Abstract
Brain-derived neurotrophic factor (BDNF) is critical in synaptic plasticity and in the survival and function of midbrain dopamine neurons. In the present study, we assessed the effects of a partial genetic deletion of BDNF on motor function and dopamine (DA) neurotransmitter measures by comparing (Bdnf+/−) with wildtype mice (WT) at different ages. Bdnf+/ and WT mice had similar body weights until 12 months of age; however, at 21 months, Bdnf+/− mice were significantly heavier than WT mice. Horizontal and vertical motor activity was reduced for Bdnf+/− compared to WT mice; but was not influenced by Age. Performance on an accelerating rotarod declined with age for both genotypes and was exacerbated for Bdnf+/− mice. Body weight did not correlate with any of the three behavioral measures studied. DA neurotransmitter markers indicated no genotypic difference in striatal tyrosine hydroxylase (TH), dopamine transporter (DAT), or vesicular monoamine transporter 2 (VMAT2) immunoreactivity at any age. However, DA transport via DAT (starting at 12 months) and VMAT2 (starting at 3 months) as well as KCl-stimulated DA release were reduced in Bdnf+/− mice and declined with age suggesting an increasingly important role for BDNF in the release and uptake of DA with the aging process. These findings suggest that a BDNF expression deficit becomes more critical to dopaminergic dynamics and related behavioral activities with increasing age.
Keywords: BDNF, aging, dopamine, uptake, striatum, mice
Introduction
Aging is associated with progressive, albeit sometimes subtle, functional and structural deterioration of neural systems, affecting both motor and memory functions (Barbeau 1973; Kluger et al. 1997; Kokmen et al. 1977; Newman et al. 1985; Richards et al. 1993; Volkow et al. 1998). Motor dysfunction and deterioration of dopamine (DA) systems have also been reported for animal models of aging (Ingram 2000). Evidence for age-related DA dysfunction includes (1) reductions in both tyrosine hydroxylase (TH) and the DA transporter (DAT) in the SN of aged rodents (Himi et al. 1995), (2) reduced DA uptake in aged Rhesus monkeys (Dejesus et al. 2001), (3) reduced DA release in aged F344 rats (Hebert & Gerhardt 1998), (4) reduction in DA and glutamine receptor binding in aged mice (Ossowska et al. 2001), (5) reduced striatal DAT and VMAT2 activity (Hebert & Gerhardt 1999; Cruz-Muros et al. 2008), and (6) marked increase in the response of aged substantia nigra (SN) DA neurons to neurotoxins (Cass et al. 2002). Moreover, alterations in many of these DA markers are associated with a conspicuous decrease in spontaneous locomotion in aged rodents (Hebert & Gerhardt 1997; Watanabe et al. 1991) although the specific factors mediating these age-related changes have not been fully elucidated.
DA neurons depend on continuous support from growth factors. Withdrawal or reduction of these growth factors, primarily glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF), increases the vulnerability of DA neurons to external stressors and toxins especially with increasing age (Yurek & Fletcher-Turner 2000; Yurek & Fletcher-Turner 2001). BDNF is involved in the development of motor coordination and the survival and maintenance of nigral DAergic neurons (Do et al. 2007; Strand et al. 2007). The expression of BDNF and its high affinity receptor, TrkB, has been shown to be decreased with normal aging and in patients with Alzheimer’s and Parkinson’s disease (Murer et al. 2001; Nagatsu & Sawada 2007; Silhol et al. 2005). These findings indicate a relationship of BDNF to the degeneration of SN neurons in PD, but do not establish whether the BDNF reduction is a result of, or a contributor to, the degenerative process.
BDNF null mutations confer severe neurological dysfunction on newborn pups, resulting in death within the first 2–3 weeks postpartum (Liebl et al. 1997). Mice heterozygous for the BDNF allele (Bdnf+/−) exhibit alterations in cortical postsynaptic responses (Abidin et al. 2006), behavioral alterations related to anxiety, appetite, and motor function (Lyons et al. 1999), reduced hippocampal serotonergic innervations (Luellen et al. 2007) and altered mRNA expression of TH in the SN and opioid peptides in the striatum (Saylor et al. 2006). Studies also document that Bdnf+/− mice exhibit (a) an enhanced aging-related decline of beam walking, spontaneous locomotion activity thought to be mediated by nigrostriatal dopaminergic functions, (b) higher total DA levels in caudate-putamen, (c) reduced K+ stimulated DA release from ex vivo slice preparations, (d) decreased neurotoxic response to methamphetamine, and (e) no changes in DAT-ligand binding (Disshon & Dluzen 1997; Dluzen et al. 2001; Dluzen et al. 2004; Dluzen et al. 1999; Joyce et al. 2004). To date, however, there are no reports of studies examining altered presynaptic DAergic function resulting from reduced BDNF expression in terms of altered motor function, DAT and VMAT2 functional activities and synaptic DA levels and K+ stimulated DA release in freely moving animals. Therefore the purpose of the current study was to assess possible differences in motor function (horizontal and vertical activity, accelerating rotarod) along with markers of nigrostriatal DAergic function in Bdnf+/− and WT mice during aging. The study included the use of striatal and substantia nigra DAergic immunohistochemical markers, extracellular basal and K+ evoked striatal DA levels using microdialysis, and functional activities of DAT and VMAT2 from striatal synaptosomes.
Materials and Methods
Animals
Pairs of Bdnf+/− mice were generously provided by L. Tessarollo (National Cancer Institute, Frederick, Maryland). They were generated by replacing the first coding exon with the pGKneo recombination cassette (Liebl et al. 1997) and have been subsequently backcrossed five times onto a C57BL/6J genetic background. Upon arrival to our animal facility, they were genotyped (backcrossed 4 times) to be 95% C57BL/6J and 5% 129S4/SvJa (service provided by Jackson labs) and were maintained at this level by heterozygous crosses. Genotyping was performed as previously described (Liebl et al. 1997). These Bdnf+/− mice, carrying a single null allele for BDNF were viable, fertile, and appear to have a normal life span. 12 breeding pairs supplied all the male Bdnf+/− and WT littermates utilized in these studies. All mice were group-housed in a temperature- and humidity- controlled room and maintained on a 12-h light/dark cycle with food and water available ad libitum. Body weight was monitored throughout the lifespan. All procedures used on animals in this study were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Experimental Design
At 3-, 12-, and 21-months of age, independent groups of male WT and Bdnf+/− mice (N=8 per group) were assessed for motor function via open-field locomotor testing and accelerating rotarod. Upon completion of these tasks, the mice were weighed, anesthetized, and the brains extracted and bisected. One hemisphere was post-fixed in 4% paraformaldehyde for 48 hrs and then processed for immunohistochemistry. The other hemisphere was frozen at −80°C and used for BDNF tissue levels. A second cohort of male 3-month-old and 12-month-old WT and Bdnf+/− mice (N=8 per group) were used to assess basal extracellular DA, as well as K+-stimulated DA release in the dorsal striatum. At the end of the microdialysis experiment, the mice were sacrificed and brains were stored in 4% paraformaldehyde for probe placement verification. DAT and VMAT2 activities were measured from fresh striatum obtained from an independent cohort of 3-month 12-month and 21-month old male WT and Bdnf+/− mice. The numbers of mice from the different Genotype × Age groups included in different experiments are summarized in Table 1.
Table 1.
Numbers of animals used in each assay.
| Assays | 3 month WT | 3 month Bdnf+/− | 12 month WT | 12 month Bdnf+/− | 21 month WT | 21 month Bdnf+/− |
|---|---|---|---|---|---|---|
| BDNF levels# | 9 | 8 | 8 | 12 | 5 | 6 |
| Body weight# | 10 | 10 | 10 | 9 | 11 | 9 |
| Horizontal activity# | 6 | 6 | 11 | 10 | 11 | 9 |
| Vertical activity# | 6 | 6 | 11 | 10 | 11 | 9 |
| Accelerating rotarod# | 8 | 5 | 11 | 10 | 11 | 9 |
| Immunohistochemistry# | 5 | 6 | 5 | 5 | 5 | 4 |
| Microdialysis$ | 7 | 7 | 7 | 5 | ND | ND |
| Striatal DAT activity¶ | 12 | 18 | 15 | 18 | 18 | 18 |
| Striatal VMAT2 activity§ | 4 | 4 | 4 | 4 | 6 | 6 |
Separate cohort for 3, 12 and 21 month-old WT and Bdnf+/− mice were used. Following completion of behavioral assays, brain tissues were used for immunohistochemistry and BDNF assays.
Separate cohort for 3 and12 month-old WT and Bdnf+/− mice were used.
Separate cohort for 3, 12 and 21 month-old WT and Bdnf+/− mice were used. DAT activity was determined from striatum of individual animals
Separate cohort for 3, 12 and 21 month-old WT and Bdnf+/− mice were used. Synaptic vesicles were prepared from pooled striatal tissue derived from three age and genotype matched animals (n=1).
ND: Not determined
Locomotor Activity Testing
Independent groups of male Bdnf+/− and WT mice were tested at 3, 12, and 21 months of age (N=8 per genotype per age) to evaluate the effects of a partial BDNF deletion on motor activity across most of the lifespan. Horizontal (total distance traveled) and vertical (rearing frequency) activity were assessed for one hour using a Digiscan Animal Activity Monitor system (Omnitech Electronics Model RXYZCM(8) TAO, Columbus, OH) contained in light- and sound- controlled chambers. The details of the apparatus have been described previously (Halberda et al. 1997). On the day of testing, the mice were transferred from the animal colony into the laboratory in groups of six and individually tested for one hour in a darkened environment. Data were collected in 15 min intervals for one hour at the same time of day (8 am to 12 pm)
Accelerating Rotarod
Motor coordination was evaluated with an accelerating rotarod (Ugo Basile, Verese, Italy) procedure. Mice from each of the six groups created by the Genotype × Age experimental independent group design (N=8 per group) were tested at 3, 12, and 21 months of age. The cross-sectional design employed allowed naive mice at each of the three ages to be behaviorally tested and then sacrificed for morphology and biochemistry. Using a rotarod procedure adapted from Rosas, et al. (Rozas et al. 1997), mice were initially trained to remain on the rotarod for 10 min at a set speed of 4 rpm one day prior to actual testing. They were then tested for three consecutive days on their ability to remain on the rotarod at increasing rotation speeds of 4, 8, 16, 24, 32, and 40 rpm. During the daily tests, mice were tested for a maximum of 5 min at each rotation speed with 5-min rest periods in their home cage between each rotation speed. Results are expressed as Kaplan-Meier survival curves representing the probability for mice of the different genotype/age groups remaining on the rotarod as time increased with fall time collapsed across the increasing rotational speeds. Statistical comparisons are described in the Statistical Methods section. In addition to determining motor coordination, examination of performance over the three-day period provided an assessment of potential genotypic difference in motor learning.
Enzyme Linked Immunosorbent Assay (ELISA)
BDNF protein levels were assessed using Promega BDNF ELISA kits (Promega, Madison, WI) according to standard laboratory protocols (Boger et al. 2006). The striatum was harvested from one hemisphere, and placed in pre-weighed Eppendorf tubes, weighed, and kept at −80°C until tested. Samples were homogenized and centrifuged in 0.5 ml lysate buffer (Promega, Madison, WI) and aliquoted. Ninety-six-welled flat-bottom NUNC-Immuno maxisorp ELISA plates were incubated with carbonate-coating buffer containing captive neurotrophic factor antibodies (Promega) overnight at 4°C. A standard curve was created from serial dilutions of known concentrations of the protein provided in the Promega kit. Duplicate samples were incubated for 6 hr at room temperature, and with secondary antibody followed by an antibody conjugated to HRP. A TMB/peroxidase solution was used as the chromogen to visualize the reaction product. The reaction was terminated with 1N HCl, and the optical density measured at 450 nm (Albeck et al. 2003). Using these kits, BDNF can be quantified in the range of 7.8–500 pg/ml. BDNF was expressed as pg/mg wet tissue.
Immunohistochemistry
For immunohistochemical analysis, mice from each genotype at each age were sacrificed using an overdose of isoflurane. The brains were quickly removed, post-fixed in paraformaldehyde (4%) for 48 hours, and then transferred to 30% sucrose in 0.1M phosphate buffered saline (PBS) for at least 24 hours before sectioning. As described above, one hemisphere was utilized for ELISA assays, while the other hemisphere from each mouse was utilized for immunohistochemical studies. The striatum was sectioned on a cryostat (Microm, Zeiss, Thornwood, NY, USA) at 45 μm. Every 6th section throughout the striatal region and every 3rd section from the midbrain (for TH only) was processed for free-floating immunohistochemistry using a rabbit polyclonal antibody against tyrosine hydroxylase (TH, 1:5000 dilution, Pel-Freeze Inc., Roger, AZ, USA), a rat monoclonal antibody against the dopamine transporter (DAT, 1:2000 dilution, Millipore Corp., Temecula, CA), or a rabbit polyclonal antibody against vesicular monoamine transporter 2 (VMAT2, 1:2000 dilution, Millipore Corp., Temecula, CA). Immunohistochemistry was performed according to our standard protocol (Granholm et al. 1997). Briefly, free-floating serial sections were treated with H2O2, methanol, and 0.01 M Tris buffer saline (TBS, pH 7.6; 1:2:7 respectively) for 15 minutes to quench endogenous peroxidase activity. Sections were permeabilized in TBST (Tris buffer with 0.9% NaCl and 0.25% Triton X-100) and treated for 20 minutes with sodium m-peroxidate (0.1 M) in TBS. To block non-specific binding sites, sections were incubated in 10% normal goat serum (NGS, Sigma-Aldrich, St. Louis, MO) in TBST for 30 minutes at room temperature. Sections were incubated with the primary antibody (1:1000) in TBST with 3% NGS at room temperature for 24 hrs. Following washing in TBST, sections were incubated with an appropriate secondary antibody, biotin-conjugated goat anti-rabbit (TH, VMAT2) or goat anti-rat (DAT) IgG (1:200, Vector Labs, Burlingame, CA) and incubated subsequently in an avidin-biotin complex (ABC kit, Vector Labs, Burlingame, CA). The reaction was developed by staining with VIP (Vector Labs, Burlingame, CA), which yields a purple reaction product. Prior to the use of each antibody, different titers were assessed to determine the optimal dilution to prevent saturation of antibody. Furthermore, all sections from all mice were processed simultaneously and rigorously timed to avoid saturation of reaction product. Sections were mounted on glass slides and cover-slipped with DPX.
Semi-quantitation of Immunohistochemistry
Semi-quantitation of striatal TH, DAT, and VMAT2 immunoreactivity from 3-, 12-, and 21-month-old mice (N=8 per genotype per age) WT and Bdnf+/− mice was conducted. Briefly, staining intensity of TH immunostaining in the dorsolateral striatum was determined using NIH Image® Software to measure a gray scale value within the range of 0 – 256, where 0 represents white and 256 black. A template covering the medial as well as lateral dorsal striatum was created and used on all brains similarly, and images were captured with a Nikon Eclipse E-600 microscope, an Olympus-750 video camera system, and a Dell Pentium III computer. Measurements were performed blinded from 5–6 sections per brain and then averaged to obtain one value per subject. Staining density was obtained when background staining was subtracted from mean staining intensities on every 6th section through the striatum.
Microdialysis Surgical Procedures
At 3- and 12-months of age, a separate cohort of Bdnf+/− and WT male mice (N=8 per group) were anesthetized using a ketamine (150 mg/kg) and xylazine (3 mg/kg) cocktail and placed in a stereotaxic instrument (Stoelting, Wood Dale, IL). Unilateral guide cannulae (CMA/7; CMA Microdialysis, Sweden) were aimed at the dorsal striatum (relative to Bregma: AP +0.8, ML +1.5, and DV −2.5; Franklin and Paxinos, 1997) and secured directly to the skull using a light-cured resin system (Kerr Corporation, Orange, CA).
In Vivo Microdialysis Study
The day before dialysate collection, a microdialysis probe (CMA/7; 1mm active membrane) was lowered into the guide cannula and mice were perfused at 0.1uL/min overnight with filtered artificial cerebral spinal fluid (aCSF: 7.4 mM glucose, 3.0 mM KCl, 140 mM NaCl, 0.5 mM MgCl2, 1.2 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4). The next day, the perfusion flow of aCSF was increased to 1.0 uL/min. After 2 hrs, dialysis samples were collected every 15 min into vials containing 2.5 uL of 750 mM perchloric acid diluted to a final concentration of 150 mM by the sample. Following the collection of 6 basal dialysate samples, 60 mM KCl-aCSF (7.4 mM glucose, 60 mM KCl, 83 mM NaCl, 0.5 mM MgCl2, 1.2 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4) was perfused for 15 min, after which 3 mM KCl-aCSF was perfused for an additional 4 dialysate collections.
Dopamine Measurement by High-Performance Liquid Chromatography
Dialysate samples were injected by an autosampler (ESA model 542, Chelmsford, MA). DA was separated from the sample with a reverse-phase column (HR 80, ESA, Chelmsford, MA) using the mobile phase (150 mM NaH2PO4, 4.76 mM citric acid, 50 uM EDTA, 10% methanol, 2.5 mM SDS, 17% acetonitrile, pH 5.6) at a flow rate of 0.9 ml/min. The column was kept in an oven at 30oC and DA detected using coulometric detection. Three electrodes were used: a guard cell (400 mV), a reduction analytical electrode (100 mV), and an oxidation analytical electrode (220 mV). Peaks were recorded, and the area under the curve was measured by a computer running ESA Chromatography Data System. DA concentrations were determined by comparing peak areas to an external standard curve.
Probe Placement Verification
After concluding the microdialysis experiment, the mice were sacrificed and brains removed and stored in 4% paraformaldehyde for 48 hrs. After transfer to 30% sucrose for 24 hrs, coronal sections (45 μM) were mounted and stained with Cresyl violet. Probe placement was determined according to Franklin and Paxinos (2001).
Synaptosome Preparations
At 3-, 12-, and 21-months of age, independent groups of male WT and Bdnf+/− mice (N=8 per group) were rapidly decapitated, and the brains were collected in ice-cooled dishes. The striatum was rapidly dissected and collected in 10 volumes (wt/vol) of cold 0.32 M sucrose. The tissue was homogenized using a Teflon-glass homogenizer under chilled water and centrifuged at 1000 × g for 15 min at 4 °C. The resulting supernatant was centrifuged at 15,000 × g for 25 min and the pellet was washed by resuspending in 0.32 M sucrose (Samuvel et al. 2005). The synaptosomes were suspended in regular Krebs-Ringer-HEPES (KRH) buffer pH 7.4 (120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2 10 mM HEPES, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5 mM Tris, and 10 mM D- glucose) saturated with 95% O2/5% CO2. Protein concentration was determined by DC protein assay (BioRad) using bovine serum albumin as standard. The synaptosomal preparation was used immediately for experiments. The striatum was pooled from 3 animals in such a way that all of the experiments were repeated at least three times.
Preparation of Synaptic Vesicles from Striatal Synaptosomes
Synaptic vesicles from synaptosomes were prepared as previously described (Sandoval et al. 2002). Synaptosomal fractions obtained from striatal tissue as described above were suspended in cold water (1 mg protein/ml) containing phosphatase inhibitor (okadaic acid, 1 μM) and other protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin and 250 μM phenylmethylsulfonyl fluoride). This solution was passed through a 20 gauge needle and incubated for 30 min at 4 °C with continuous shaking. HEPES and potassium tartate, pH 7.5 were added slowly to the mixture to the final concentration of 25 mM and 100 mM, respectively. The lyzed synaptosomal fraction was centrifuged at 22,000 g for 30 min at 4 °C. The supernatant containing the synaptic vesicular fractions was further concentrated by a MgSO4 (1 mM) salt-out method followed by centrifugation at 100,000 g for 1 hr. The pellet represents vesicular fractions used for VMAT2 analysis (Sandoval et al. 2002).
DAT Activity Assay
DA uptake was performed as described previously (Samuvel et al. 2005). Briefly, 20 μg of synaptosomes were incubated for three minutes in 250 μl of KRH buffer pH 7.4 containing 0.1 mM ascorbic acid and 0.1 mM pargyline and 40 nM [3H]DA. Synaptosomes were pre-incubated with the DAT inhibitor nomifensine (100 μM) or GBR12909 (0.2 μM) at 37 °C for 10 min followed by the addition of [3H]DA to determine the nonspecific DA uptake. Uptake was terminated with the addition of 3 ml ice-cold PBS followed by rapid filtration using vacuum 10 mm Hg over 0.3% polyethylenimine coated GF-B filters on a Brandel Cell Harvester. Filters were washed rapidly with 5 ml cold PBS and radioactivity bound to filter was counted using a liquid scintillation counter. Nonspecific uptake was defined as the uptake in the presence of DAT blocker and subtracted from the total accumulation to yield specific DAT mediated DA uptake. Mean values of specific uptake ± SEM of at least three separate experiments were determined. Our initial optimizing assay conditions for nomifensine (100 μM) or GBR12909 (0.2 μM) sensitive DAT activity in striatal synaptosomes showed linear DA uptake with respect to uptake time (1 to 8 min) and protein concentration (5 to 30 μg) using 50 nM labeled DA. Therefore, we utilized a 5 min uptake time using 20 μg synaptosomal proteins in all subsequent DAT assays.
VMAT2 Activity Assay
VMAT2 activity was determined as previously described (Sandoval et al. 2002). Synaptic vesicles were incubated in 25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid and 2 mM ATP-Mg, pH 7.5, in the presence of 50 nM [3H]DA at 37 C. To establish our VMAT2 assay conditions, we optimized the specificity of [3H]DA transport using synaptic vesicular fractions by (a) examining [3H]DA transport in the presence of DAT specific blocker, GBR12909 (0.1 μM) to reveal the DAT specific [3H]DA transport component, and (b) testing [3H]DA transport in the presence of reserpine and or in the absence of ATP, to isolate VMAT2 specific [3H]DA transport. DA uptake in the presence of GBR12909 and reserpine was subtracted from total uptake to obtain specific VMAT2 activity as described (Finn et al. 1998; Sandoval et al. 2002). GBR12909/reserpine-sensitive VMAT2 activity in synaptic vesicles showed linear DA uptake with respect to uptake time (1 to 5 min) and protein concentration (5 to 25 μg) using 50 nM [3H]DA. These multiple controls were adapted to ensure that the [3H]DA uptake by synaptic vesicles is mediated by VMAT2 within the linear range of time and protein concentration.
Statistical Methods
Striatal BDNF tissue concentration, body weight, total distance traveled, vertical activity, immunohistochemistry, and DAT/VMAT2 activity were analyzed with independent group 2(Genotype) × 3(Age) ANOVAs. Student-Neuman-Keul’s post-hoc tests were used for group comparisons following significant Genotype × Age or Genotype × Drug Treatment Interactions. Rotarod data were analyzed as total survival time across the six rotational speeds with a maximum survival time of 1800 if the mouse did not fall during any of the six rotational speed tests. Data for the full Age × Genotype × Day factorial with, and without, adjustment for body weight were analyzed with a Cox regression analysis (STATA 9.0) with Days clustered within subjects to account for the correlated responses across the three days (Lee et al. 1992).). The type of analysis for in vivo microdialysis data depended on whether the data were collected prior to (basal level), or after K+ -stimulation. Basal DA level data were analyzed with a 2(Genotype) × 2(Age) × 6 (Sample Interval) mixed factor ANOVA with sample interval being a repeated measure. Because the two genotypes had different basal DA levels, data collected after K+ -stimulation were analyzed with a 2(Genotype) × 2(Age) × 4(Sample Interval) mixed factor ANCOVA using Baseline-Sample-Interval number 6 as a covariate. Again, the Sample Interval factor was a repeated measure.
Results
Striatal BDNF concentration
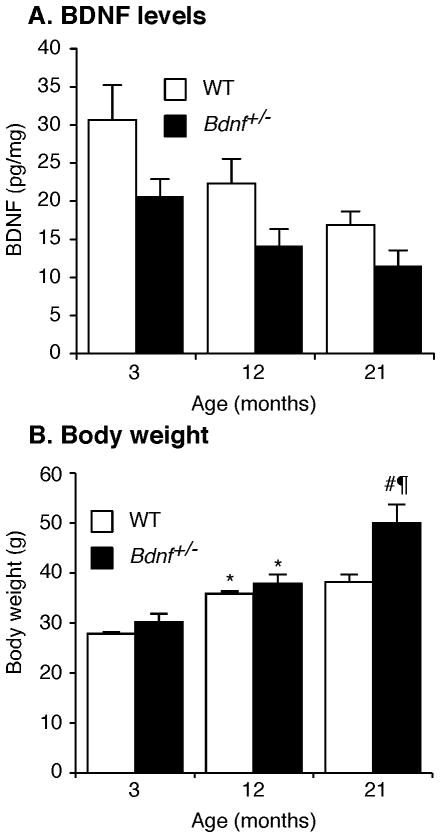
BDNF protein levels in striatal tissue are summarized in Fig 1A and were significantly lower in Bdnf+/− mice than WT littermates [Genotype: F(1,32)= 6.76, p≤ 0.05]. In addition, the BDNF protein declined across age [F(1,32)=5.53, p<0.01]. Although BDNF levels declined by ~25% in WT mice and ~40% in Bdnf+/− mice; this apparent interaction was not statistically supported [Genotype × Age: F(2,32)= 0.19, p> 0.10]
Figure 1. Variations in striatal BDNF levels and body weight with aging in Bdnf+/− vs. WT mice.
(A) BDNF protein levels were lower in Bdnf+/− than WT littermates at all ages tested with no significant effect of age. (B) Body weight (grams) depended on a Genotype × Age interaction. Post-hoc analysis indicated body weight increases for both genotypes between 3 and 12 months of age (*p≤ 0.05). At 21 months of age, Bdnf+/− mice weighed more than age-matched WT mice (#p≤ 0.01) and more than 12-month-old Bdnf+/− mice (¶p≤ 0.01).
Body weight
As noted in Fig 1B, weight gain across age was greater for Bdnf+/− than WT littermates [Genotype × Age: F(2,50)= 5.67, p≤ 0.01]. Student-Neuman-Keul’s post-hoc analysis revealed that 12 month-old mice weighed significantly more than 3 month-old mice, regardless of genotype (p≤ 0.05). Importantly, at 21 months of age, Bdnf+/− mice weighed significantly more than either age-matched WT littermates (p≤ 0.01), or 12 month-old Bdnf+/− mice (p≤ 0.01). Although body weight became elevated for aging Bdnf+/− compared to WT mice, the availability of the same number of Bdnf+/− mice at 21 vs. 3 or 12 months of age suggests that genotype did not alter mortality rate.
Locomotor activity
The first behavior experiment completed for this study was evaluation of motor activity. Horizontal and vertical motor activity data from independent groups of WT and Bdnf+/− mice tested in open-field locomotor activity chambers at 3, 12, and 21 months of age are summarized in 15-min intervals across hourly tests in Fig 2. Horizontal motor activity, expressed as “distance traveled” is summarized in Fig 2A and vertical activity expressed as “rearing response frequency is summarized in Fig 2B. Data for each of the measures were analyzed with 2(Genotype) × 3(Age) × 4(15-min Interval). Both Distance traveled, Fig 2A, and Rearing Response frequency, Fig 2B, declined across the hour session [Distance Traveled: F(3,19)= 19.4, p< 0.001; Rearing Responses: F(3,19)= 97.3, p< 0.001]. The decline across the session was influenced by only the Age factor, and this influence of age was restricted to the rearing response measure, which declined more rapidly for the older mice [Age × Interval: F(4.65)= 3.15, p≤ 0.01]. Both horizontal and vertical motor activity also declined with the Age [Distance Traveled: F(2.65)= 3.86, p≤ 0.03; Rearing Responses: F(2.65) = 3.37, p≤0.04]. Although the Genotype factor did not impact the distance traveled measure [F(1.65)= 1.02, p≥ 0.01], it did influence rearing response frequency [F(1.65)= 6.27, p≤ 0.01], fewer rearing responses for Bdnf+/− mice. Finally, the pattern of change in distance traveled with age for the two genotypes suggested in Fig 2A reached only a 0.09 level of significance [F (2,65)= 2.50, p< 0.09].
Figure 2. Motor activity [A: Horizontal; B: Rearing Responses] of Bdnf+/− and WT mice at 15-minute intervals during one-hour open-field activity tests at 3, 15, and 21 months of age.
Both Distance Traveled, Fig 2A, and Rearing Response frequency, Fig 2B, declined across the hour session. The only effect of the Genotype or Age on the decline in activity across the tests was an Age by Interval interaction for the Rearing Response measure with the decline more rapid for older mice. Both Distance Traveled and Rearing Responses declined with age. The Genotype factor was associated with reductions in Rearing Response frequency; however, did not impact Distance Traveled. Finally, the apparent difference in the pattern of change in Distance Traveled across age for the two genotypes suggested by Fig 2A reached only a 0.09 level of significance.
Since relative body weights of WT and Bdnf+/− mice varied according to age, as noted in Fig 1, correlations of total distance traveled and vertical activity with body weights were determined within each age group. Spearman/Rho correlation analysis indicated no relationship of body weight to either of the activity measures at any of the ages tested (p> 0.05). Thus, the elevated body weight of Bdnf+/− mice did not likely account for their compromised motor performance (data not shown).
Accelerating rotarod performance
Since BDNF deficiency reduced horizontal and vertical activity at 21 months of age (Fig 2), we further evaluated motor behavior using accelerated rotarod performance to assess motor coordination and balance. Rotarod performance, is summarized in Figs 3A&B as Cox regression survival plots with the proportion of mice not falling during tests (ordinate) across time (abcissa), with time reflecting the seconds the animal avoided falling off the rotarod summed across the 6 rotational speeds. These data are also expressed as median survival times across rotation speeds and days for each Genotype and Age combination in Fig 3C. In general, rotarod performance improved across the three days (C2(2)= 9.61, p= 0.01) with the most improvement occurring between day 2 and day 3; however, neither genotype nor age significantly altered this improvement. Further analysis of the genotype and age factorial across days (retaining the clustering of day within subject in the model) indicated no significant age by genotype interaction (X2(2)= 2.3, p= 0.25). The Cox regression plots for the two genotypes across ages (Fig 3A) indicates that Bdnf+/− mice were less able than WT controls to remain on the rotarod with increasing rotation speed (X2(1)= 16.7, p< 0.0001). The Cox regression plots with genotypes combined for the three ages (Fig 3B) indicated a highly significant (X2(2)= 11.07, p= 0.004) reduction in performance with increasing age. Post hoc evaluation confirmed the substantial reduction in performance between 3- vs. 12- (p< 0.001) or 22- (p= 0.002) month-old mice, with no difference between the two older groups (p= 0.86). Finally, Fig 3C summarizes the genotype by age interaction as median survival time. Though the age curves diverge slightly from the parallel, as noted above, the age by genotype interaction was not significant.
Figure 3. Accelerating rotarod performance was reduced for Bdnf+/− and aging mice and was independent of body weight.
Data are expressed as Kaplan-Meier survival curves representing the probability of mice within each group remaining on the rotarod collapsed across the 6 increasing rotational speeds with increasing time up to 300 sec, and across the three test days. The proportion of mice remaining on the rotarod (ordinate) with increasing time across the increasing rotation speeds (abcissa) declined more rapidly for Bdnf+/− than for WT mice (Fig 3A), and more rapidly for 12- and 22-month-old compared to 3-month-old mice (Fig 3B). Although change in median survival times across the three ages differed somewhat for Bdnf+/− and WT mice and were independent of body weight (Fig 3C), the different pattern was not supported by a Genotype × Age interaction.
Adjusting the Cox regression analysis to account for the body weight differences between the two genotypes and across age did not alter any of the results noted above and body weight did not predict rotation performance (B= −0.013(0.02), p= 0.529).
Immunohistochemistry
Our data (Fig 2 and 3) indicate the role of BDNF in the maintenance of age dependent decline of motor activity. Nigrostriatal DAergic functions have been strongly implicated in motor behavior. Notably, dysregulation of several DAergic markers have been documented in aging and several neurological disorders. Therefore, we examined whether BDNF deficiency exhibit any effects on age-dependent alterations of DAergic markers including TH, DAT, VMAT2 expressions. TH-ir, DAT-ir, and VMAT2-ir levels in the striatum of the two genotypes at 3, 12, and 21 months of age were analyzed. TH-ir levels declined with Age [F(2,29)= 6.86, p< 0.01], as did DAT-ir [F(2,23)= 7.71, p< 0.01] but were not influenced by Genotype or its interaction with Age. Contrary to the Age effect on striatal TH-ir and DAT-ir, VMAT2-ir was not influenced by Age, Genotype, or a Genotype × Age interaction (see Supplemental Figures 1–3).
Furthermore, the assessment of TH-ir in DA neurons of the SN at the three ages indicated no effect of Genotype, Age, or Genotype × Age Interaction (see Supplemental Figure 4).
Striatal extracellular DA and K+ stimulated DA release
To determine if the BDNF reduction in Bdnf+/− mice affected in vivo basal synaptic DA homeostasis and evoked DA release, we conducted microdialysis on freely moving 3 and 12 months old Bdnf+/− and WT control mice. Data for extracellular DA in the striatum during basal assessment and following stimulated release by a 60 mM KCl infusion are shown for the two genotypes in Fig 4A and C for 3-month-old mice and Fig 4B and D for 12-month-old mice. Analysis of data for basal extracellular DA levels with a 2(Genotype) × 2(Age) × 6(Baseline) ANOVA with repeated measures on the Baseline factor supported the reduced level of extracellular DA for Bdnf+/− mice noted in Figs 4A and B [F(1,22)= 13.45, p< 0.001]. Basal DA levels did not differ across Age or Baseline interval [Fs ≤ 1], nor according to interactions of the factors. KCl-stimulated DA release data obtained from the four 15-min samples after the 60 mM KCl infusion were analyzed with a 2(Genotype) × 2(Age) × 4(Time Interval) ANCOVA. Because the genotypes had different basal levels of DA, Baseline Sample #6 was used as a co-variate for ANCOVA to account for the difference in baseline in our evaluation of KCl-stimulated DA release. Potassium stimulation increased extracellular DA during the first 15-min interval for WT and Bdnf+/− mice, respectively, by 232 ± 36% and 197 ± 27% at 3 months and by 179 ± 43% and 166 ± 20% at 12 months. Although genotype and age did not alter the degree to which DA was elevated above basal levels, the rate at which the elevated extracellular DA declined across the four 15-min intervals differed according to genotype [F(3,63)= 4.02, p< 0.01]. Follow-up analysis indicated significant differences among the four Genotype × Age groups during the first 15-min period after the KCl infusion [F(3,25) = 5.13, p< 0.018]. Using the 3-month-old WT value as a base, extracellular DA levels during this time were reduced for Bdnf+/− mice of both ages [Dunnette’s test p< 0.05]. Additional analysis of the slopes of declining extracellular DA for the four groups indicated genotype differences with −0.43 ± 0.05 and −0.33 ± 0.05, respectively, for 3 and 12 months old WT mice and −0.26 ± 0.05 and −0.24 ± 0.06 for the aged matched Bdnf+/− mice. A 2(Genotype) × 2(Age) ANOVA indicated that slope depended on Genotype [F(1,37) = 7.09, p< 0.01] but not Age or the interaction of the two factors.
Figure 4. Extracellular DA levels in striatum were lower for Bdnf+/− than WT mice during basal assessment, and following KCl-stimulated release at 3 and 12 months of age.
Extracellular DA levels determined from six 15-min interval basal collections are summarized for 3-month-old mice in (Fig 4A) and 12 month-old mice in (Fig 4B). Basal DA was lower for Bdnf+\− than WT mice with no difference related to Age or its interaction with Genotype. Because basal DA differed for the two genotypes, data collected at intervals following infusion of 60 mM KCl-aCSF summarized in Figs 4C & D, respectively, for 3- and 12-month-old mice were analyzed with an ANCOVA using Baseline Sample #6 (Time=0 min) as a covariate. ANCOVA indicated that extracellular DA varied according to a Genotype × Time Interval Interaction with no effect of the Age factor. Additional analysis of the regression slopes indicated a more rapid return to basal levels for WT than Bdnf+\− mice regardless of age.
DA uptake via DAT and VMAT2
The immunohistochemical analysis noted above indicated that total protein expression of TH and DAT declined with age, but did not differ for WT and Bdnf+/− mice. However, microdialysis experiments revealed lower basal level of DA in the Bdnf+/− compared to WT mice. Moreover, though KCl-stimulated DA release was similar for the two genotypes, the rate at which the elevated DA levels produced by potassium stimulation were cleared was slower for Bdnf+/− than WT mice. These observations suggest the possibility that altered activity, but not total expression of DAT and VMAT2, may contribute to the mechanisms accounting for Bdnf+/− mice exhibiting lower DA basal levels and reduced DA clearance rate. Thus next we examined the functional activity of DAT and VMAT2 proteins using striatal synaptosomes. The influence of partial BDNF deletion on DA uptake mediated by DAT and by VMAT2 across the three ages is summarized, respectively, in Fig 5A and B. As noted in Fig 5A, DAT activity declined progressively with age, and was lower for Bdnf+/− mice compared to age-matched WT mice in the older age groups. Analysis of striatal DAT activity revealed a significant Genotype × Age interaction [F(2,93)= 4.29, p< 0.05]. Student-Newman-Keuls comparison of group means provided statistical support for DAT-mediated DA uptake difference across each of the three age groups for both genotypes noted in Fig 5A (p≤ 0.01). Comparisons of genotype at each age established statistical support for lower DAT activity for Bdnf+/− than WT mice at the 12 and 21 months time points (p≤ 0.01, Student-Newman-Keuls post hoc analysis), but not for the young adult mice.
Figure 5. Age- and genotype-related differences in striatal DAT and VMAT2 activity in Bdnf+/− and WT mice.
DA-transport via DAT (A) and VMAT2 (B) are expressed as pmol/mg protein/min. DAT activity declined with age for both genotypes but the degree of reduction was greater for Bdnf+/− than for WT mice (WT: *p< 0.05 (12 months), *p< 0.001 (21 months) compared with 3 months; #p< 0.001 compared with 12 months WT; Bdnf+/−: ¶p< 0.001(12 months), ¶p< 0.001(21 months) compared with 3 months Bdnf+/− ®p< 0.001 compared with 12 months Bdnf+/−). The greatest reduction in DAT activity observed in Bdnf+/− mice compared to WT mice at 12 and 21 months of age (§p< 0.01 (12 months), §p< 0.007 (21 months) compared with 12 months and 21 months WT respectively). VMAT2 activity also declined with age for both genotypes with the extent of the reduction depending on genotype (WT: *p<0.001 (12 months), *p< 0.001 (21 months) compared with 3 months WT; #p< 0.05 compared with 12 months WT; Bdnf+/−: ¶p< 0.001 (12 months), ¶p< 0.001(21 months) compared with 3 months Bdnf+/−). VMAT2 activity was lower for the Bdnf+/− at 3 and 12 months of age compared with 3 and 12 months WT (§p< 0.001 (3 months), §p< 0.001 (12 months) compared with 3 months and 12 months WT respectively).
In addition, vesicular VMAT2-specific DA uptake (Fig 5B) was also dependent on a Genotype × Age interaction [F(2,22)= 18.38, p< 0.0001]. Striatal VMAT2 activity declined with age, and was lower for Bdnf+/− mice compared to age-matched WT mice in young adult (3 month) and mid-aged (12 month) mice. Subsequent Student-Newman-Keuls post hoc comparisons established significant reductions for 12 and 21 months compared with 3 month-old mice of both genotypes (p≤ 0.01). A further reduction in VMAT2 activity was evident from 12 to 21 months of age for WT (p≤ 0.01) but not Bdnf+/− mice (p≥ 0.10).
Discussion
This study confirms reports of motor deficits for male Bdnf+/− mice and that BDNF reduction advances the motor system decline with aging (Dluzen et al. 2004). The study focuses on how the aging-related decline in three different measures of motor behavior might relate to altered DA system function in a specific Bdnf+/− mouse genotype (i.e., the exon-1 region of the BDNF gene replaced with recombinant neomyocin; mice backcrossed ≥ 10 backcrosses on the B6 strain; (Liebl et al. 1997; Lyons et al. 1999). Male B6 mice with the noted BDNF gene modification in our experiments had motor function deficits that became more evident with aging, and were accompanied by functional changes in the nigrostriatal DA system. Young adult Bdnf+/− mice were deficient on a complex motor task (rotarod), had lower basal levels of extracellular DA and a less rapid decline in DA following K+-stimulated release, and lower VMAT2 activity relative to WT controls. With increasing age, motor deficits for Bdnf+/− mice were observed on less challenging tasks (open-field activity) and were accompanied by reductions in DAT-mediated DA uptake, neither of which were evident in the young mice. Reported studies include mice with different manipulations of the BDNF gene, e.g., exon-1 (Liebl et al. 1997; Lyons et al. 1999) vs exon-5 (Dluzen et al. 2002; Dluzen et al. 2004; Ernfors et al. 1995; Joyce et al. 2004; Liu et al. 1995) vs the entire BDNF coding region (Bennett et al. 1999), different inbred strains for backcrosses, e.g., B6 (Liebl et al. 1997) vs BALB/c (Ernfors et al. 1995; Liu et al. 1995), as well as different experimental protocols. A recent article by Crusio et al. (2009) and a Nature Neuroscience editorial (Crusio et al. 2009; Editorial 2009) reviews the importance of these factors in gene knockout experiments.
General characteristics of Bdnf+/− mice on the C57BL/6 background
Bodyweight gain was similar for Bdnf+/− and WT mice from 3 to 12 months of age; however, from 12 to 21 months increased by 43% for Bdnf+/− while remaining constant for WT mice. The greater weight for the Bdnf+/− mice is consistent with elevated fat and insulin (Kernie et al. 2000; Lyons et al. 1999) and is independent of strain background (Bartoletti et al. 2002; Ernfors et al. 1994; Liebl et al. 1997). Elevated body weight for the Bdnf+/− mice occurred later in life in our study than reported by Lyons et al. (1999) (2–4 months). Differences in the diets used (i.e. 9% crude fat in the Lyons et al. (1999) study vs. 4.40% fat in our study (Harlan Teklad Rodent Diet 8604) is likely accountable (Lyons et al. 1999). Another possible contributing factor could be animal housing differences with single caging in the Lyons et al. (1999) study, and 4–5 per cage in ours which alters feeding and resultant weight gain (Lyons et al. 1999). The elevated bodyweights seems to require a general BDNF reduction since it did not occur when restricted to the forebrain in a conditional KO mouse (Monteggia et al. 2004). Elevated body weight for Bdnf+/− mice is consistent with elevated leptin, an important regulator of feeding behavior, in the hypothalamus and increased adipocyte cell size reported for a similar mouse genotype at six months (Kernie et al. 2000), and also for obese humans (Caro et al. 1996; Friedman 1997).
Behavioral characteristics of Bdnf+/− mice across the life span
Locomotor activity (horizontal) of Bdnf+/− mice was comparable to that of age-matched WT controls at 3 and 12 months of age but was lower at 21 months of age. In contrast, their performance on a more challenging motor coordination task, accelerating rotarod, was deficient at 3 months of age. Such a pattern of age-related motor deficiency is common for aging humans (Bennett et al. 1996; Volkow et al. 1998). The possible contribution of elevated body weight to the motor deficits observed for Bdnf+/− mice eliminated via correlation analysis on the locomotor activity and rotarod measures, and factoring out the weight difference on rotarod performance indicating other peripheral or central nervous system abnormalities.
The absence of a motor activity deficit for the younger Bdnf+/− mice in our study is consistent with other reports: 1) young adult Bdnf+/− mice on a C57BL/6 genetic background (Macqueen et al. 2001), 2) young female Bdnf+/− mice on a mixed B6/SV129 background (Chourbaji et al. 2004), and 3) mixed sexes on a mixed BALB/C/SV129 background (Dluzen et al. 2001). The lower motor activity for older Bdnf+/− mice in our study is consistent with a study using BDNFlacZ/neo+ mice with a different BDNF manipulation but a similar behavioral paradigm (Saylor et al. 2006), and with the poor performance of older, but not young, Bdnf+/− mice on a beam walk task (Dluzen et al. 2004). In contrast, Kernie et al. (Kernie et al. 2000) reported hyperactivity for six-month-old Bdnf+/− mice with normal body weight, and activity comparable to WT controls for 50% of the Bdnf+/− mice classified as obese. These variable effects of a genetic reduction in BDNF on motor activity across different strains emphasizes the importance of considering the influence of different parental genetic backgrounds noted in the recent articles by Crusio et al. (2009) and the editorial in Nature Neuroscience (Crusio et al. 2009; Editorial 2009), as well as different behavioral testing strategies noted above when interpreting the outcome of experiments on genetically manipulated animals.
Motor coordination is one of the first behavioral components to deteriorate with normal aging (Bickford et al. 1992) and the poor rotarod performance of Bdnf+/− mice suggests a role for BDNF in this decline. The poor coordination of the Bdnf+/− mice in our experiments is consistent with a report of vertical activity and accelerating rotarod performance deficits associated with low BDNF protein expression in the cerebellum of mice in a Classic Rett syndrome model (Kondo et al. 2008); and with a report of low striatal and cerebellar BDNF levels for aged rats with motor problems (Katoh-Semba et al. 1998). In contrast, Baker et al. reported no rotarod performance deficit for Bdnf+/− mice although performance of the mice declined with age (Baker et al. 2005). In addition, Canals et al. (2004) reported that although BDNF reduction alone did not alter their measure, it did enhance aging-related motor dysfunction in a double mutant mouse model of Huntington’s disease (Canals et al. 2004). Mice used in the Baker and Canals studies were backcrossed on the BALB/c rather than the B6 strain used in our experiment and the influence of strain on open-field testing and rotarod behavior (Carola et al. 2002; Depino & Gross 2007; Griebel et al. 2000) could contribute to different outcome of these experiments. Given the influence of gender on behavioral measures (Archer 1975; Fernandes et al. 1999), it should also be noted that the Chourbaji et al. study which observed no coordination deficits in young Bdnf+/− mice used only females (Chourbaji et al. 2004). Finally, the particular experimental protocol including data analysis used in rotarod assessment can influence experimental outcomes (Rustay et al. 2003) and differed with data from each animal across each test and each day analyzed with sensitive Kaplan-Myer survival curves in our experiment.
The possibility that learning deficits contributed to the poor rotarod performance for Bdnf+/− mice is unlikely because performance improved at the same rate across days for WT and Bdnf+/− mice tested at 3 months of age in the current study. Studies focusing on more focused behavioral evaluation and perhaps location of BDNF loss in different brain regions could help clarify the impact of reducing this neurotrophic factor on motor systems.
Neuroanatomical & neurochemical characteristics of Bdnf+/− mouse DA systems across the life span
TH-ir, DAT-ir, or VMAT2-ir in the nigrostriatal system of Bdnf+/− mice did not differ from WT controls at any age as was previously reported for young Bdnf+/− mice (Joyce et al. 2004). TH-ir was lower in striatum of older mice regardless of genotype. The aging-related decline in striatal TH-ir was accompanied by reduced DAT and VMAT2 activity, with substantial reductions at 21 months. Further, in comparison to WT controls, activity of both transporters was reduced for Bdnf+/− mice at 12 months, and VMAT2 was also reduced at 3 months of age. Activity of DAT and VMAT2 has shown to be dynamically regulated by cellular kinases, phosphatase and transporter associated proteins. It is possible that constitutive reductions in BDNF and its receptor linked signaling cascades can affect transporter activity independent to total expressions (Jayanthi et al., 2007; Krantz et al., 1997). In vivo microdialysis focused on young adult and middle-aged mice. These experiments clearly established lower basal levels of extracellular DA for Bdnf+/− mice regardless of age. Extracellular DA following KCl stimulation was increased above basal levels to roughly the same extent for both genotypes with DA greater for WT than Bdnf+/− mice during the first 15-min interval. An important genotypic difference, however, was the slower return of the elevated DA level to pre-stimulus basal levels for the Bdnf+/− compared to WT mice. Results of our in vivo experiment differs from the report of an in vitro experiment indicating that K+-stimulated DA release was lower in ex vivo nigrostriatal tissue preparations from 12 and 20 month-old Bdnf+/− mice than from age-matched WT mice (Dluzen et al. 2004). Our results also differ from the results obtained from the ex vivo experiment in that KCl-stimulated release did not change significantly between the young adult and mid-aged mice in our experiment whereas it increased substantially in tissue from mid-aged compared to young adults in the in vitro report. The reasons for the different results of the two experiments are not clear but could include: 1) possible differences in neurotransmitter dynamics ex vivo vs in vivo assessments, 2) the different genetic background strains (BalbC vs C57) which have different DA characteristics, or 3) slight differences in the particular DA areas assessed. Although the slower decline of extracellular DA following KCl- stimulation for Bdnf+/− compared to WT mice is consistent with the lower DAT transporter activity noted for the genotype, the observed lower basal DA levels combined with lower DAT activity for the Bdnf+/− mice remains difficult to explain. Possible explanations include some adaptive changes resulting from prolonged reduction in BDNF for the Bdnf+/− mice. For example, the constitutive reduction in brain BDNF levels in Bdnf+/− mice could decrease DA-synthetic pathway activity, or perhaps increase DA-degradative pathways. The higher DA levels in striatal tissue and the unaltered DA cell bodies or axons noted for Bdnf+/− mice (Baker et al. 2005; Dluzen et al. 1999) suggest that DA synthesis or DA-neuronal loss are not likely accountable for the low levels of synaptic DA in Bdnf+/− mice. While DAT is considered to clear DA primarily via influx/uptake process, DAT also has regulated capacity to efflux cytosolic DA to synapse (Defelice & Goswami 2007; Khoshbouei et al. 2004). In addition, other transporters such as the norepinephrine transporter (NET) and organic cation transporter (OCT) might contribute toward the clearance of synaptic DA (Busch et al. 1996; Chemuturi & Donovan 2007; Moron et al. 2002). Studies related to DAT-mediated DA efflux, NET and OCT3 activities and active levels of DA-degradative enzymes have yet to be conducted in Bdnf+/− mice and are warranted for future investigations. In addition, systematic use of no net flux microdialysis approach is necessary to confirm the role of amine transporters in relevance to lower basal DA levels in Bdnf+/− mice. Furthermore, in contrast to the decreased basal DA levels found in young and middle aged Bdnf+/− mice, higher basal extracellular 5-HT levels in the Bdnf+/− ventral hippocampus have been reported (Benmansour et al. 2008; Daws et al. 2007; Guiard et al. 2008).
DAT activity reductions from 3 to 12 months for Bdnf+/− mice (59%) and WT mice (37%) were not accompanied by reduced striatal TH-ir. DAT knockout mouse studies indicate a direct relationship between DAT and TH expression (Jaber et al. 1999; Jones et al. 1998) with DAT depletion producing a 90–96% reduction in striatal TH-ir. Thus, maintenance of TH protein expression in the striatum appears to have a DAT activity threshold. Current data suggest that BDNF is extensively involved in the complex functions of DA release and re-uptake.
The precise relationship of the DA system deficits to the motor system deficits noted in Bdnf+/− remain unclear, however, both the mesoaccumbens and nigrastriatal systems are involved in mediating various aspects of motor function (Volkow et al. 1998). Clearly the advanced decline of motor function with age for Bdnf+/− mice is consistent with the reduced levels of extracellular DA, and with the reduced DAT and VMAT2 activity, all of which suggest a less functional DA system. The aging-related decline in motor coordination is also related to striatal DA, as well as cerebellar NE (Bickford et al. 1992). The particular behavioral abnormalities noted and the type of Bdnf+/− mice used our study differed from those obtained on the Bdnf+/− mouse type used in studies reported by Dluzen and colleagues (Dluzen et al. 2002; Dluzen et al. 1999); however, the primary conclusion are similar in that aging-related changes in DA system function and the behaviors they mediate are accentuated by reduced BDNF levels (Dluzen et al. 2004).
In conclusion, the present study indicates that the constitutive BDNF reduction noted in Bdnf+/− mice exacerbates the aging-related dysfunctions of motor systems and DAergic dynamics in the striatum. The accentuated aging dysfunctions were indicated in Bdnf+/− mice; behaviorally, by lower locomotor activity and deficient performance on the accelerating rotarod, and neurochemically, by reductions in extracellular DA and activity of DAT and VMAT2 transporters. To help further elucidate the consequences of genetic manipulations on behavior and mediating neurochemistry, it will be important to attend to details of the genetic manipulation, the genetic background of the knockout mice, other biogenic amine transporters, as well as the details of the particular behavioral protocols employed.
Supplementary Material
Acknowledgments
The authors wish to thank Mrs. Claudia Umphlet and Mr. Alfred Moore for their technical assistance. This work was supported by National Institutes of Health Grant PO1 AG023630 (to SR, A-ChG, JFM, BR), MH062612, MH083928 (to S.R) and GM081054 (to L.D.J)
References
- Abidin I, Kohler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, Mittmann T. Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur J Neurosci. 2006;24:3519–3531. doi: 10.1111/j.1460-9568.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- Albeck D, Mesches MH, Juthberg S, Browning M, Bickford PC, Rose GM, Granholm AC. Exogenous NGF restores endogenous NGF distribution in the brain of the cognitively impaired aged rat. Brain Res. 2003;967:306–310. doi: 10.1016/s0006-8993(03)02272-8. [DOI] [PubMed] [Google Scholar]
- Archer J. Rodent sex differences in emotional and related behavior. Behav Biol. 1975;14:451–479. doi: 10.1016/s0091-6773(75)90636-7. [DOI] [PubMed] [Google Scholar]
- Baker SA, Stanford LE, Brown RE, Hagg T. Maturation but not survival of dopaminergic nigrostriatal neurons is affected in developing and aging BDNF-deficient mice. Brain Res. 2005;1039:177–188. doi: 10.1016/j.brainres.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Aging and the extrapyramidal system. J Am Geriatr Soc. 1973;21:145–149. doi: 10.1111/j.1532-5415.1973.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Bartoletti A, Cancedda L, Reid SW, Tessarollo L, Porciatti V, Pizzorusso T, Maffei L. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22:10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Bickford P, Heron C, Young DA, Gerhardt GA, De La Garza R. Impaired acquisition of novel locomotor tasks in aged and norepinephrine-depleted F344 rats. Neurobiol Aging. 1992;13:475–481. doi: 10.1016/0197-4580(92)90075-9. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F, Koepsell H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 1996;395:153–156. doi: 10.1016/0014-5793(96)01030-7. [DOI] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Bailey SL. Enhanced effects of 6-hydroxydopamine on evoked overflow of striatal dopamine in aged rats. Brain Res. 2002;938:29–37. doi: 10.1016/s0006-8993(02)02481-2. [DOI] [PubMed] [Google Scholar]
- Chemuturi NV, Donovan MD. Role of organic cation transporters in dopamine uptake across olfactory and nasal respiratory tissues. Mol Pharm. 2007;4:936–942. doi: 10.1021/mp070032u. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, Henn FA, Hortnagl H, Gass P. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101:641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- Dejesus OT, Endres CJ, Shelton SE, Nickles RJ, Holden JE. Noninvasive assessment of aromatic L-amino acid decarboxylase activity in aging rhesus monkey brain in vivo. Synapse. 2001;39:58–63. doi: 10.1002/1098-2396(20010101)39:1<58::AID-SYN8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Depino AM, Gross C. Simultaneous assessment of autonomic function and anxiety-related behavior in BALB/c and C57BL/6 mice. Behav Brain Res. 2007;177:254–260. doi: 10.1016/j.bbr.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Dluzen DE. Estrogen as a neuromodulator of MPTP-induced neurotoxicity: effects upon striatal dopamine release. Brain Res. 1997;764:9–16. doi: 10.1016/s0006-8993(97)00418-6. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI, McDermott JL, Kucera J, Walro JM. Striatal dopamine output is compromised within +/− BDNF mice. Synapse. 2002;43:112–117. doi: 10.1002/syn.10027. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNF mutant mice. Exp Neurol. 2001;170:121–128. doi: 10.1006/exnr.2001.7698. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Anderson LI, Kucera J, Joyce JN, Osredkar T, Walro JM. Age-related changes in nigrostriatal dopaminergic function are accentuated in +/− brain-derived neurotrophic factor mice. Neuroscience. 2004;128:201–208. doi: 10.1016/j.neuroscience.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Story GM, Xu K, Kucera J, Walro JM. Alterations in nigrostriatal dopaminergic function within BDNF mutant mice. Exp Neurol. 1999;160:500–507. doi: 10.1006/exnr.1999.7225. [DOI] [PubMed] [Google Scholar]
- Do T, Kerr B, Kuzhikandathil EV. Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J Neurochem. 2007;100:416–428. doi: 10.1111/j.1471-4159.2006.04249.x. [DOI] [PubMed] [Google Scholar]
- Editorial, Nature Neuroscience. Troublesome variability in mouse studies. Nat Neurosci. 2009;12:1075. doi: 10.1038/nn0909-1075. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39:799–807. [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Finn JP, 3rd, Merickel A, Edwards RH. Analysis of neurotransmitter transport into secretory vesicles. Methods Enzymol. 1998;296:144–162. doi: 10.1016/s0076-6879(98)96012-8. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Leptin, leptin receptors and the control of body weight. Eur J Med Res. 1997;2:7–13. [PubMed] [Google Scholar]
- Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Guiard BP, David DJ, Deltheil T, Chenu F, Le Maitre E, Renoir T, Leroux-Nicollet I, Sokoloff P, Lanfumey L, Hamon M, Andrews AM, Hen R, Gardier AM. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11:79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Himi T, Cao M, Mori N. Reduced expression of the molecular markers of dopaminergic neuronal atrophy in the aging rat brain. J Gerontol A Biol Sci Med Sci. 1995;50:B193–200. doi: 10.1093/gerona/50a.4.b193. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagne C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Renish L, Osredkar T, Walro JM, Kucera J, Dluzen DE. Methamphetamine-induced loss of striatal dopamine innervation in BDNF heterozygote mice does not further reduce D3 receptor concentrations. Synapse. 2004;52:11–19. doi: 10.1002/syn.10309. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci Res. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, Ferris SH, Reisberg B. Motor/psychomotor dysfunction in normal aging, mild cognitive decline, and early Alzheimer’s disease: diagnostic and differential diagnostic features. Int Psychogeriatr. 1997;9(Suppl 1):307–316. doi: 10.1017/s1041610297005048. discussion 317–321. [DOI] [PubMed] [Google Scholar]
- Kokmen E, Bossemeyer RW, Jr, Barney J, Williams WJ. Neurological manifestations of aging. J Gerontol. 1977;32:411–419. doi: 10.1093/geronj/32.4.411. [DOI] [PubMed] [Google Scholar]
- Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome--Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- Lee EW, Wei LJ, Amato D. In Survival Analysis, State of the Art. Netherlands: Kluwer; 1992. Cox-type regression analysis for large number of small groups of correlated failure time observations; pp. 237–247. [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J Neural Transm Suppl. 2007:113–120. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- Newman RP, LeWitt PA, Jaffe M, Calne DB, Larsen TA. Motor function in the normal aging population: treatment with levodopa. Neurology. 1985;35:571–573. doi: 10.1212/wnl.35.4.571. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wolfarth S, Schulze G, Wardas J, Pietraszek M, Lorenc-Koci E, Smialowska M, Coper H. Decline in motor functions in aging is related to the loss of NMDA receptors. Brain Res. 2001;907:71–83. doi: 10.1016/s0006-8993(01)02601-4. [DOI] [PubMed] [Google Scholar]
- Richards M, Bell K, Dooneief G, Marder K, Sano M, Mayeux R, Stern Y. Patterns of neuropsychological performance in Alzheimer’s disease patients with and without extrapyramidal signs. Neurology. 1993;43:1708–1711. doi: 10.1212/wnl.43.9.1708. [DOI] [PubMed] [Google Scholar]
- Rozas G, Guerra MJ, Labandeira-Garcia JL. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc. 1997;2:75–84. doi: 10.1016/s1385-299x(97)00034-2. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22:8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, Meredith GE, Vercillo MS, Zahm DS, McGinty JF. BDNF heterozygous mice demonstrate age-related changes in striatal and nigral gene expression. Exp Neurol. 2006;199:362–372. doi: 10.1016/j.expneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, Beal MF, Jones L, Kooperberg C, Olson JM, Jones KR. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohta H, Imamura L, Asakura W, Matoba Y, Matsumoto K. Effect of Panax ginseng on age-related changes in the spontaneous motor activity and dopaminergic nervous system in the rat. Jpn J Pharmacol. 1991;55:51–56. doi: 10.1254/jjp.55.51. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol. 2000;161:392–396. doi: 10.1006/exnr.1999.7274. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.