diseases. Ernst Schering Res Found Workshop. 2006;56:1–27. doi: 10.1007/3-540-37673-9_1. [DOI] [PubMed] [Google Scholar]
- Solichova D, Melichar B, et al. Biochemical profile and survival in nonagenarians. Clin Biochem. 2001;34:563–569. doi: 10.1016/s0009-9120(01)00261-2. [DOI] [PubMed] [Google Scholar]
- Sono M, Cady SG. Enzyme kinetic and spectroscopic studies of inhibitor and effector interactions with indoleamine 2,3-dioxygenase. 1. Norharman and 4-phenylimidazole binding to the enzyme as inhibitors and heme ligands. Biochemistry. 1989;28:5392–5399. doi: 10.1021/bi00439a012. [DOI] [PubMed] [Google Scholar]
- Stewart JC, et al. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- Sucher R, Schroecksnadel K, et al. Neopterin, a prognostic marker in human malignancies. Cancer Lett. 2010;287(1):13–22. doi: 10.1016/j.canlet.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Sullivan DT, Bell LA, Paton DR, Sullivan MC. Genetic and functional analysis of tryptophan transport in malpighian tubules of Drosophila. Biochem Genet. 1980;18:1109–1130. doi: 10.1007/BF00484342. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Mattei J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KJ, Oxenkrug GF, et al. Neopterin, pyridoxal-5′-phosphate and insulin resistance in adult community dwellers of Puerto Rican origin. Ann N Y Acad Sci. 2010 doi: 10.1166/ajnn.2011.1024. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbańska EM, Luchowski P, Luchowska E, et al. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep. 2006;58:507–511. [PubMed] [Google Scholar]
- Ursavaş A, Karadag M, Oral AY, et al. Association between serum neopterin, obesity and daytime sleepiness in patients with obstructive sleep apnea. Respir Med. 2008;102:1193–1197. doi: 10.1016/j.rmed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Voelker DR. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu Rev Biochem. 2009;78:827–856. doi: 10.1146/annurev.biochem.78.081307.112144. [DOI] [PubMed] [Google Scholar]
- Walsh HA, Daya S. Inhibition of hepatic tryptophan-2,3-dioxygenase: superior potency of melatonin over serotonin. J Pineal Res. 1997;23:20–23. doi: 10.1111/j.1600-079x.1997.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest. 2003;33:550–554. doi: 10.1046/j.1365-2362.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- You T, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SD, Odenwald WF. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc Natl Acad Sci USA. 1995;92:5525–5529. doi: 10.1073/pnas.92.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]