Abstract
We report here that des-methyl, des-amino pateamine A (DMDA-PatA), a structurally simplified analogue of the marine natural product pateamine A, has potent antiproliferative activity against a wide variety of human cancer cell lines while showing relatively low cytotoxicity against nonproliferating, quiescent human fibroblasts. DMDA-PatA retains almost full in vitro potency in P-glycoprotein-overexpressing MES-SA/D×5-R×1 human uterine sarcoma cells that are significantly resistant to paclitaxel, suggesting that DMDA-PatA is not a substrate for P-glycoprotein-mediated drug efflux. Treatment of proliferating cells with DMDA-PatA leads to rapid shutdown of DNA synthesis in the S phase of the cell cycle. Cell-free studies show that DMDA-PatA directly inhibits DNA polymerases α and γ in vitro albeit at concentrations considerably higher than those that inhibit cell proliferation. DMDA-PatA shows potent anticancer activity in several human cancer xenograft models in nude mice, including significant regressions observed in the LOX and MDA-MB-435 melanoma models. DMDA-PatA thus represents a promising natural product-based anticancer agent that warrants further investigation.
Introduction
Marine natural products hold great promise as anticancer agents; the therapeutic utilities of several marine natural product-based compounds are currently being evaluated in clinical trials for cancer (1, 2). Pateamine A is one such marine natural product with potent antiproliferative and immunosuppressive activities. Pateamine A was first isolated from the sponge Mycale sp. by bioassay-guided fractionation based on its cytotoxic activity against P388 murine leukemia cells (IC50, 0.27 nmol/L; ref. 3) but remarkably showed ~2,000 times differential activity against a quiescent cell line (BSC kidney epithelial cells; IC50, ~0.54 μmol/L; ref. 3). Consistent with its cytotoxic activity, pateamine A was subsequently shown to induce apoptosis in several cancer cell lines; interestingly, the apoptosis induction was more pronounced in either ras- or bcr-abl-transformed 32D myeloid cells (4). Pateamine A also displayed potent immunosuppressive activity through affecting T-cell receptor-mediated interleukin-2 production (5). Des-methyl, desamino pateamine A (DMDA-PatA; Fig. 1A) is a structurally simplified, chemically stabilized synthetic analogue of pateamine A with equivalent or greater in vitro activity as judged by its ability to inhibit interleukin-2 reporter gene expression (6).
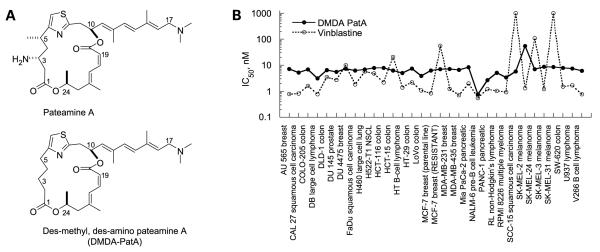
Figure 1.
DMDA-PatA has broad anticancer activity in vitro. A, chemical structures of pateamine A and DMDA-PatA. B, in vitro cell growth inhibition in human cancer cell lines. Human cancer cell lines were incubated in the presence of different concentrations of DMDA-PatA (0.3 nmol/L-10 μmol/L) or vinblastine (0.03 nmol/L-1 μmol/L) or DMSO-containing vehicle as control for 4 d. Methods of evaluation of cell growth are presented in Materials and Methods. IC50 values were calculated as the concentration of a test compound resulting in cell growth equal to 50% of DMSO control treated cell preparations.
Biological activity of pateamine A has been attributed to inhibition of cap-dependent translation initiation by two independent groups. In one study, pateamine A was identified as a translation inhibitor through a screening of natural product extracts (7). The inhibitory activity of pateamine A on protein translation was shown by pulse-chase labeling. The eukaryotic initiation factor 4A (eIF4A) family of RNA helicases was identified as the molecular target of pateamine A using affinity chromatography by attaching pateamine A directly to a resin (7). In parallel, Lowet al. also identified the translation initiation factor eIF4A as a molecular target for pateamine A by using an affinity purification approach with a biotin-pateamine A conjugate (8, 9). In addition, the precise effect of pateamine A on eIF4A, which includes increasing its ATPase and helicase activities, was proposed to involve binding of pateamine A to eIF4A and as a result stabilizing the interaction between eIF4A and mRNA. Thus, by sequestering eIF4A, pateamine A prevents formation of the eIF4F complex necessary for translation initiation (8). Further studies revealed that the increase of enzymatic activities associated with binding of pateamine A to eIF4A is caused by the induction of global conformational changes within eIF4A (10). Parallel studies by another group corroborated the main points of this mechanism with some minor differences (11). In addition, pateamine A was shown to induce stress granule formation by a novel pathway not involving eIF2α (12, 13). eIF4A is part of the eIF4F initiation factor responsible for the recruitment of ribosomes to mRNA (14). Translation initiation, particularly the eIF4F complex, has attracted great interest for anticancer drug development in large part due to the clinical success of mammalian target of rapamycin inhibitors that indirectly target the assembly of eIF4F (15).
We undertook a systematic approach to characterize the in vitro and in vivo anticancer activity of the pateamine A analogue DMDA-PatA. We report here that DMDA-PatA has potent antiproliferative activity against a broad panel of human cancer cell lines as well as marked in vivo anticancer activity in human tumor xenograft models. In addition to the reported activity of inhibition of translation, we also found that DMDA-PatA rapidly inhibits DNA synthesis during the S phase of the cell cycle.
Materials and Methods
Cell Culture and Cell Growth Inhibition Assay
The 32 cancer cell lines used in this study were cultured in standard tissue culture medium for each cell line and supplemented with 10% fetal bovine serum as described (16). DMDA-PatA was synthesized as described (6). For the cell growth inhibition assay, cells were seeded in 96-well tissue culture plates at 1,500 per well. Test compounds at concentrations of 0.03 nmol/L to 10 μmol/L (or DMSO control) were added to each well. Cells were incubated for a period of 96 h in the presence and absence of the test agents. Following the incubation period, CellTiter-Glo reagent (Promega) was added to all wells to assess cell proliferation. Luminescence was measured using a Victor V microplate reader (Perkin-Elmer). IC50 values were calculated as the concentration that inhibited cell growth to 50% of untreated cell preparations.
P-glycoprotein Sensitivity Assay
Test compounds were compared side-by-side in drug-sensitive MES-SA human uterine sarcoma cells (American Type Culture Collection) and a P-glycoprotein (PgP)-overexpressing drug-resistant subline, MES-SA/D×5-R×1. Growth-inhibitory IC50 values were then obtained as described above, and drug resistance ratios were calculated using the following formula: resistance ratio = IC50 in resistant cells / IC50 in parental cells. Resistance ratio values of >1 were interpreted as evidence that the test agent shows susceptibility to the PgP-based drug resistance mechanism.
IMR-90 Cytotoxicity Assay
To evaluate the effect of anticancer agents on nonproliferating cells, an in vitro cytotoxicity assay using quiescent IMR-90 human fibroblasts was used (17). Briefly, IMR-90 human fibroblasts, obtained from American Type Culture Collection, were grown for 4 days to confluency in MEM containing 10% fetal bovine serum and supplemented with L-glutamine and penicillin/streptomycin. After washing, the medium was replaced with complete MEM containing 0.1% fetal bovine serum and cells were cultured for 3 additional days under these lowserum conditions to achieve complete quiescence. Test compounds were then added followed by incubation for 24 h at 37°C. Cell viability was assessed by measurement of cellular ATP levels using the ATPLite assay kit (Perkin-Elmer).
Cell Cycle Analysis
Cell cycle analysis of exponentially growing U-937 histiocytic lymphoma cells was done by flow cytometric DNA content analysis as described (16), except that single-channel flowcytometry was done on a Becton Dickinson Canto flowcytometer, with collection and analysis of data done using Becton Dickinson FACSDiva 4.1 software for the Windows platform. Doublet events were eliminated from analyses by proper gating on PE-W/PE-A primary plots before histogram analysis of DNA content (measured on the PE-A channel). For DNA content analysis of COLO 205 colorectal adenocarcinoma cells and MDA-MB-435 melanoma cells, cells were treated with DMDA-PatA as monolayers and then detached from culture dishes by standard trypsin digestion procedures. Cells were then processed for the cell cycle analysis as above for U-937 cells.
Bromodeoxyuridine Incorporation Study
MDA-MB-435 cells were plated in 6-well tissue culture plates at 3 × 105 per well and incubated overnight. The following day, cells were preincubated with 300 nmol/L DMDA-PatA for 0 to 4 h before addition of 10 μmol/L bromodeoxyuridine (BrdUrd; Sigma) for 30 min pulse labeling. Cells were harvested with trypsin/EDTA, washed, and then fixed in 70% ethanol for 30 min on ice. Cells were then pelleted and ethanol was removed. The pellet was dispersed and 1 mL of 2 N HCl/0.5% Triton X-100 was added for 30 min incubation at room temperature. Cells were pelleted and resuspended in 0.1 mol/L disodium tetraborate (Na2B4O7; pH 8.5) for 5 min. One million cells were removed, pelleted, and resuspended in 50 μL of 0.5% Tween 20 in PBS containing 1% bovine serum albumin followed by addition of 20 μL FITC-conjugated anti-BrdUrd (BD Biosciences) for a 30 min incubation at room temperature. After one wash, cells were resuspended in 1 mL PBS containing 5 μg/mL propidium iodide (Sigma) and analyzed on a FACScan flow cytometer (BD Biosciences).
[3H]Thymidine Incorporation Assay
For U-937 cells, cells were plated in 96-well plates at 40,000 per well in 50 μL volumes. Cells were preincubated with compounds for 0 to 230 min before addition of 1 μCi [3H]thymidine for 10 min pulse labeling. The final concentrations of compounds used in the time course studies were empirically determined IC80 values for inhibiting a 10 min [3H]thymidine pulse following a 110 min preincubation: 60 nmol/L DMDA-PatA, 60 nmol/L aphidicolin, 80 nmol/L SN-38, 50 μmol/L methotrexate, 8.0 μmol/L floxuridine, and 100 μmol/L hydroxyurea. After harvesting samples onto filters, filters were dried and counted using Wallac 1450 microbeta scintillation counter. For MDA-MB-435 cells, cells were plated in a 96-well plate at 30,000 per well in 50 μL volumes. DMDA-PatA at 300 or 1,000 nmol/L was added to cells 18 h later followed by preincubation for different periods: 0, 10, 20, 30, 50, 80, and 110 min. At the end of each preincubation time, [3H]thymidine was added to corresponding wells for 10 min in the continued presence of DMDA-PatA. One group of wells received [3H]thymidine in the absence of any DMDA-PatA for approximation of a true time zero. At the end of the 10 min [3H]thymidine pulses, cells were harvested and processed for liquid scintillation counting as above for U-937 cells.
DNA Polymerase Assay
DNA polymerase assays were done under a contract by Replizyme. For this assay, poly(dA) was bound to the wells of a 96-well microtiter plate. These plates were then used for the oligo(dT)-primed incorporation of the nucleotide analogues Br(d)UTP. Br(d)UTP concentrations were standardized at 1.5 × Km [Br(d)UTP] for each polymerase. The quantitative detection of incorporated Br(d)U product is done immunologically using alkaline phosphatase-conjugated anti-BrdUrd monoclonal antibody. The alkaline phosphatase substrate pNPP is used for colorimetric detection. In all assays, measurements are taken on the linear portion of the progress curve.
In vivo Tumor Xenograft Studies
Anticancer effects of DMDA-PatA were evaluated in five human cancer xenograft models as follows.
In the LOX human melanoma model, NCr female nu/nu mice (Charles River) were injected s.c. with 1 × 106 LOX melanoma cells. Tumor-bearing mice were randomized into 6 groups of 10 mice each. Mean group tumor size was ~100 mm3 when treatments began on day 13 after tumor implantation. Drug treatments were by i.v. tail vein injection on a qd×5 × 2 schedule (2 cycles of 5 daily injections with a 2-day rest period between cycles) and consisted of DMDA-PatA at 1.25, 0.94, 0.70, or 0.53 mg/kg, paclitaxel at 12.5 mg/kg (positive control), or vehicle control (10% ethanol, 10% Cremophor, 4% glucose).
In the MDA-MB-435 melanoma5 model, NCr female nu/nu mice were injected s.c. with 2 × 106 MDA-MB-435 H8 melanoma cells. Tumor-bearing mice were randomized into 7 groups of 10 mice each. Treatments began when tumors reached ~100 mm3 in size. Drug treatments were by i.v. tail vein injection on a MWF × 3 schedule (3 weekly cycles of 3 injections given every other day with a 2-day rest period between cycles) and consisted of DMDA-PatA at 1.25, 0.94, 0.70, or 0.53 mg/kg, paclitaxel at 20 mg/kg (positive control), or vehicle (10% ethanol, 10% Cremophor, 4% glucose).
In the DLD-1 human colon cancer model, NCr female nu/nu mice were injected s.c. with 2 × 106 DLD-1 human colon cancer cells. Mice bearing tumors were randomized into 8 groups of 10 mice each. Mean group tumor size was 170 mm3 on day 13 when treatment began. Drug treatments were by i.v. tail vein injection on a q4d × 6 schedule (6 sequential injections administered on every fourth day) and consisted of DMDA-PatA at 1.67, 1.25, and 0.94 mg/kg, CPT-11 at 40 mg/kg (positive control), or vehicle control (10% ethanol, 10% Cremophor, 4% glucose).
In the H522-T1 human non-small cell lung cancer model, NCr female nu/nu mice were injected s.c. with 2 × 106 H522-T1 human non-small cell lung cancer cells. Mice bearing tumors were randomized into 8 groups of 10 mice each. Treatments began on day 13 after tumor implantation when mean group tumor size reached 244 mm3. Drug treatments were by i.v. tail vein injection on a MWF × 3 schedule (see above) and consisted of DMDA-PatA at 1.25, 1.10, and 0.94 mg/kg, paclitaxel at 20 mg/kg (positive control), or vehicle control (10% ethanol, 10% Cremophor, 4% glucose). Three additional groups were treated with DMDA-PatA at 1.80, 1.60, and 1.40 mg/kg on a q4d × 6 schedule (see above).
To evaluate DMDA-PatA in the NALM-6 leukemia model, CB-17 SCID female mice were injected i.v. with 2 × 106 NALM-6 human leukemia cells. Compound administration was by i.v. injection starting on day 3 using a MWF × 3 schedule (see above). Mice were randomly divided into 6 treatment groups of 10 mice each. DMDA-PatA was administered at 0.56, 0.70, 0.88, and 1.1 mg/kg formulated in 10% ethanol, 10% Cremophor, and 4% glucose. One group was treated with vincristine at 1.2 mg/kg in 2.5% DMSO/97.5% saline (positive control). Mice in the control group were treated with vehicle (10% ethanol, 10% Cremophor, and 4% glucose).
Results
DMDA-PatA Displays Antiproliferative Activity against a Large Panel of Human Cancer Cell Lines
The marine natural product pateamine A was previously shown to have antiproliferative activity and to induce apoptosis in several cancer cell lines (3, 4). More recent studies also showed antiproliferative activity of both pateamine A and DMDA-PatA in a selected panel of cancer cell lines (8). We thus sought to determine the range of anticancer activity of the structurally simplified, chemically stabilized analogue DMDA-PatA using a panel of 32 human cancer cell lines. The chosen cell lines represented a wide variety of cancer types including both solid tumors and hematologic malignancies. As shown in Fig. 1B, DMDA-PatA exhibited consistent and potent antiproliferative activity across all cell lines tested with IC50 values generally between 5 and 8 nmol/L, whereas the potency of the standard anticancer agent vinblastine showed a significantly greater variation across cell lines. The relative lack of differential activity across the 32 cancer cell lines tested suggests that DMDA-PatA targets a cellular process fundamental to cell proliferation.
DMDA-PatA Is Not Sensitive to PgP-Mediated Drug Efflux
Cross-resistance of tumors to a spectrum of cytotoxic agents, termed multidrug resistance, is a significant clinical problem. A major known driver of multidrug resistance is the drug efflux transporter PgP. Anticancer agents that are not substrates of the PgP drug efflux pump are thus expected to be more effective as chemotherapeutic agents for cancers with multidrug-resistant phenotypes associated with PgP overexpression. We therefore evaluated the susceptibility of DMDA-PatA to PgP-mediated drug efflux by determining if growth-inhibitory activity of DMDA-PatA is reduced in PgP-overexpressing human cancer cells. A pair of human uterine sarcoma cell lines was used: parental, non-multidrug-resistant MES-SA human uterine sarcoma cells and MES-SA/D×5-R×1, a PgP-overexpressing subline derived from MES-SA after long-term exposure to doxorubicin. The MES-SA/D×5-R×1 subline was confirmed to express high levels of PgP (data not shown). As shown in Fig. 2A, DMDA-PatA was relatively insensitive to PgP-mediated drug efflux. The IC50 values for growth inhibition in the PgP-expressing MES-SA/D×5-R×1 cells were only 2-fold higher than the IC50 values in the parental MES-SA cells. The positive control paclitaxel, a known PgP substrate, showed a 326-fold difference between IC50 values for cell growth inhibition in MES-SA/D×5-R×1 and MES-SA cells. These data thus indicate that DMDA-PatA has little or no susceptibility to PgP-mediated drug efflux.
Figure 2.
A, DMDA-PatA is not susceptible to PgP-mediated drug efflux. Compounds were tested side-by-side for growth-inhibitory effects in the drug-sensitive MES-SA cell line and its drug-resistant subline MES-SA/D×5-R×1. Growth-inhibitory IC50 values were obtained. Drug resistance ratios were calculated as described in Materials and Methods. Paclitaxel, a known PgP substrate, was included as a positive control. B, DMDA-PatA is not cytotoxic to quiescent fibroblasts at doses that lead to antiproliferative effects. Cytotoxic effects of 24 h treatment with DMDA-PatA (closed circles) or carbonyl cyanide (closed squares; positive control) against quiescent IMR-90 human fibroblasts were determined as described in Materials and Methods. C, DMDA-PatA has potent antiproliferative activity against proliferating fibroblasts. IMR-90 human lung fibroblast cells were incubated in the presence of different concentrations of DMDA-PatA or DMSO control for 4 d. Methods of evaluation of cell growth are presented in Materials and Methods. IC50 value was calculated as the concentration of a test compound resulting in cell growth equal to 50% of DMSO control treated cell preparations.
DMDA-PatA Shows Differential Activity between Proliferating Cancer Cells and Quiescent Human Fibroblasts
It was previously observed that pateamine A had significantly reduced activity against BSC renal epithelial cells grown to near confluence, suggesting that pateamine A may have differential activity against rapidly proliferating cells (3). To extend this observation to DMDA-PatA, we tested this agent for cell-killing ability against fully quiescent IMR-90 human fibroblasts in a cytotoxicity assay developed by us to distinguish between true antiproliferative activity and general cellular cytotoxicity not related to proliferation (17). For this assay, IMR-90 human fibroblast cells were induced to enter full quiescence by culturing to contact inhibition and then subsequent exposure to very low serum conditions. Under these conditions, incorporation of [3H] thymidine is negligible, confirming complete quiescence (17). Effects of compounds on cellular viability of the completely quiescent cells were then assessed by measuring ATP levels after 24 h exposure to compounds. In this assay, carbonyl cyanide is routinely used as a positive control for cytotoxicity that is not associated with proliferation. As shown in Fig. 2B, DMDA-PatA showed little or no cytotoxicity at the lownanomolar levels associated with its antiproliferative activities against dividing cells (see Fig. 1B); cytotoxic effects against quiescent IMR-90 cells were seen only at concentrations close to those required for cell killing by carbonyl cyanide (IC50 values for DMDA-PatA and carbonyl cyanide, 8 and 16 μmol/L, respectively). In contrast, DMDA-PatA showed low nanomolar potency against proliferating IMR-90 cells (Fig. 2C). Thus, DMDA-PatA has a wide in vitro therapeutic window between proliferating and quiescent cells (~1,000-fold), indicating that the cell growth-inhibitory effects observed against human cancer cells result from inhibited proliferative processes rather than indiscriminant proliferation-independent cytotoxicity.
DMDA-PatA Affects the S Phase of the Cell Cycle in a Cell Type-Specific Manner
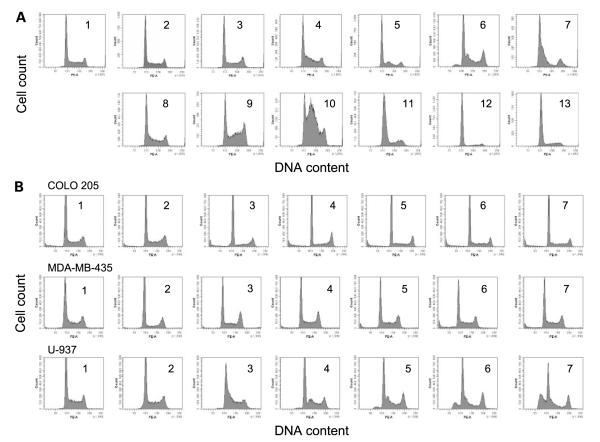
To better understand the observed antiproliferative effects of DMDA-PatA, we investigated whether DMDA-PatA targets a particular phase or phases of the cell cycle. Flow cytometric cell cycle evaluations were thus done on untreated and DMDA-PatA-treated U-937 human histiocytic lymphoma cells. As shown in Fig. 3A, treatment of U-937 cells in suspension culture with DMDA-PatA for 0 to 24 h caused increases in the number of cells in the early S-phase population in a time-dependent fashion. To determine if such effects were limited to suspension cell cultures, similar studies were done on COLO 205 and MDA-MB-435 cells, which grow in monolayer culture. Interestingly, in contrast to U-937 cells, DMDA-PatA did not cause significant observable changes in the cell cycle profile of COLO 205 or MDA-MB-435 cells after a 24 h compound treatment time (Fig. 3B) despite the fact that these cells show virtually identical growth-inhibitory IC50 values (Fig. 1B). It is possible that, unlike U-937 cells that showclear early S-phase peaks induced by DMDA-PatA, COLO 205 and MDA-MB-435 cells may simply stop cycling while remaining equally populated within the S phase. There does not appear to be a correlation between DMDA-PatA-induced S-phase arrest with the expression levels of p53, p21, p16, PTEN, and Rb tumor suppressor proteins (Supplementary Data S1).6
Figure 3.
Cell cycle effects of DMDA-PatA. A, exponentially growing U-937 cells were left untreated (1) or were treated with DMDA-PatA (2–7; concentrations of 0.3 to 700 nmol/L, respectively, corresponding to 0.3- to 100-fold IC50 obtained in a 4-d cell growth inhibition assay) or aphidicolin (8–13; concentrations of 90 nmol/L to 30 μmol/L, respectively, corresponding to 0.3- to 100-fold IC50 obtained in a 4-d cell growth inhibition assay). After 24 h of compound treatment, samples were collected and subject to flow cytometric cell cycle analysis as described in Materials and Methods. Relative number of cells (counts; Y axis) as a function of fluorescence intensity representing DNA content (PE-A; X axis). B, exponentially growing COLO 205, MDA-MB-435, or U-937 cells were left untreated (1) or were treated with DMDA-PatA (2-7; at concentrations corresponding to 0.3- to 100-fold IC50 obtained in a 4-d cell growth inhibition assay, respectively). After 24 h compound treatment, samples were collected and subject to flow cytometric cell cycle analysis as described in Materials and Methods. Relative number of cells (counts; Y axis) as a function of fluorescence intensity representing DNA content (PE-A; X axis).
DMDA-PatA Caused Rapid Shutdown of DNA Synthesis
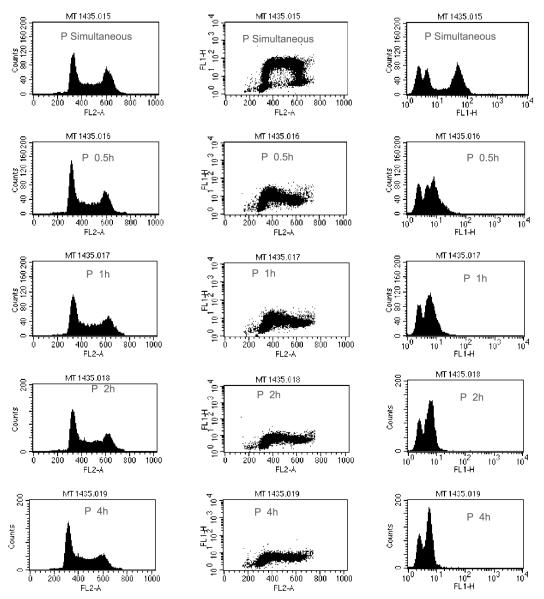
To further clarify the effect of DMDA-PatA on the S phase of the cell cycle, two types of studies were conducted to assess whether DMDA-PatA inhibits S-phase DNA synthesis. In the first approach, effects of short-term DMDA-PatA exposure on BrdUrd incorporation in MDA-MB-435 cells were investigated. As shown in Fig. 4, pretreatment of cells with DMDA-PatA for as little as 30 min led to dramatic decreases in BrdUrd incorporation indicative of rapid inhibition of S-phase DNA synthesis.
Figure 4.
Rapid inhibition of BrdUrd incorporation by DMDA-PatA in MDA-MB-435 cells. A 30 min pulse of BrdUrd was added simultaneously with DMDA-PatA or after the indicated preincubation time. FL2-A histograms show the intensity of propidium iodide staining and indicate little change in the distribution of cells in the G1, S, and G2-M stages of the cell cycle with increased preincubation time. FL1-H histograms show the uptake of BrdUrd is essentially stopped after 0.5 h preincubation indicating a shutdown of DNA synthesis. The multiparameter FL-2/FL-1 plot combines the cell cycle and BrdUrd uptake effects.
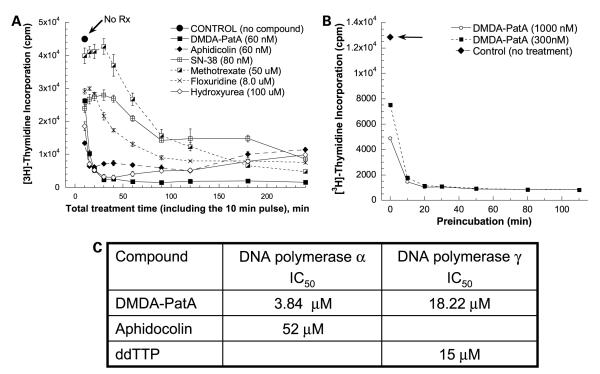
In the second approach, we tested the effects of DMDA-PatA and other known S-phase agents on [3H]thymidine incorporation by U-937 and MDA-MB-435 cells. In the U-937 studies, cells were preincubated with compounds for 0 to 230 min before addition of [3H]thymidine for final 10 min pulse-labeling periods. As shown in Fig. 5A, DMDA-PatA treatment caused very rapid inhibition of [3H]thymidine incorporation, with >85% inhibition seen with as little as 30 min total incubation time. This effect was similar to that seen with the known DNA synthesis inhibitor aphidicolin. Inhibition of DNA synthesis by other known S-phase agents, including topoisomerase I inhibitor SN-38, thymidylate synthase inhibitor floxuridine, and dihydrofolate reductase inhibitor methotrexate, was somewhat slower and less complete. Consistent with the BrdUrd incorporation effects shown above, treatment of MDA-MB-435 cells with DMDA-PatA also led to rapid inhibition of [3H]thymidine incorporation in this cell type (Fig. 5B). Taken together, the BrdUrd and [3H]thymidine incorporation studies in U-937 and MDA-MB-435 cells show that treatment of cells with DMDA-PatA leads to rapid and profound shutdown of S-phase DNA synthesis. Whereas this rapid shutdown of DNA synthesis led to a clear S-phase cell cycle effect in U-937 cells, no detectable effect on S phase was observed in MDA-MB-435 and COLO 205 cells as mentioned above. One possible explanation is that other phases of the cell cycle were also affected in these two cell lines due to inhibition of protein synthesis caused by DMDA-PatA.
Figure 5.
Inhibition of cellular DNA synthesis by DMDA-PatA. U-937 cells (A) or MDA-MB-435 cells (B) were preincubated with compounds at indicated concentrations for 0 to 230 and 0 to 120 min, respectively, before the addition of [3H]thymidine for a 10 min pulse labeling. Cells were collected using a cell harvester and processed as described in Materials and Methods. DNA synthetic activity ([3H]thymidine incorporation; Y axis) as a function of total compound treatment time including the 10 min pulse labeling with [3H]thymidine (X axis). C, inhibition of DNA polymerase α and γ in vitro. DMDA-PatA and two positive controls compounds [aphidicolin and 2′,3′-dideoxythymidine 5′-triphosphate (ddTTP)] were evaluated at a range of concentrations in an in vitro DNA polymerase assay as described in Materials and Methods.
Effects of DMDA-PatA on Known S-Phase Cancer Drug Targets
Having established rapid effects of DMDA-PatA treatment on S-phase DNA synthesis, we sought to determine if DMDA-PatA targets well-established S-phase cancer drug targets. Investigation of DMDA-PatA effects on DNA topoisomerase I and II and dihydrofolate reductase failed to show any inhibition of these three standard cancer drug targets (data not shown). In contrast, in an in vitro cell-free assay, DMDA-PatA inhibited both DNA polymerase α (IC50, ~3.84 μmol/L) and DNA polymerase γ (IC50, ~18.22 μmol/L), although no significant inhibition of DNA polymerase β was observed (Fig. 5C; data not shown). As expected, known DNA polymerase α inhibitor aphidicolin and DNA polymerase γ inhibitor 2′,3′-dideoxythymidine 5′-triphosphate used as positive controls showed IC50 values of 52 and 15 μmol/L in these assays, respectively.
Concentrations of DMDA-PatA required for inhibition of DNA polymerases α and γ in vitro were considerably higher than those that elicit antiproliferative effects against cells, so further work will be required before relationships, if any, between the two observations can be defined. Nevertheless, it is interesting to note that DMDA-PatA is selective for DNA polymerases α and γ, both being processive replicative DNA polymerases, whereas it is not inhibitory against polymerase β, which plays roles in DNA nick excision and repair.
DMDA-PatA Has Potent In vivo Anticancer Activity
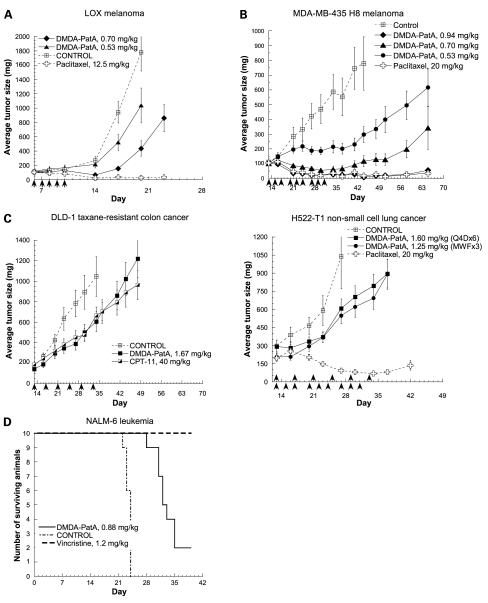
Having shown potent antiproliferative activity and a wide in vitro window between proliferating and quiescent cells, we then sought to address the question if DMDA-PatA exerts in vivo anticancer effects against human cancer xenografts grown in nude mice. As shown in Fig. 6A, DMDA-PatA was very effective in inhibiting growth of LOX melanoma xenografts. At the maximum tolerated dose (MTD) of 0.7 mg/kg, administration of DMDA-PatA resulted in tumor regression. It was noted that, in the LOX xenograft model, regression caused by DMDA-PatA was not as durable as that induced by paclitaxel at its empirically determined MTD in this model. In the MDA-MB-435 melanoma xenograft model, tumor regression was achieved with DMDA-PatA treatment at the MTD of 0.94 and 0.7 mg/kg, a dose below MTD on a MWF × 3 schedule (Fig. 6B). If evaluated based only on average tumor size, DMDA-PatA caused similar tumor regression levels as paclitaxel dosed at its respective MTD in this model. However, in this study, DMDA-PatA treatment actually led to more tumor-free survivors. DMDA-PatA at 0.94 mg/kg caused 9 of 10 mice being tumor-free on day 44 and 5 mice remaining tumor-free at the time of study termination (day 65) compared with 7 of 10 mice tumor-free on day 44 and 2 mice tumor-free on day 65 as a result of paclitaxel treatment. Thus, DMDA-PatA treatment led to greater numbers of tumor-free mice whether analyzed midway through the study (day 44) or at its termination (day 65).
Figure 6.
In vivo anticancer activity of DMDA-PatA in human tumor xenograft models. Nude mice bearing LOX melanoma (A), MDA-MB-435 melanoma (B), DLD-1 colon cancer, or H522-T1 non-small cell lung cancer (C) xenografts were treated with the indicated concentrations of DMDA-PatA and either paclitaxel or CPT-11 control agents using dosing routes and regimens described in Materials and Methods. X axis, arrows, days on which compounds were administered. D, CB-17 SCID mice bearing NALM-6 human leukemia cells were treated with the indicated concentration of DMDA-PatA as described in Materials and Methods. Vincristine (1.2 mg/kg) served as a positive control (see text).
Modest tumor growth inhibition was observed in the DLD-1 taxane-resistant colon cancer model and the H522-T1 non-small cell lung cancer model (Fig. 6C). In the DLD-1 model, modest effects of DMDA-PatA were comparable with those of the positive control CPT-11. DMDA-PatA did not showsignificant antitumor effects in either MiaPaca-2 pancreatic cancer or HT-29 colon cancer xenograft model (data not shown).
We also evaluated the effect of DMDA-PatA in a survival-based NALM-6 leukemia model. As shown in Fig. 6D, DMDA-PatA at the MTD of 0.88 mg/kg prolonged the survival of NALM-6-bearing SCID mice by somewhat more than a week. The known antileukemic drug vincristine was used a positive control in this study; however, the dose of vincristine selected proved to be above MTD, so not all injections were administered due to toxicity. Nevertheless, at this dose, vincristine showed a 100% survival benefit to NALM-6-bearing mice.
Overall, results from these in vivo studies indicate that DMDA-PatA has potent anticancer activity against a variety of in vivo human xenograft models, although not all models respond to this agent.
Discussion
The marine natural product pateamine A was first isolated in 1991 and its in vitro anticancer and immunosuppressive activities have been previously reported (3). To our knowledge, in vivo anticancer activities of pateamine A have not been previously reported. Studies described in this report represent the first systematic characterization of both in vitro and in vivo anticancer activities of not pateamine A itself but a structurally simplified, chemically stabilized analogue, DMDA-PatA. In contrast to a previous report that indicated that pateamine A did not have a significant effect on DNA synthesis (7), our studies here showthat DMDA-PatA causes a rapid and profound shutdown of S-phase DNA synthesis based on inhibition of both BrdUrd and [3H]thymidine incorporation and the S-phase cell cycle effect observed in at least one cell line, U-937. The basis for such differences is unknown but could be related to the different cell lines used in the two studies.
Previous work by others has identified eIF4A RNA helicase, a component of protein translation initiation machinery, as a cellular target of natural pateamine A (7–9, 11). A natural product extract containing pateamine A was identified as a positive hit in a screen for chemical inhibitors of translation, and the inhibitory activity of pateamine A on Cap-dependent protein translation in cells was subsequently shown by pulse labeling using radiolabeled amino acid. The eIF4A family of RNA helicases was also identified as a molecular target of pateamine A following affinity chromatography using pateamine A bound to a resin (7). In an independent study, Lowet al. used a biotin-pateamine A conjugate in a different affinity purification approach; their results also identified eIF4A as a cellular binding target of pateamine A (8).
As discussed above, our current results show that DMDA-PatA treatment of cells leads to rapid shutdown of S-phase DNA synthesis. Our in vitro data indicate direction inhibition of DNA polymerases α and γ, although it is not clear that these in vitro effects, requiring 100- to 1,000-fold more DMDA-PatA than required for cellular antiproliferative effects, are related. More importantly, our results showing rapid shutdown of DNA synthesis seem to be at odds with the previously reported identification of eIF4A as a cellular target of pateamine A in multiple laboratories. One explanation might be that pateamine A and DMDA-PatA may inhibit DNA synthesis and activate eIF4A helicase activity independently. In such a scenario, DNA synthesis might be inhibited via still unknown targets in the DNA synthetic machinery, or even by direct inhibition of DNA polymerases α and γ, if DMDA-PatA was concentrated in cells as is known to be the case for some tubulin-targeted agents. One intriguing possibility in this regard is that pateamine A and DMDA-PatA might target not only RNA helicases such as eIF4A but also DNA helicases involved in DNA replication machinery at the replicative fork. If so, however, explanations for why DNA helicases were not identified in the original mechanistic studies with pateamine A would have to be found. An alterative explanation for our observations of rapid DNA synthetic shutdown by DMDA-PatA might be that hitherto unknown signaling pathways exist between eIF4A-related steps in protein synthesis and continuation of ongoing DNA synthesis. Consistent with this explanation, some elegant work in the 1960s identified several protein translation inhibitors that inhibited both protein and DNA synthesis, and it was hypothesized that protein synthesis inhibition was the primary effect (18, 19). Such coupling of DNA synthesis to protein synthesis might make biological sense, in that unexpected shutdown of protein synthesis while a cell was in the middle of DNA synthesis might be a critical enough insult to result in a survival response, which shuts down DNA synthesis until protein synthesis has resumed. In this regard, it is notable that newly synthesized DNA is assembled on newly synthesized histones, which are made simultaneously with new DNA. Although our current results shed no light on this mystery, it is possible that, like many natural products before it, pateamine and its synthetic analogues may represent a door to new regulatory pathways impinging on cellular regulation. The dual inhibition of protein synthesis and DNA synthesis by DMDA-PatA represents a unique mechanism of action distinct from other cytotoxic anticancer drugs with mechanisms ranging from inhibition of DNA topoisomerases, antimetabolites, to disrupting microtubule functions.
Although DMDA-PatA displayed little differential activity across a broad range of cultured human cancer cell lines in vitro, differential activity was nevertheless observed in vivo. DMDA-PatA caused significant regression in both LOX and MDA-MB-435 melanoma xenograft models. On the other hand, it had no significant anticancer activity in the Mia-Paca-2 pancreatic and HT-29 colorectal xenograft models. Future studies will be required to determine the reasons for the selective in vivo anticancer activity observed in our studies. Our observations also highlight the limitation of using in vitro cell line data to predict in vivo differential activity of anticancer agents. Perhaps most importantly, our work provides the first demonstration of in vivo anticancer activity for the pateamine A class of compounds. Although the mechanism of action of DMDA-PatA and relationships of that mechanism to those established for pateamine A remain to be worked out, our results underscore the fact that the pateamine class of compounds represents an interesting and unique class of agents with anticancer therapeutic potential worthy of further investigation.
Supplementary Material
Acknowledgments
This work was done under license to Eisai Research Institute of Boston from Texas A&M University. The licensed subject matter included a U.S. patent application covering DMDA-PatA and related compounds and methods of making such compounds (USSN 10/388,257). The aforementioned license terminated on December 17, 2005. D. Romo thanks the NIH (GM52964) for initial support of these investigations leading to the discovery of DMDA-PatA.
Footnotes
MDA-MB-435 was routinely referred to as a breast cancer cell line in the past. However, recent gene expression analyses have provided strong evidence that MDA-MB-435 is actually a melanoma cell line. This cell line is thus referred to as a melanoma line in this article. The accumulated evidence for melanoma origin of MDA-MB-435 can be found at http://dtp.nci.nih.gov/docs/misc/common_files/mda-mb-435-update.html.
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest All authors except D. Domo were employees of Eisai Research Institute of Boston, Inc. (ERI) during the period in which the reported work was conducted. No other potential conflicts of interest were disclosed.
References
- 1.Newman DJ, Gordon MG. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004;67:1216–38. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- 2.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4:333–42. [PubMed] [Google Scholar]
- 3.Northcote PT, Blunt JW, Munro MHG. Pateamine: a potent cytotoxin from the New Zealand marine sponge, Mycale sp. Tetrahedron Lett. 1991;32:6411–4. [Google Scholar]
- 4.Hood KA, West LM, Northcote PT, Berridge MV, Miller JH. Induction of apoptosis by the marine sponge (Mycale) metabolites, mycalamide A and pateamine. Apoptosis. 2001;6:207–19. doi: 10.1023/a:1011340827558. [DOI] [PubMed] [Google Scholar]
- 5.Romo D, Rzasa RM, Shea HA, et al. Total synthesis and immunosuppressive activity of (−)-pateamine A and related compounds: implementation of a β-lactam-based macrocyclization. J Am Chem Soc. 1998;120:12237–54. [Google Scholar]
- 6.Romo D, Choi NS, Li S, Buchler I, Shi Z, Liu JO. Evidence for separate binding and scaffolding domains in the immunosuppressive and antitumor marine natural product, pateamine A: design, synthesis, and activity studies leading to a potent simplified derivative. J Am Chem Soc. 2004;126:10582–8. doi: 10.1021/ja040065s. [DOI] [PubMed] [Google Scholar]
- 7.Bordeleau ME, Matthews J, Wojnar JM, et al. Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc Natl Acad Sci U S A. 2005;102:10460–5. doi: 10.1073/pnas.0504249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low WK, Dang Y, Schneider-Poetsch T, et al. Inhibition of eukaryotic translation initiation by the marine natural product pateamine A. Mol Cell. 2005;20:709–22. doi: 10.1016/j.molcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Low WK, Dang Y, Scheider-Poetsch T, et al. Isolation and identification of eukaryotic initiation factors 4A as a molecular target for the marine natural product pateamine A. Methods Enzymol. 2007;431:303–24. doi: 10.1016/S0076-6879(07)31014-8. [DOI] [PubMed] [Google Scholar]
- 10.Low WK, Dang Y, Bhat S, Romo D, Liu JO. Substrate-dependent targeting of eukaryotic translation initiation factor 4A by pateamine A: negation of domain-linker regulation of activity. Chem Biol. 2007;14:715–27. doi: 10.1016/j.chembiol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Bordeleau ME, Cencic R, Lindqvist L, et al. RNA-mediated sequestration of the RNA helicase eIF4A by pateamine A inhibits translation initiation. Chem Biol. 2006;13:1287–95. doi: 10.1016/j.chembiol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Dang Y, Kedersha N, Low WK, et al. Eukaryotic initiation factor 2α-independent pathway of stress granule induction by the natural product pateamine A. J Biol Chem. 2006;281:32870–8. doi: 10.1074/jbc.M606149200. [DOI] [PubMed] [Google Scholar]
- 13.Mazroui R, Sukarieh R, Bordeleau ME, et al. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2 phosphorylation. Mol Biol Cell. 2006;17:4212–9. doi: 10.1091/mbc.E06-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 15.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209–22. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 16.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo antitumor activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–21. [PubMed] [Google Scholar]
- 17.Rudolph-Owen LA, Salvato K, Cheng C, et al. A 96-well plate cell-based assay to quantify undesired cytotoxic effects against quiescent non-dividing cells. Proc Am Assoc Cancer Res. 2004;45:264–5. [Google Scholar]
- 18.Young CW. Inhibitory effects of acetoxycycloheximide, puromycin, and pactamycin upon synthesis of protein and DNA in asynchronous populations of HeLa cells. Mol Pharmacol. 1966;2:50–5. [PubMed] [Google Scholar]
- 19.Brega A, Falaschi A, De Carli L, Pavan M. Studies of the mechanism of action of pederine. J Cell Biol. 1968;36:485–96. doi: 10.1083/jcb.36.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.