Abstract
Normal aging is accompanied by global as well as regional structural changes. While these age-related changes in grey matter volume have been extensively studied, less has been done using newer morphological indices such as cortical thickness and surface area. To this end, we analyzed structural images of 216 healthy volunteers, ranging from 18 to 87 years of age, using a surface-based automated parcellation approach. Linear regressions of age revealed a concomitant global age-related reduction in cortical thickness, surface area and volume. Cortical thickness and volume collectively confirmed the vulnerability of the prefrontal cortex, whereas in other cortical regions such as in the parietal cortex, thickness was the only measure sensitive to the pronounced age-related atrophy. No cortical regions showed more surface area reduction than the global average. The distinction between these morphological measures may provide valuable information to dissect age-related structural changes of the brain, with each of these indices probably reflecting specific histological changes occurring during aging.
Keywords: Aging, Magnetic resonance imaging, morphology, cortical thickness, cortical volume, cortical surface
1. Introduction
Normal brain aging is characterized by an overall cerebral atrophy. This atrophy is associated with shrinkage of grey matter (GM) and white matter (WM) volumes and enlargement of the cerebrospinal fluid (CSF) spaces. As shown by postmortem studies, histological changes underlying these age-related macroscopic variations are more likely related to a loss of neuropil associated with a reduction of dendrites and synapses, and a loss of nerve fibers, rather than related to a direct loss of neurons which is relatively limited with age (Pakkenberg, et al., 2003, Peters, et al., 1998). In vivo studies using Magnetic Resonance Imaging (MRI) have consistently shown an age-related decrease in GM and WM volume concomitant with an increase in CSF volume (Courchesne, et al., 2000, Good, et al., 2001, Lemaitre, et al., 2005, Raz, et al., 1997, Smith, et al., 2007,K. B. Walhovd, et al., 2005). Studies looking at the effect of age using either manual drawing of regions of interest (ROI) (Allen, et al., 2005, Raz, et al., 2004, Raz, et al., 1997) or automated/semi-automated methods such as voxel-based morphometry (VBM) (Good, et al., 2001, Smith, et al., 2007, Tisserand, et al., 2002) have reported regionally variable vulnerability to aging across the whole brain (Raz and Rodrigue, 2006, Kristine B. Walhovd, et al., 2005).
The literature related to regional heterogeneity in age-related changes in brain morphometry can be grouped into two categories based on the regions involved. The first reflects the vulnerability of the prefrontal region to aging (Tisserand and Jolles, 2003). This region has been shown to be one of the most affected with advancing age (Abe, et al., 2008, Allen, et al., 2005, Good, et al., 2001, Raz, et al., 1997, Tisserand, et al., 2002). Prefrontal vulnerability to aging is supported by evidence of pronounced age-related decline in several cognitive processes such as speed of processing, working memory, cognitive control including response inhibition and interference suppression all of which depend on the integrity of the prefrontal cortex (Raz, et al., 1998, West, 1996). The second set of regions repeatedly implicated in normal aging includes the hippocampus and the medial temporal lobe. Similar to the prefrontal cortex, numerous studies have found an age-related reduction in the volume of the hippocampal region (Bigler, et al., 2002, Du, et al., 2006,K. B. Walhovd, et al., 2005). The medial temporal lobe has received much attention as it is involved in both normal as well as pathological aging (Fox, et al., 2001, Killiany, et al., 2002). The growing interest of the affect of aging on this region is also due to its important role in episodic memory, which has been shown to decline with age (Verhaeghen, et al., 1993). Studies have also shown that atrophy of medial temporal structures predicts future memory decline in healthy aging (Rodrigue and Raz, 2004, Rusinek, et al., 2003). Reliable characterization of the extent and rate of atrophy, therefore, is an important tool for understanding normal human aging and therapeutic interventions targeted at disorders associated with accelerated atrophy, such as the dementias, making a study of the methodology used to quantify atrophy in humans in vivo important.
VBM is a classical quantitative method based purely on a volumetric representation of the brain. The local amount of tissue is simply measured as the intensity within each voxel and can be influenced by local cortical folding as well as local cortical thickness. Computational advances in surface reconstruction of inner and outer cortical boundaries have allowed the development of surface-based morphometry (SBM) methods which provide more specific morphological measures such as cortical thickness, surface area of the cortical mantle and cortical volume by product of these two first measurements (Dale, et al., 1999, Fischl and Dale, 2000, Fischl, et al., 1999). SBM has been shown to be more sensitive to age-related decline than VBM which within the cortex provides a local measure of grey matter dependent on both cortical folding and thickness (Hutton, et al., 2009). The SBM approach may lead to better understanding of global and regional structural changes in the brain associated with normal aging. Sorting out the different cortical features given by SBM on the entorhinal and the posterior parahippocampal cortices, Dickerson et al. (2009) reported that while volume reduction was seen in both normal aging and Alzheimer’s disease (AD), surface and thickness reductions were exclusively associated with normal aging and AD respectively. Thus, it appears that exploring age-related changes in thickness and surface area, the two components of volume, independently may be more informative. In a whole brain study of cortical thickness, Salat et al. (2004) reported marked age-related reductions in prefrontal cortex thickness and relative conservation of temporal and parahippocampal cortical thickness. They also reported thinning of the precentral and calcarine cortices. These results have since been replicated by other groups measuring cortical thickness (Fjell, et al., 2009, Ziegler, et al., 2008).
To date, no study has directly assessed over the whole brain the effect of age on the different cortical measurements provided by a surface-based reconstruction approach. In the present study, we proposed to examine age-related cortical thickness, surface area, and cortical grey matter volume changes across all cortical regions in a sample of 216 healthy volunteers ranging from age 18 to 87 years.
2. Methods
2.1 Participants
Two hundred sixteen healthy volunteers were included in this study with ages ranging from 18 to 87 years (mean age = 39.86 ± 16.51 years; 119 women; education level = 16.85 ± 2.73 years). Subjects were recruited nationwide as part of an ongoing study at the National Institute of Mental Health, Bethesda, MD, which included a neurological exam and Structured Clinical Interview for the DSM-IV (SCID) (First, et al., 1995). Exclusion criteria included a current or past history of neurological or psychiatric disorders, hypertension or drug abuse. All subjects provided written informed consent, and participated according to the guidelines of the National Institute of Mental Health Institutional Review Board.
2.2 Imaging and Preprocessing
MRI acquisition
Three-dimensional structural MRI scans were acquired on a 1.5T GE scanner using a T1-weighted SPGR sequence (TR/TE/NEX 24/5/1, flip angle 45º, matrix size 256 × 256, FOV 24 × 24 cm), with 124 sagittal slices (0.94 × 0.94 × 1.5 mm resolution). Images were reconstructed and visually checked for major artifacts (e.g. motion, ringing, wrap around and neurological abnormalities) before further processing.
MRI preprocessing
Images were processed using FreeSurfer version 4.0.1 (Dale, et al., 1999, Fischl, et al., 1999), which produced surface-based data for each subject. MRI data were re-sampled to a 3D coronal image with 1mm isotropic voxel size, followed by a series of preprocessing steps previously described (Dale, et al., 1999). Briefly, images were first corrected for intensity nonuniformity using N3 (Sled, et al., 1998) and registered via affine transformation (12 parameters) to Montreal Neurological Institute (MNI) space (Collins, et al., 1994). Then, images underwent a further intensity normalization using a different automated algorithm and were automatically skull stripped (Dale, et al., 1999).
At this juncture, images were visually inspected and data from 71 subjects were deemed to require manual correction which included manually realigning each subject’s image to the MNI template, setting intensity normalization control points where brain matter was erroneously skull stripped, and adjusting watershed parameters of the skull strip. Using a fully-automated method (Fischl, et al., 2002, Fischl, et al., 2004), images were segmented into different cerebral compartments and regions of interest, some of which were used to aid surface reconstruction.
Cortical surfaces were then reconstructed using a validated algorithm (Dale, et al., 1999, Fischl, et al., 2001, Fischl, et al., 1999, Segonne, et al., 2005). Briefly, binary white matter masks were generated using information from the automated segmentation and corrected in areas that commonly produce topological defects (particularly basal ganglia and lateral ventricles). The interhemispheric cutting plane was used to isolate the two hemispheres and to produce their corresponding binary white matter masks (Dale, et al., 1999). A triangle-based mesh of the white matter surface was then produced, and smoothed to alleviate the voxel-based nature of the initial curvature (Dale, et al., 1999). After automated correction of topological defects (Fischl, et al., 2001, Segonne, et al., 2005), a deformation algorithm was used to produce final white matter and pial surfaces (Fischl, et al., 1999). Finally, each surface was spherically inflated and automatically registered to a canonical spherical template using cortical features (Fischl, et al., 1999). An automated algorithm then parcellated the surface into 33 gyrally-based regions of interest (as well as two non-cortical regions, “corpus callosum” and “unknown, ” that we did not analyze) based on a cortical atlas, spatial relationship between regions, and knowledge of curvatures and sulcal depths (Desikan, et al., 2006, Fischl, et al., 2004). Mean thickness, cortical volume, and surface area were then extracted for each brain region from each individual subject’s brain image in its native space.
2.3 Statistical Analysis
Age effect on all data was analyzed in SPSS 15.0 using the General Linear Model. First, total cortical volume, total surface area and global average thickness for the whole brain were derived by combining all cortical regions previously identified. Then, looking at each cortical region independently, we explored the regional distribution of age-related changes on surface area, cortical volume, and average thickness. Since some regions covered a larger portion of the brain than others, we standardized the age-related changes in surface area and volume by reporting them as a percentage of the regions’ mean surface area and volume respectively (% of region per year). This was not an equivalent concern for age-related changes in thickness, which was independent of the regions’ size and kept on a scale of mm reduction per year. Finally, since we expected all three global morphometric measures to show nearly universal reductions with age, we scaled the regional values by their total brain values. In this way, we were not only considering regions for which age-related changes exceeded the global variations but we were also adjusting for head size difference across subjects. For all analyses, age was entered as covariate of interest and gender and years of education as confounding variables. Due to the large number of regions examined, results within each regional measure were corrected for multiple comparisons using a False Discovery Rate (FDR) threshold of 0.05.
3. Results
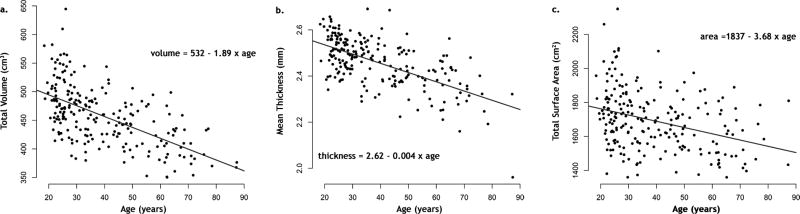
The average total cortical volume was 458±52 cm3 (See Table). The average total surface and thickness for the whole cortex were 1692±117 cm2 and 2.46±0.11 mm respectively. Linear regressions revealed significant age-related reductions (p < 0.001) in total cortical grey matter volume (r = −0.59), total surface area (r = −0.34), and average cortical thickness (r = −0.62). These effects represented global reductions of 1.89 cm3 per year in total cortical grey matter volume, 3.68 cm2 per year in total surface area, and 0.004 mm per year in global average thickness (see Table and Figure 1).
Table 1.
Age effects on total surface area, global average thickness and total cortical volume
| Descriptives | Age Effects | ||||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Effect Size | r | p | |
| Total surface area (cm2) | 1692.54 | 177.13 | 3. 68 cm2/year | −0.34 | 3.9 × 10−7 |
| Global average Thickness (mm) | 2.46 | 0.11 | 0.004 mm/year | −0.62 | 5.2 × 10−24 |
| Total cortical volume (cm3) | 458.17 | 52.19 | 1.89 cm3/year | −0.59 | 1.3 × 10−21 |
Figure 1.
Scatter plots and simple linear regressions of total volume (a.), mean thickness (b.) and total surface area (c.) on age for both cerebral hemispheres.
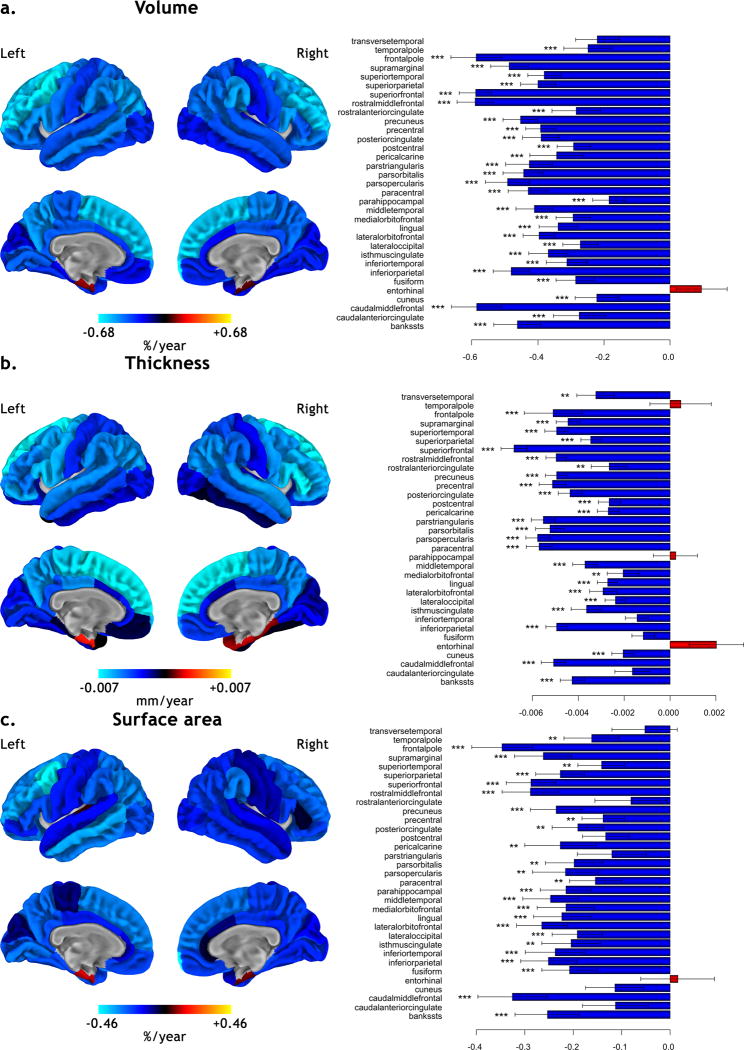
The regional counterpart of these global age-related changes is represented in Figure 2. The cortical regions showing the greatest age-related volume reduction were the middle frontal gyrus, the superior frontal gyrus and the frontal pole (superior to −0.58 % of label’s volume per year, p < 0.001 FDR). For the regional age-related changes in cortical thickness, predominant reductions were seen in the superior frontal gyrus, the paracentral gyrus and the pars opercularis and triangularis of the inferior frontal gyrus (superior to −0.0055 mm per year, p < 0.001 FDR). The frontal pole, the middle frontal gyrus and the superior frontal gyrus showed the greatest age-related reduction in surface area (superior to −0.28 % of label’s surface per year, p < 0.001 FDR).
Figure 2.
Effects of age on cortical volume (a.), thickness (b.) and surface area (c.) for each region of the brain. On the left, regression maps are superimposed onto an average pial surface (n = 216) and displayed without statistical threshold in % of region per year for cortical volume and surface, and in mm per year for cortical thickness (blue color scale for reductions and red color scale for increases). On the right, horizontal barplots of the corresponding age-related effect on the 33 bilateral cortical regions as parcellated by the automatic labeling procedure in FreeSurfer (*** p < 0.001, ** p < 0.01 corrected for False Discovery Rate).
In addition, we looked at the pattern of cortical regions with more pronounced age effects as compared to the global average; this is illustrated in Figure 3. Significantly greater-than-average reductions in volume were seen in the left middle frontal gyrus, the left frontal pole and the right temporal pole (blue regions in Figure 3). For thickness, more marked reductions than the global average reduction were identified in the left hemisphere in the paracentral gyrus, the posterior cingulate gyrus, the precuneus, the precentral gyrus, and the pars opercularis of the inferior frontal gyrus, and in the right hemisphere in the paracentral gyrus, the inferior parietal gyrus and the inferior frontal gyrus (red regions in Figure 3). Regions showing more marked reductions in both volume and thickness were the left and right superior frontal gyri and the rostral part of the right middle frontal gyrus (green regions in Figure 3). No cortical regions showed more surface area reduction than the global average.
Figure 3.
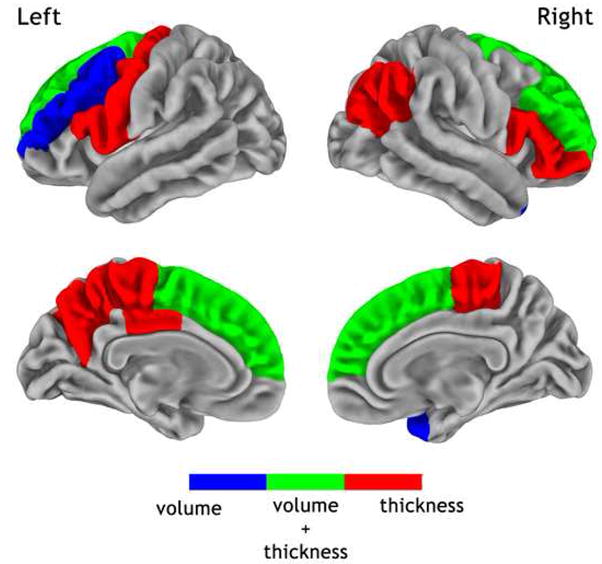
Cortical regions where the age-related reductions in volume (blue), thickness (red) or both (green) exceeded the global variations at the statistical level of p < 0.05 corrected for False Discovery Rate. Maps are superimposed onto an average pial surface (n = 216).
4. Discussion
Aided by automated surface reconstruction (Dale, et al., 1999, Fischl, et al., 2001, Fischl, et al., 1999), the present study examined age-related structural changes upon several distinct morphometric measures. Total and regional cortical thickness and surface area were measured in addition to cortical grey matter volume. Previously, the effects of aging on neuroanatomy have been extensively studied using volumetric techniques such as ROI (Jernigan, et al., 2001, Raz, et al., 1997,Kristine B. Walhovd, et al., 2005) and VBM analysis (Good, et al., 2001, Grieve, et al., 2005, Kalpouzos, et al., 2007), and more recently through surface-based measures such as thickness (Fjell, et al., 2006, Salat, et al., 2004), surface area (Dickerson, et al., 2009), and sulcal characteristics (Rettmann, et al., 2006). However, studies analyzing the effect of aging on these surface-based metrics remain scarce, especially for changes in surface area. In the current study, all three morphometric measured components presented a nearly-ubiquitous global reduction with age. Furthermore, the regional age-related changes for these three measures presented convergent patterns but also notable disparities, which are discussed below.
Starting with regions commonly highlighted by these three indicators, of all the brain regions the prefrontal cortex was by far the region that showed the most striking reduction in all three indicators. Particularly, the superior frontal and middle frontal gyri consistently showed the greatest age-related reduction in volume, thickness and surface area. When compared to the global trend, the prefrontal cortex showed a significantly accelerated decrease in volume and thickness with age (as illustrated in Figure 3). Our results are in line with several reports of a preferential structural vulnerability of the prefrontal cortex (Good, et al., 2001, Raz, et al., 1997, Tisserand, et al., 2002). Thus, the current study supports the longstanding “last in, first out” hypothesis otherwise known as the phylogenetic/ontogenetic model according to which the brain regions that are the last to mature or develop are the first to be affected by aging (Raz, 2005). Thus, based on the Flechsig’s myelination precedence, the prefrontal cortex is one of the last regions to undergo complete maturation.
Related to disparities between indicators, cortical thickness changes compared to volume changes showed a more diffuse range of marked age-related reduction. The cortical thickness reduction was observed in the inferior, middle and superior frontal gyri, as well as in the precentral, paracentral, precuneus, and posterior cingulate gyri. These results are not only contradictory to the anterior-posterior gradient theory as posterior regions such as the precuneus and inferior parietal gyrus also showed accelerated decline (Raz and Rodrigue, 2006), but also to the “first in last out” theory as the precentral gyrus - a primary cortex with early maturation – also showed pronounced age-related thinning. The age-related structural changes in the parietal region that we observed have been more consistently reported by cortical thickness studies (Fjell, et al., 2009, Salat, et al., 2004, Ziegler, et al., 2008) than by volume-based studies such as VBM (Good, et al., 2001, Resnick, et al., 2003). Thus, cortical thickness might be a more sensitive indicator of morphological aging than volume for cortical regions presenting less pronounced age-related atrophy as within posterior brain areas. One possible explanation could be that the histological parameters involved in the age-related cortical changes affect more specifically cortical thickness than volume per se which is a more comprehensive measure that integrates changes in cortical folding and thickness. Similarly, for the use of structural neuroimaging phenotypes in the field of imaging genetics, Winkler et al. (2009) suggest that image processing methods that only provide measurements of grey matter volume may be less sensitive for gene identification than those that allow measurement of differences in cortical thickness and/or surface area. With regard to the precentral gyrus, the relative accelerated thinning of this region with age might be driven by sample characteristics. Prior studies that had a large number of subjects over 60 years of age tended to include the primary cortices in their patterns of regions prominently affected by age (Lemaitre, et al., 2005, Resnick, et al., 2003, Salat, et al., 2004, Ziegler, et al., 2008). This is in contrast to studies with a scarce representation of individuals after the sixth decade (Fjell, et al., 2009, Good, et al., 2001, Sowell, et al., 2003). Based on this, we could propose the hypothesis that primary cortices also decline with age but much later in life, and is therefore noticeable only if the period of time covered by the study includes the late ages. Additionally, the involvement of the precentral cortex subserving motor functions could possibly explain the motor slowness observed with increasing age (Mattay, et al., 2002) even in the absence of peripheral sensory or motor changes (Kolev, et al., 2006). Morphometric studies that report on age-related changes in the primary cortices quite often find a concomitant age-related reduction in the sensorimotor and visual cortex (Lemaitre, et al., 2005, Salat, et al., 2004). In our study, we did not find a significant age-related reduction in volume, thickness or surface area of the occipital cortex. One could bring up the same reasoning as described above for the premotor cortex in this case too. Though we included subjects over 60 years, they represent only 17 % of the sample and may be not enough to detect significant late age reduction in grey matter of the visual cortex (Fjell, et al., 2009), given that reduction in this region appears to be not as strong as in in the precentral region and thereby possibly more difficult to detect (Salat, et al., 2004).
With reference to the cortical regions that show relative preservation with aging, an interesting result in our study is the relative sparing of the medial temporal lobe including the entorhinal and parahippocampal cortices. This result is consistent with the observations of Salat et al. (2004) who examined cortical thickness in healthy volunteers in a similar age range as in our study (18 to 97 years old). A combination of two factors may explain this finding. The first is the relative preservation of these structures with aging as confirmed by other studies. One of them, in a large sample of subjects ranging from 7 to 79 years, found stable hippocampal volume across its entire age range (Grieve, et al., 2005). Another study reported a very late decline in the volume of the hippocampal region; its volume remained steady until the 7th decade and then showed a sharp decrease after the 8th decade (Allen, et al., 2005). In our study, we did not assess age-related-hippocampal atrophy as the cortical surfaces derived from FreeSurfer do not include this region. However, the surrounding cortical regions provide an indirect insight into the age-related changes of this structure. The second factor could be related to a technical limitation of the methodology currently used. The narrow separation between the medial temporal structures (i.e. hippocampus, entorhinal and parahippocampal regions) may hinder accurately outlining the GM/WM surface of these structures, which could increase the variability in the derived-measurements of volume, thickness and surface area (Han, et al., 2006). If this limitation is systematically related to age, it may falsely enhance or even create age-relationships (Fjell, et al., 2009). Therefore, these two factors may lead to the observed but artificial preservation of the entorhinal cortex.
Throughout the brain, surface area did not show any regions of more pronounced reduction with aging. Since the coefficients of variation are smaller on global and regional measurements of surface (10.46 % and 10.31 % respectively) than of volume (11.39 % and 11.48 % respectively), this negative result can not simply be explained by a higher variability in the measurement of surface. This implies that while a global reduction of surface area was observed during aging, the rates of decline were relatively homogeneous across most of the cortical areas. This makes cortical thickness and grey matter volume quantitatively more informative for age-related morphometric changes across the brain. Measurement of surface area may be less sensitive to morphometric variations with aging than thickness and volume measurements. To some extent, an analogy to a dry apple could help understand the possible mechanisms underlying these age-related morphometric changes. When an apple dries, its flesh and thickness reduce and its skin shrivels keeping the surface area relatively constant but gaining spatial complexity from a flat to an uneven surface. Applied to cerebral aging, the width and depth of cortical sulci might influence the complexity metric, such that more atrophied brains might exhibit an increase in gyral complexity but not a decrease in surface area (Narr, et al., 2004). Indeed, a study characterizing the geometric shape of cortical changes associated with aging is in agreement with this hypothesis (Rettmann, et al., 2006). Among a set of eight sulcal regions, they found age-related reductions in thickness and volume but no changes in surface area. They also found changes in curvatures such as an age-related increase in inward bending of the surface. It is therefore possible that this mechanism might provide an explanation to the relative conservation of surface area in cortical areas such as the prefrontal regions which show significant atrophy with aging.
The morphometric measures - volume, thickness and surface area - are linked and inter-related to each other by a simple mathematical equation, the volume being the product of the surface area by the thickness of the cortical mantle. However, they may not be equally sensitive to factors associated to cortical atrophy such as in aging or neuropsychiatric disorders, and thus may present their own specificity. For example, Dickerson et al. (2009) looking at the entorhinal cortex have shown that normal aging exerted a preferential effect on surface area, while Alzheimer’s disease was associated with larger thickness reduction. In our study, we put forward the hypothesis that these measures, particularly volume and thickness, would behave differentially across the cortical regions of the brain to aging. The histological correlates of these morphometric measures are not well defined and warrant further investigation. However, it is likely that thickness may be related, at least in part, to the integrity of cellular elements within the cortical mantle; and surface area to the size of intracortical elements or to local subcortical factors, such as the volume of the white matter adjacent to the given gyrus or sulcus (Feczko, et al., 2009). In those conditions, alterations in volume could be associated with at least one of these histological parameters or with a still undiscovered parameter which would equally target surface and thickness. In any case, these measures put together provide complementary pieces of information, which may help to understand mechanisms underlying the differential brain aging across cortical regions.
A possible limitation of our study is inherent in its cross-sectional design. In such studies, age effect is inferred from measurements made from subjects of different ages, which may bias results due to potential cohort differences. For this reason, longitudinal designs, where each subject serves as his or her own control, are preferable but are also more difficult to conduct and may suffer from other issues (Klauschen, et al., 2009). The method employed in the present study has been shown to provide accurate surface-based measures of the cortex as confirmed by using postmortem brains (Rosas, et al., 2002). These measures have also been shown to be highly reliable across MRI scanners and field strengths (Han, et al., 2006), and across different samples of subjects (Fjell, et al., 2009).
5. Conclusion
The present study provides a detailed description of global and regional changes of cortical volume, thickness and surface area occurring during normal aging. These three cortical measures showed common patterns of age-related decline in the prefrontal cortex but also distinct patterns such as noticed in the parietal lobe with only an age-related reduction of thickness. These differences may account for the specificity of each of these morphological measures which may reflect different age-related histological changes. Thus, the distinction between cortical volume, thickness and surface area is an interesting strategy to dissect age-related changes in brain structure and may also be beneficial in the context of pathological aging and other brain disorders.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, NIH, Bethesda, MD 20892, USA.
Footnotes
Disclosure Statement: All subjects provided written informed consent, and participated according to the guidelines of the National Institute of Mental Health Institutional Review Board. None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29(1):102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. S0197-4580(06)00330-7 [pii] [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26(9):1245–60. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 79–82. S0197-4580(05)00169-7 [pii] [DOI] [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23(2):255–66. [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of computer assisted tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30(3):432–40. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27(5):733–40. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol Aging. 2009;30(3):420–31. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. The Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute; New York: 1995. Patients Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE transactions on medical imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM. Selective increase of cortical thickness in high-performing elderly--structural indices of optimal cognitive aging. Neuroimage. 2006;29(3):984–94. doi: 10.1016/j.neuroimage.2005.08.007. S1053-8119(05)00597-5 [pii] [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High Consistency of Regional Cortical Thinning in Aging across Multiple Samples. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358(9277):201–5. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. S1053-8119(01)90786-4 [pii] [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48(2):371–80. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22(4):581–94. doi: 10.1016/s0197-4580(01)00217-2. S0197458001002172 [pii] [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.019. S0197-4580(07)00230-8 [pii] [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58(8):1188–96. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp. 2009;30(4):1310–27. doi: 10.1002/hbm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev V, Falkenstein M, Yordanova J. Motor-response generation as a source of aging-related behavioural slowing in choice-reaction tasks. Neurobiol Aging. 2006;27(11):1719–30. doi: 10.1016/j.neurobiolaging.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26(3):900–11. doi: 10.1016/j.neuroimage.2005.02.042. S1053-8119(05)00145-X [pii] [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58(4):630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Kim S, Thompson PM, Szeszko P, Robinson D, Luders E, Toga AW. Abnormal gyral complexity in first-episode schizophrenia. Biological psychiatry. 2004;55(8):859–67. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Experimental gerontology. 2003;38(1–2):95–9. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8(4):295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Raz N. Ageing and the brain. The Encyclopedia of life sciences. 2005:1–6. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25(3):377–96. doi: 10.1016/S0197-4580(03)00118-0. S0197458003001180 [pii] [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–48. doi: 10.1016/j.neubiorev.2006.07.001. S0149-7634(06)00070-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettmann ME, Kraut MA, Prince JL, Resnick SM. Cross-sectional and longitudinal analyses of anatomical sulcal changes associated with aging. Cereb Cortex. 2006;16(11):1584–94. doi: 10.1093/cercor/bhj095. bhj095 [pii] [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004;24(4):956–63. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229(3):691–6. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. bhh032 [pii] [DOI] [PubMed] [Google Scholar]
- Segonne F, Grimson E, Fischl B. A genetic algorithm for the topology correction of cortical surfaces. Inf Process Med Imaging. 2005;19:393–405. doi: 10.1007/11505730_33. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28(7):1075–87. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–15. doi: 10.1038/nn1008. nn1008 [pii] [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex; a journal devoted to the study of the nervous system and behavior. 2003;39(4–5):1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17(2):657–69. S1053811902911730 [pii] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: a quantitative integration of research findings. Journal of gerontology. 1993;48(4):P157–71. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 75–8. S0197-4580(05)00167-3 [pii] [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Quinn BT, Salat D, Makris N, Fischl B. Neuroanatomical aging: Universal but not uniform. Neurobiology of Aging. 2005;26(9):1279–82. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological bulletin. 1996;120(2):272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]