Abstract
Background and Purpose
Our previous studies have shown that bone marrow-derived cells (BMDCs) home to the brain angiogenic focus. Angiogenic response to vascular endothelial growth factor (VEGF) stimulation is reduced in matrix metalloproteinase-9 (MMP-9) knockout mice. We hypothesized that BMDCs contribute to VEGF-induced angiogenesis by supplying MMP-9.
Methods
Bone marrow (BM) transplantation was conducted using MMP-9 knockout (MMP-9 KO) or wild-type (WT) mice as donor and recipient. Adeno-associated viral vectors expressing VEGF or LacZ was injected into the striatum 4 weeks after BM transplantation. Circulating white blood cells (WBCs), microvessel density, number of BMDCs, and MMP-9 activity around the injection site were analyzed.
Results
Circulating WBCs increased in WT mice but not in MMP-9 KO mice 2 weeks after vector injection. Overexpressing VEGF increased microvessel density by 38% in WT mice 4 weeks after vector injection. Transplantation of MMP-9 KO BM to WT mice reduced angiogenic response by 80% of that in WT mice. The microvessel density only increased 18% after VEGF stimulation with MMP-9 activity reduced to 35% of the level of WT mice. There was no significant angiogenic response in the brain of MMP-9 KO. Transplantation of WT BM to MMP-9 KO mice restored their brain angiogenic response to 92% of the WT level, indicated by a 30% increase of microvessel density in the VEGF-treated group with MMP-9 activity close to the level of WT mice.
Conclusion
BM-derived MMP-9 plays an important role in BM cell mobilization and focal angiogenesis in the brain in response to VEGF stimulation.
Keywords: angiogenesis, bone marrow-derived cells, brain angiogenesis, metalloproteinase-9, vascular endothelial growth factor
Introduction
Increasing evidence indicates that bone marrow-derived cells (BMDCs) are associated with angiogenesis-related disorders, including tumorgenesis1–3 and brain arteriovenous malformations (AVM).4 BMDCs are recognized as a major supplier of matrix metalloproteinases (MMPs),2, 5 a family of zinc-dependent endopeptidases responsible for the degradation of extracellular matrix component, for tissue angiogenesis.
Our previous studies have demonstrated that cerebral angiogenesis can be induced by adeno-associated viral vector (AAV)-mediated vascular endothelial growth factor (VEGF) gene transfer.6, 7 This angiogenic response to VEGF stimulation was reduced in MMP-9 knockout (KO) mice,7 suggesting that MMP-9 plays a role in cerebral angiogenesis.
Studies have shown that MMP-9 facilitates mobilization of BM cells into the circulation8 and their homing to the tumorangiogenic foci.3 In this study, we provide direct evidence that BMDC-derived MMP-9 is crucial for VEGF-induced brain.
Our primary motivation for undertaking this study of the association of BM-derived MMP-9 and angiogenesis is in the context of various brain vascular malformations, especially brain AVM. The other neurological diseases, including tumor, are secondary areas of potential relevance.
Materials and Methods
Experimental Design
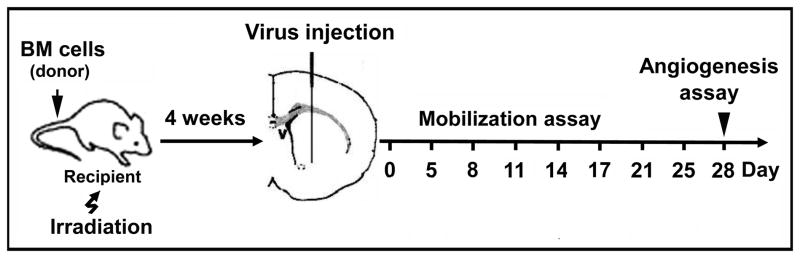
The experimental design and groups are shown in Figure 1 and Table 1. All experimental procedures for using laboratory animals were approved by the Institutional Animal Care and Use Committee, University of California, San Francisco. Adult male mice at age 8–10 weeks in the C57BL/6 background carrying homozygous null mutations of the MMP-9 gene9 and their wild-type littermates were used as BM donors and recipients in all the experiments.
Figure 1. Experimental design.
Recipients were lethally irradiated and transplanted with donor BM cells. AAV-VEGF or AAV-LacZ was injected into the right basal ganglia 4 weeks after BM transplantation. Circulating blood was collected on the days indicated and WBCs were quantified using HEMAVET. Mice were sacrificed 4 weeks after gene transfer, and BM and brain samples were collected for analyses.
Table 1.
Experimental groups
| Groups | BM Recipient | BM Donor |
|---|---|---|
| WT+WT BM | WT | WT |
| KO+WT BM | KO | WT |
| WT+KO BM | WT | KO |
| KO+KO BM | KO | KO |
For Methods and Statistical Analysis, please see Supplemental Methods at http://ahajournals.org.
Results
MMP-9 Facilitates the Mobilization of BM Cells into the Circulation
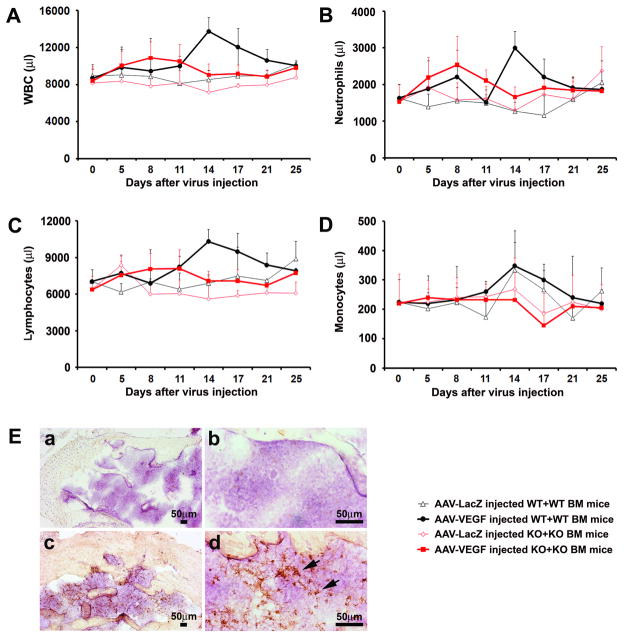
WT+WT BM and MMP-9 KO+KO BM mice were used to explore the effects of MMP-9 on BM cell mobilization in response to VEGF stimulation. The mice in these two groups had a similar baseline of total circulating WBCs. Total WBCs increased to 160% of baseline (P=0.0015) in WT+WT BM mice 14 days after intra-brain injection of AAV-VEGF, decreased thereafter, then returned to baseline on day 25 (Figure 2A). The total WBCs increased slightly on day 8 only (135% of baseline, P=0.08) in MMP-9 KO+KO BM mice. Thus, VEGF could not induce a significant increase in total WBCs in KO+KO BM mice.
Figure 2. Increase in circulating WBCs in response to VEGF stimulation was impaired in MMP-9 KO+KO BM mice.
Circulating WBCs (A), neutrophils (B), lymphocytes (C), and monocytes (D) were quantified by HEMAVET every 3–4 days after vector injection. The data show that all of these cells significantly increased in AAV-VEGF-injected WT+WT BM mice compared to that of similarly treated MMP-9 KO+KO BM mice at day 14. All values are given as mean±SD. N=6 in each group. E. Representative images of immunohistochemical-stained BM sections collected 4 weeks after AAV-VEGF injection. MMP-9 positive staining (brown) was detected in WT mice (c, d), but not in MMP-9 KO mice (a, b).
Both neutrophils and lymphocytes peaked on day 14 and returned to baseline on day 25 in VEGF-treated WT+WT BM mice (Figure 2B, C). They were the major cell-types responsible for the increase of total WBCs at this time point. These cells did not increase in KO+KO BM mice. Monocytes increased in WT+WT BM mice but not in KO+KO BM mice on day 14 after AAV-VEGF and AAV-LacZ vector-injection (Figure 2D). These data suggest that MMP-9 facilitates BM cell mobilization in response to VEGF stimulation.
VEGF Treatment Induces MMP-9 Expression in BM Cells
We then determined whether the increased mobilization of BM cells in VEGF-treated WT+WT BM mice was due to the upregulation of MMP-9 in the BM cells. We analyzed MMP-9 expression in BM tissue collected from WT+WT BM and MMP-9 KO+KO BM mice. Very few MMP-9-positive cells were detected in the BM of LacZ-treated WT+WT BM mice (data not shown). MMP-9 positive signals were detected in BM sections of VEGF-treated WT+WT BM mice (Figure 2E, c and d), but not in KO+KO BM mice (Figure 2E, a and b). These results suggest that VEGF upregulated MMP-9 expression in BM cells.
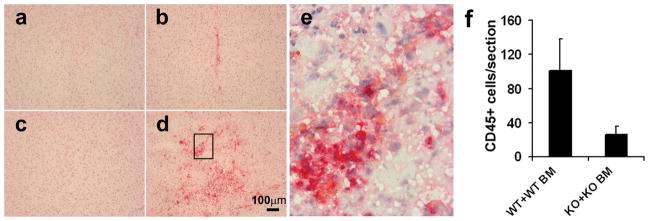
MMP-9 promotes the Recruitment of BMDCs to the Angiogenic Foci
We then explored the role of MMP-9 in the recruitment of BMDCs from the circulation to the angiogenic foci in the mouse brain. The brain sections were stained with an antibody specific to CD45, a common leukocyte marker. CD45+ cells were observed in the VEGF-treated brain (Figure 3, b and d), but not in the LacZ-treated brain (Figure 3, a and c). Numerous CD45+ BMDCs were observed in the WT+WT BM brain around the injection site (Figure 3, d, e, and f, 101±37/hemisphere section), but few were detected around the injection site in the brain of MMP-9 KO+KO BM mice (Figure 3, b, f, 26±9/hemisphere section), indicating that MMP-9 promoted the recruitment of BMDCs (CD45+ cells) into the VEGF-induced angiogenic foci.
Figure 3. Homing of CD45+ cells to VEGF-induced mouse brain angiogenic foci was impaired in MMP-9 KO mice.
Representative images of immunohistochemical-stained mouse brain sections from WT+WT BM mice (c, d) and MMP-9 KO+KO BM mice (a, b) Many CD45+ cells (red) were detected around the AAV-VEGF injection site in the WT+WT BM brain (d), a few in the MMP-9 KO+KO BM brain (b), none in the AAV-LacZ-injected WT+WT BM brain (c) or KO+KO BM brain (a). High magnification photo (e) shows the boxed region in d. Bar graph shows the quantification of CD45+ cells around the injection sites (f), Data are mean±SD. N=4 per group.
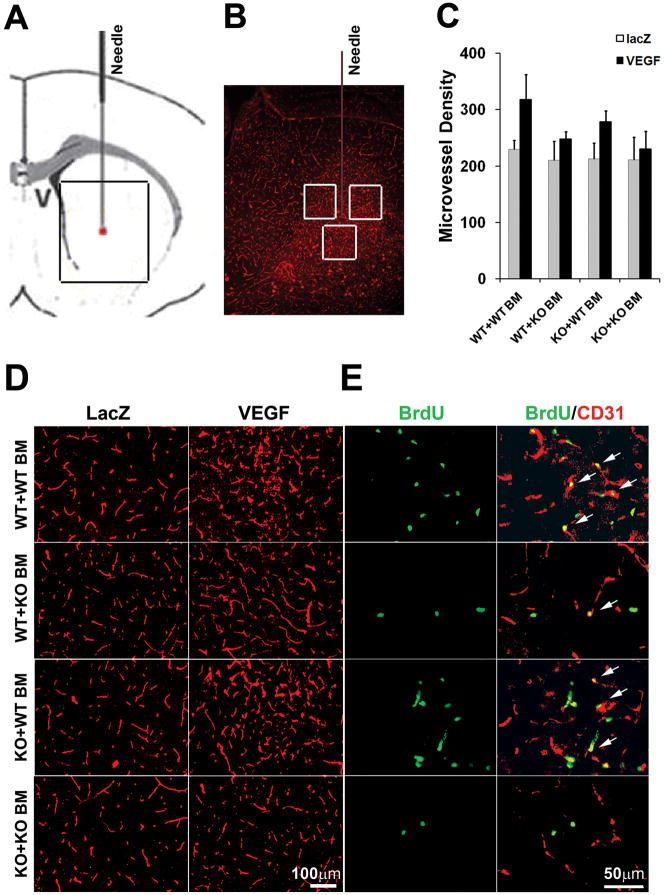
Transplantation of WT BM Rescued the impaired Brain Angiogenesis of MMP-9 KO Mice
Microvessel density increased 38% in the brain of VEGF-treated WT+WT BM mice (Figure 4C and D, 318±44 vs. 230±16; VEGF vs. LacZ, P=0.0001), and 18% in VEGF-treated WT+KO BM mice (Figure 4C, 248±13 vs. 210±34, VEGF vs. LacZ, P=0.037). VEGF did not induce significant angiogenesis in the brain of KO+KO BM mice (231±31 vs. 211±40, VEGF vs. LacZ, P=0.28). Transplantation of WT BM partially rescued the impaired angiogenic response in MMP-9 KO mice, indicated by an increase of microvessel density to 30% in the brain of AAV-VEGF-injected mice compared to that of AAV-LacZ-injected mice (Figure 4C, 278±19 vs. 213±29, VEGF vs. LacZ, P=0.0006). These results suggest that BMDC homing is crucial for VEGF-induced brain focal angiogenesis.
Figure 4. Transplantation of WT BM cells rescued the impaired angiogenic response to VEGF in MMP-9 KO mice.
A. A cartoon depicts a coronal section surrounding the needle injection site. B. A microimage of a real section of the brain in the boxed region in A covering an entire AAV-VEGF-induced angiogenic focus. The three white boxes demonstrate the areas that were used for quantification of vascular density. C. Bar graph shows the quantification of vascular densities around the injection sites. Data are mean±SD. N=6 per group. D. Representative pictures of lectin-stained brain sections (ipsilateral side) showing a higher vascular density in the AAV-VEGF-injected brain compared to AAV-LacZ-injected brain. E. Representative images of AAV-VEGF-injected brain sections (ipsilateral side) stained with anti- BrdU antibody (green) and anti-CD31 antibody (red). There were more BrdU positive endothelial cells in WT+WT BM mice and MMP-9 KO+WT BM mice than in WT+KO BM and KO+KO mice. Arrows indicate dual positive cells.
We then used antibodies specific to CD31 and cell proliferating marker BrdU to show the proliferating endothelial cells. The numbers of BrdU/CD31 double-positive cells in each group paralleled the data for microvessel density: there were more double-positive cells in KO+WT BM compared to KO+KO BM mice, and fewer double-positive cells in WT+KO BM compared to WT+WT BM mice (Figure 4E).
We also analyzed the correlation of microvessel density with injected vectors, recipient genotypes and donor BM genotypes using three-way ANOVA. We observed that injection of AAV-VEGF increased microvessel density (p=0.0001, AAV-VEGF vs. AAV-LacZ). VEGF was more effective in WT+WT BM mice compared to MMP-9 KO +KO BM mice (p=0.0642), and WT mice with MMP-9 KO BM (p=0.0037) genotypes. Linear regression revealed that both recipient (p=0.022) and donor BM (P=0.002) genotype influenced the microvessel density in the VEGF-treated brain (Figure 4C).
BMDCs are Important Sources of MMP-9 in the Brain Angiogenic Foci
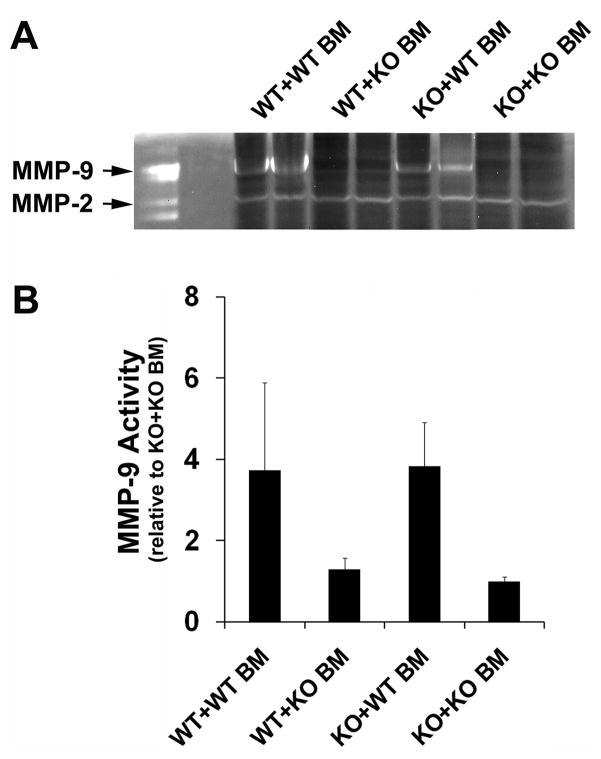
To investigate whether BMDCs are important sources of MMP-9 in VEGF-induced angiogenic foci in the mouse brain, we examined the level of MMP-9 activity in the VEGF-treated brain using zymography. A higher level of MMP-9 was detected in VEGF-treated WT+WT BM mice (3.7±2.2 ) than in MMP-9 KO+KO BM mice (1.0±0.1 ) (Figure 5, P<0.01). The MMP-9 level was significantly reduced in WT+KO BM mice (1.3±0.3), but was greatly increased in KO mice transplanted with WT BM (3.8±1.1, Figure 5, P<0.01). These results suggest that BMDCs provided a significant amount of MMP-9 to the VEGF-induced brain angiogenic foci.
Figure 5. MMP-9 activity in the treated brain.
A. Representative picture of zymogram gel. First lane = MMP-2, -9 standard. B. Bar graph demonstrates densitometry analysis of the band mean intensity of MMP-9. Data are mean±SD. N=6 per group.
Discussion
This study is the first to show that following VEGF administration in the adult mouse brain: (1) circulating WBCs increased in WT+WT BM mice (accompanied by MMP-9 expression in the BM), but not in MMP-9 KO+KO BM mice, suggesting that MMP-9 activation facilitates mobilization; (2) numerous BMDCs (CD45+ cells) were observed in the brain of WT+WT BM mice and few in the brain of KO+KO BM mice, suggesting that MMP-9 promotes the recruitment of BMDCs to VEGF-induced angiogenic foci; (3) focal brain angiogenesis decreased in WT mice transplanted with KO BM, accompanied by a reduced level of brain MMP-9, whereas transplantation of WT BM partially rescued the impaired angiogenic response of MMP-9 KO mice, with an increased level of brain MMP-9. Taken together, our results demonstrate that BM-derived MMP-9 facilitates the mobilization of BM cells into the circulation and their homing to the angiogenic foci in response to VEGF stimulation. BMDCs contribute to angiogenesis by supplying MMP-9.
MMP-9 is Crucial for VEGF-induced BM Cell Mobilization
Stem cells are quiescent in the BM niche, but they are mobilized to the peripheral blood in response to specific signals. Single-dose intravenous injection of Ad-VEGF increases circulating WBCs in WT mice, which is MMP-9-dependent and is associated with an elevation of VEGF plasma level.8 Our results showed that intra-brain injection of AAV-VEGF increased circulating WBCs to 160% of baseline in WT+WT BM mice; this effect was not observed in MMP-9 KO+KO BM mice (Figure 2A). The number of circulating WBCs peaked on day 14, likely the peak of plasma VEGF, as Heissig et al showed in their article that the peak of the mature WBCs in circulation correlated with the peak of plasma VEGF level.8 Further studies are needed to prove this in our model.
Activated MMP-9 within the BM liberates soluble kit-ligand (sKitL), thereby mobilizing BM cells into the circulation.8, 10 We showed that injection of AAV-VEGF into the mouse brain increased MMP-9 expression in BM cells (Figure 2E), resulting in increased mobilization of BM cells, as demonstrated by increased circulating WBCs. While sKitL released by induced MMP-9 in BM cells seems to be the key factor in the process, further studies to investigate whether exogenous sKitL rescues impaired BM mobilization in MMP-9 KO mice may provide more evidence.
BMDCs Contribute to VEGF-induced Focal Brain Angiogenesis by Supplying MMP-9
We have previously studied whether BMDCs (tracked by GFP expression after transplantation of BM cells harvested from GFP mice to wild type mice) can be recruited into AAV-VEGF-induced angiogenic foci, and whether they incorporate into the neovasculature.7 We demonstrated that MMP-9 activity is necessary for the VEGF-induced focal angiogenesis by injecting AAV-VEGF into the brain of MMP-9 KO mice.7 But we have no direct evidence to show that BMDCs contribute to MMP-9 in the VEGF-induced angiogenic foci.
In the current study, we used a BM transplantation mouse model to test our hypothesis that MMP-9 derived from BMDCs is crucial for VEGF-induced brain angiogenesis. We found that transplanting MMP-9 KO BM to WT mice resulted in a reduction of their brain angiogenic response to VEGF stimulation, whereas transplanting WT BM to MMP-9 KO mice partially rescued their brain angiogenic response. Thus, this study provides solid evidence that BMDC-derived MMP-9 contributes to VEGF-induced brain focal angiogenesis. In addition, we demonstrate that MMP-9 facilitates the mobilization of BM cells into the circulation, which subsequently may facilitate their recruitment into the mouse brain angiogenic foci.
To what extent BMDCs incorporate into the neovasculature remains controversial. Despite the fact that only small fractions of circulation cells are monocytes, we found that most of BMDCs homing to VEGF-stimulated brain angiogenic focus are CD45 (94%) and CD68 (71%) positive cells.7 Others have also found that BMDCs infiltrating into the brain give rise primarily to microglia.11–14 Studies including ours have shown that BMDCs are recruited to the angiogenic foci but are rarely incorporated into the vessel structure,1, 7, 15, 16 suggesting a paracrine angiogenic role for BMDCs.
In this study, transplantation of BM cells from MMP-9 KO mice into WT recipient mice and vice versa was conducted to study the effects of BM-derived MMP-9 on VEGF-induced focal brain angiogenesis. Brain angiogenesis indicated by microvessel density decreased in VEGF-treated WT+KO BM mice compared to WT+WT BM mice, accompanied by a decrease in MMP-9 level to 35% of that in WT +WT BM mice; the impaired angiogenesis in MMP-9 KO mice was partially rescued by transplantation of WT BM. A near-to-normal level of MMP-9 activity was also detected in the brain of MMP-9 KO mice transplanted with WT BM. These results suggest that BM-derived MMP-9 is crucial for cerebral angiogenesis, and that BMDCs are an important source of MMP-9 in the VEGF-induced angiogenic foci. MMP-9 provided by BMDCs promotes local angiogenesis by degrading the extracellular matrix (ECM), resulting in capillary branching. It also cleaves membrane-bound cytokines17 such as VEGF, which, in turn, promotes angiogenesis.18 Moreover, BMDCs themselves express VEGF and other angiogenic factors.19 MMP-9, the major player, delivered by recruited BMDCs along with other BMDC-derived proangiogenic factors, modulates proliferation of local endothelial cells.
Interestingly, microvessel density slightly increased in the brain of VEGF-treated WT mice transplanted with MMP-9 KO BM mice, and transplantation of WT BM partially rescued the impaired angiogenesis in MMP-9 KO mice. Our results suggest that although BMDCs are the major participant in VEGF-induced focal brain angiogenesis, resident, non-BM cells of the recipient brain may compensate partly for the lack of BMDC-derived MMP-9. The statistical analysis confirms that both the somatic genotype and BM genotype influenced the microvessel density in the VEGF-treated brain.
A limitation of our study is that we did not clarify the contribution of various resident cell types in the target brain tissue. We did observe a weak band of MMP-9 in the brain of WT mice transplanted with MMP-9 KO BM (Figure 5A), which is probably derived from other resident cells in the brain such as neurons, astrocytes, or endothelia. Another limitation of our study is that we did not clarify how bone marrow and the brain communicate, although this phenomenon has been observed by other investigators.20 The possibility exists that the signal from the brain to the bone marrow derives from circulating VEGF. Heissig et al have shown that plasma VEGF level increased after Ad-VEGF injection (iv).8 We believe that an increase of plasma VEGF was present in our mice, which will be addressed in our future studies.
The important point that our study makes is that a biologically significant fraction of MMP9 activity arrives at an angiogenic focus from the extracranial BM site. Not only is this important for understanding the precise mechanisms and procedural considerations for a focal angiogenic response, but it opens up a potential line of inquiry to target this blood-borne source of protease activity for eventual new therapeutic approaches for angiogenic-related neurological disorders.
Summary
In this study, we showed that BM-derived MMP-9 plays a key role in VEGF-induced brain angiogenesis, including BM cell mobilization and BMDC recruitment to the angiogenic foci. BMDCs contribute to focal angiogenesis by supplying MMP-9. Our study suggests that modifying MMP-9 activity of BMDCs may be a therapeutic strategy for the treatment of angiogenic-related disorders, such as brain AVM and brain tumor.
Supplementary Material
Acknowledgments
The authors thank Charles E. McCulloch, PhD, for assistance with statistical analysis; Voltaire Gungab for assistance with manuscript preparation; and the other members of the UCSF BAVM Study Project (http://avm.ucsf.edu.) for their support.
Sources of Funding
This study was supported in part by grants from the National Institutes of Health: R01 NS27713 (W.L.Y.) and P01 NS44155 (W.L.Y., H.S.).
Footnotes
Disclosures
None.
References
- 1.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, Declerck YA. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, Hashimoto T, Yang GY, Young WL. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62:1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhs BE, Gagne P, Plitas G, Shaw JP, Shamamian P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and -9 activity: a potential mechanism for ischemia induced MMP activation. J Surg Res. 2004;117:249–254. doi: 10.1016/j.jss.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 7.Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, Lee CZ, Young WL, Yang GY. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90:570–576. doi: 10.1160/TH03-03-0188. [DOI] [PubMed] [Google Scholar]
- 11.Beck H, Voswinckel R, Wagner S, Ziegelhoeffer T, Heil M, Helisch A, Schaper W, Acker T, Hatzopoulos AK, Plate KH. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J Cereb Blood Flow Metab. 2003;23:709–717. doi: 10.1097/01.WCB.0000065940.18332.8D. [DOI] [PubMed] [Google Scholar]
- 12.Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 14.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 15.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–3554. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 16.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 18.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41:524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.