-
Structure Tear lipocalin, or von Ebner’s gland protein, tear specific prealbumin, (GenBank Accession Number: BC074925; Ref Seq ID: NM_002297; PDB ID: 3EYC, 1XKI) is a 17.45 kDa member of a superfamily christened lipocalins because lipophiles are enclosed within their cup shaped cavity or calyx. Tear lipocalin is the predominant lipid carrier in human tears and is critical to functions involving lipids in protection of the ocular surface. The protein exploits a number of structural elements to bind an extremely broad range of ligands that includes a variety of lipids with long alkyl chains, charged moieties, and even polycyclic structures. At ~75 μM, tear lipocalin is second only to lysozyme as the most concentrated protein in human tears. Lipocalin-1 (LCN-1), the name of the gene that encodes tear lipocalin, resides in a lipocalin gene cluster on chromosome 9q34. Two other genes LCN1b and LCN1c are not translated. Three isoforms have been consistently identified in protein sequence data and mass spectrometry in human tears. Genetic polymorphism is unlikely to be the source of these isoforms; at least three laboratories have found only one identical cDNA sequence in several tissues. Mass spectrometry and protein sequencing data combined with the inter-study variability of other isoforms suggest proteolytic action on the major isoform may account for the other forms. In one study tear lipocalin was proffered as the predominant phosphoprotein in tears although phosphorylation of the major isoforms was not evident by mass spectrometry in another study.
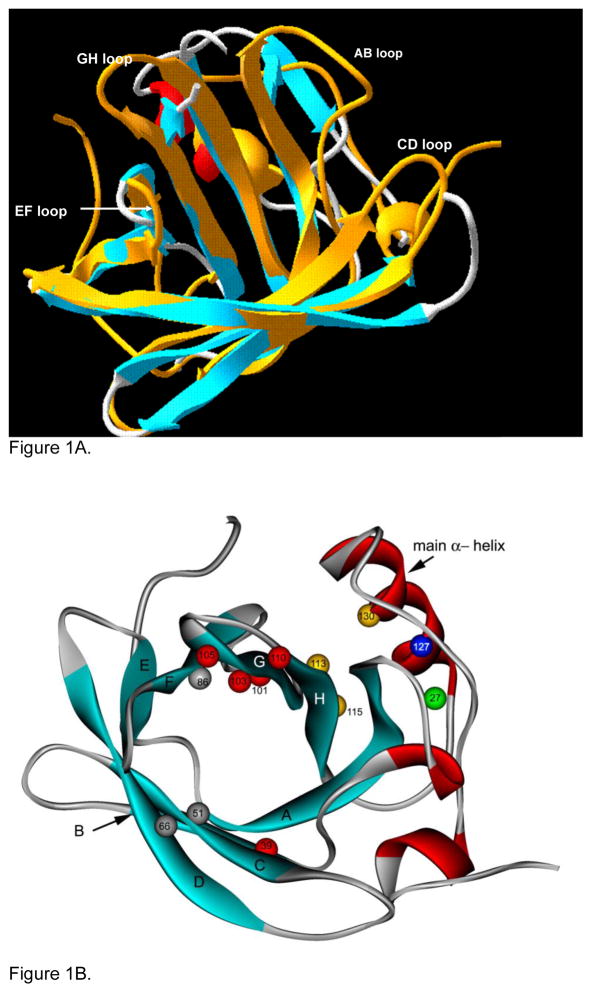
The solution structure of tear lipocalin was first elucidated by a new method called site directed tryptophan fluorescence.(Gasymov et al., 2009) Spectral changes defined the secondary structure in a series of mutants in which tryptophan had been substituted along the entire protein’s polypeptide chain. The information was combined with homology modeling to yield the 3D structure. Four years later conventional X-crystallography of a single serine mutant of tear lipocalin confirmed the solution structure.(Breustedt et al., 2009) Eight anti-parallel β strands, with interconnecting loops and distinct α helical regions (Figure 1) were in accord. Fine details were also in agreement such as the interaction of the H strand’s Val113 and Leu115 with Phe130 (Figure 1B yellow balls) on the main α-helix, identification of β bulges in strands A and F, the short loop EF (Figure 1A) as well as critical positioning of Ala 51,66 and 86 (Figure 1B grey balls) in the cavity mouth that permit ligand promiscuity. The positions of four flexible loops, AB, CD, EF, and GH at the mouth of the calyx were better resolved in solution structure than in crystal structure presumably due to loop motion inherent in apo-tear lipocalin. The size of the cavity by both solution and crystal structure are commensurate with its ligand binding function. The depth of the cavity by crystal structure is 15 Å approximating the 22.5 Å inferred from increased binding up to an alkyl chain length of 18. The functional mouth diameter has been measured at 10 Å in crystal structure in close agreement with a 9.1 Å limitation deduced because the complex multi-ring structure of perylene is not admitted. Recently, the solution structure of tear lipocalin bound to a native ligand was revealed by fluorescence quenching of site directed tryptophan mutations.(Gasymov et al., 2009) Dynamic and static quenching mapped the favored binding sites and the entire binding energy landscape. Residues on AB and GH loops show marked static quenching by nitroxide labeled ligands and are therefore preferred sites for conformational selection of ligands for protein excited states (Figure 1B, red balls). The crystal structure of tear lipocalin bound to a non-native ligand was determined contemporaneously and confirmed the flexibility and role of these loops in binding.(Breustedt et al., 2009) Both solution and crystal studies show that the AB loops harbors a proton activated switch at Glu27 (Figure 1B green ball) that is triggered by protonation and moves the AB and GH loop to a closed conformation over the calyx. Similar pH driven movements for loops CD and EF have been described. Knowledge of the loop movements have great significance for understanding ligand binding in lipocalin.
-
Function Tear lipocalin is a multi-functional protein expressed mainly in the lacrimal glands, packaged in granules accompanied by lysozyme and lactoferrin and secreted from the lacrimal gland into tear fluid. Tear lipocalin is also secreted in Von Ebner’s gland and possibly in Meibomian glands of the eyelid.
Tear lipocalin is one of the few lipocalins for which the native ligands, phospholipids, fatty acids, alcohols, glycolipids, and cholesterol, have been identified. Tear lipocalin’s functions are inexorably linked to its ligands. At the corneal surface tear lipocalin rapidly retrieves phospholipids and fatty acids, preventing desiccation; lipid renders the corneal surface unwettable.(Glasgow et al., 2010) Tear lipocalin solubilizes lipids in the divalent cation rich milieu of the tear film and promotes clarity and stability. At the aqueous-lipid-air interface, tear lipocalin may integrate into Meibomian lipids with implications for film stability and retardation of evaporation.(Millar et al., 2009) A trigonal cluster of positively charged residues in lipocalin may bind negatively charged residues on phospholipids to stabilize the lipid layer. Tear lipocalin can carry steroid hormones, vitamin E, and Vitamin A in tears. Tear lipocalin is both an enzyme and enzyme inhibitor. Tear lipocalin is the principal endonuclease in tears. The enzymatic activity is conferred by a conserved LEDFXR domain of divalent ion dependent nucleases with a magnesium water cluster involving the α-helical segment residue Glu127 (Figure 1B, blue ball). Tear lipocalin also inhibits cysteine proteinase activity. The functional significance is clouded because more potent cystatins have been isolated from tears. Furthermore, crystallographic studies show the presumed inhibitory motifs are either disordered or buried in the cavity of tear lipocalin. Tear lipocalin is highly conserved in humans reflecting its multiple roles in health and disease.
Disease involvement Tear lipocalin is decreased in tears in disorders with reduced lacrimal secretion such as Sjogren’s disease and LASIK induced dry eye disease. Tear lipocalin is diminished or absent when lacrimal gland mass is reduced because of surgical removal of the lacrimal gland or in syndromes with congenital absence of the lacrimal gland, congenital alacrima. Tear lipocalin is quite active in removing lipid from abnormal corneal surfaces in dry eye disease as well bullous keratopathy. Lipocalin may also provide a natural defense for fungal infections. The protein binds siderophores that are generated by fungus to compete for iron capture. Fungal siderophores, particularly rhodotorulic acid, have stronger affinity for iron than does lactoferrin but are captured by tear lipocalin and sequestered in macrophages through a lipocalin interacting membrane receptor. Tear lipocalin may also be important in pulmonary diseases. Tear lipocalin is upregulated in patients with cystic fibrosis presumably to scavenge excess lipids in the airways.
Future studies Tear lipocalin has multiple and specific roles at the cornea and tear film surfaces that need further elucidation. The detailed mechanisms and interactions with other proteins and specific tear film lipids are critical for understanding its functions. The unique structure of flexible loops and capacious calyx make tear lipocalin an archetype for protein engineering to enhance drug binding and delivery. So called anticalins have been developed to utilize the scaffold of β strands of various lipocalins. Combinatorial mutations of the entrance loops alter the conformations to fit a variety of molecules such as digoxin, and fluorescein, useful in clinical in treatments and assays. Tear lipocalin has been modified to bind T cell co-receptor CD4, vascular endothelial growth factor, and interleukin 4 receptor. Potential clinical applications include neoplasms, proliferative retinopathy, and allergic reactions. Duocalins are designed as a fusion protein of two lipocalin derivatives, one to carry ligands and one with target specificity for a receptor. Tear lipocalin also naturally binds to rifampin in tears but releases the drug in a milieu of fatty acids, raising potential for targeted drug delivery to granulomata without structural alteration. Tear lipocalin is an example of a protein where basic knowledge of structure has uncovered functions, generated methodologies applicable for general protein dynamics and created opportunities for genetic manipulation for novel diagnostic and therapeutic interventions covering a wide range of disorders.
Figure 1.
A. Solution structure of tear lipocalin showing the positions of the 4 flexible loops (Gasymov et al. 2001 Biochemistry 40, 14754–14762) is superimposed on the crystal structures of tear lipocalin without the loops (adapted from Breusted et al. 2005 J. Biol Chem 280, 484–493). B. Cartoon structure of tear lipocalin illustrates the positions of the anti-parallel β strands (aqua ribbons), α-helical segments (red ribbons), loops (thin grey segments). The five amino acids with the greatest static quenching constants (indicative of strong ligand binding) for fatty acids are shown in red. Other key amino acid positions are discussed in text (numbered and colored balls). Adapted from solution structure (Gasymov et al., 2001 Biochemistry 40, 14754e14762).
Acknowledgments
Supported by Research grants RO1 EY11224 and the Edith and Lew Wasserman Endowed Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breustedt DA, Chatwell L, Skerra A. A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr. 2009;65:1118–1125. doi: 10.1107/S0907444909031011. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48:7219–7228. doi: 10.1021/bi9005557. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow BJ, Gasymov OK, Abduragimov AR, Engle JJ, Casey RC. Tear lipocalin captures exogenous lipid from abnormal corneal surfaces. Invest Ophthalmol Vis Sci. 2010;51:1981–1987. doi: 10.1167/iovs.09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–151. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]