Abstract
Adiposity is associated with chronic low-grade systemic inflammation and increased inflammation in the hypothalamus, a key structure in feeding behavior. It remains unknown whether inflammation impacts other brain structures that regulate feeding behavior. We studied 44 overweight/obese and 19 lean individuals with MRI and plasma fibrinogen levels (marker of inflammation). We performed MRI-based segmentations of the medial and lateral orbitofrotal cortex (OFC) and hippocampal volumes. Gray matter (GM) volumes were adjusted for head size variability. We conducted logistic and hierarchical regressions to assess the association between fibrinogen levels and brain volumetric data. Using diffusion tensor imaging (DTI), we created apparent diffusion coefficient (ADC) maps and conducted voxelwise correlational analyses. Fibrinogen concentrations were higher among the overweight/obese (t[61]=-2.33, P=0.023). Lateral OFC associated together with fibrinogen correctly classified those with excess of weight (accuracy=76.2%, sensitivity=95.5%, specificity=31.6%). The lateral OFC volumes of overweight/obese were negatively associated with fibrinogen (r=-0.37, P=0.016) and after accounting for age, hypertension, waist/hip ratio and lipid and sugar levels, fibrinogen significantly explained an additional 9% of the variance in the lateral OFC volume (β=-0.348, ΔR2=0.093, ΔF P=0.046). Among overweight/obese the associations between GM ADC and fibrinogen were significantly positive (P<0.001) in the left and right amygdala and the right parietal region. Among lean individuals these associations were negative and located in the left prefrontal, the right parietal and the left occipital lobes. This is the first study to report that adiposity-related inflammation may reduce the integrity of some of the brain structures involved in reward and feeding behaviors.
Keywords: gray matter integrity, apparent diffusion coefficient (ADC), obesity, inflammation, fibrinogen, feeding behavior
1. Introduction
Worldwide, a billion adults are overweight and at least 300 million are obese (World health organization, 2010). The prevalence of overweight people has increased dramatically in the US adult population and obesity-related diseases reached epidemic proportions in the last decade (Flegal et al., 2010). Excess body fat increases the risk of cardiovascular disease, hypertension, fatty liver, type 2 diabetes, and may contribute to a decline in cognitive abilities, and dementia (Ikeoka et al., 2010; Rizvi et al., 2010). Due to the meteoric increase in overweight and obese individuals, it is increasingly important to elucidate the cause and effects of excessive adipose deposition. All of the pathological conditions associated with excess weight may be related to adiposity-induced inflammation as obesity is characterized by a state of chronic low grade inflammation (Vachharajani and Granger, 2009). The adipose tissue of obese individuals is comprised of hypertrophied fat cells and infiltrating macrophages and lymphocytes. These immune cells secrete several pro-inflammatory cytokines and chemokines that may block insulin signaling and recruit other macrophages, which may propagate the low-grade inflammation and lead to further insulin resistance (Olefsky and Glass, 2010). High-fat diet has been found in experimental animal models to induce an inflammatory response in the hypothalamic areas that control feeding behavior and energy homeostasis by regulating downstream neurons (Velloso, 2009). Hypothalamic inflammation may result in the breakdown of the circuitry that maintains balance between energy intake and energy expenditure. Therefore, chronic low grade inflammation, in conjunction with a high calorie diet, is a possible mechanism that may contribute to the diseases associated with obesity (Hirai et al., 2010).
Several previous studies (Gunstad et al., 2008; Soreca et al., 2009; Ward et al., 2005) report that elevations in BMI are associated with decreased volume in the frontal lobes and hippocampi (Raji et al., 2010), as well as reductions in GM density in several regions involved in the taste and reward pathways (Pannacciulli et al., 2006). Additionally, Tataranni et al. (1999) suggest key roles in motivational feeding behavior for the paralimbic and limbic areas, such as the orbitofrontal cortex and the hippocampal formation. Recent rodent and human studies also demonstrate that peripheral inflammation is associated with reductions in hippocampal gray matter volume (Marsland et al., 2008). Based on these observations, we hypothesized that the low grade inflammation present among overweight and obese individuals may be associated with structural/functional alterations to the orbitofrontal, limbic, and paralimbic brain structures that regulate feeding behavior. We used fibrinogen, a pleiotropic glycoprotein, as our marker of inflammation as it is associated with neuroinflammation (Ryu et al., 2009) and predicts weight gain in middle-aged adults (Duncan et al., 2000).
To test our hypotheses we utilized Diffusion Tensor Imaging (DTI) to generate brain apparent water diffusion coefficient (ADC) maps for lean and overweight groups. After the ADC maps were created we utilized a voxelwise correlational analysis, which is a fully automated whole-brain unbiased method, to ascertain regionally specific associations between fibrinogen and ADC value for each of the two groups. In addition, by utilizing structural images and operator-determined volumes, or using FreeSurfer, we derived the gray matter (GM) volume of medial and lateral OFC and hippocampus, structures that are involved in the circuitry of behavioral feeding control. We assessed group differences and their associations to inflammation in these specific regions of interest.
2. Results
The participants' demographic and endocrine characteristics are summarized in Table 1. The groups were matched on gender, age, years of education, blood pressure (BP), hypertension, cholesterol, triglycerides, periventricular WMH and deep WMH. As expected, relative to lean subjects, overweight and obese individuals had significantly greater BMI (t[58]=-9.69, P<0.0001), as well as, larger waist/hip ratios and higher fibrinogen concentrations (t[61]=-2.33, P=0.023).
Table 1. Demographic and biological data.
| Lean | Overweight & Obese | T-Test | Effect size | |
|---|---|---|---|---|
| n=19 | n=44 | P | d | |
| Male gender * | 42% | 52% | 0.459 | 0.2 |
| Age (years) | 57.6±6.7 | 58.7±7.7 | 0.605 | 0.14 |
| Education (years) | 16.0±2.7 | 15.5±2.2 | 0.42 | 0.22 |
| BMI (kg/m2) | 21.7±1.9 | 31.4±5.9 | <0.0001 a | 1.92 |
| Waist/Hip ratio | 0.94±0.08 | 0.99±0.06 | 0.003 a | 0.99 |
| Fibrinogen (mg/dl) | 321±48.8 | 371±86.6 | 0.023 a | 0.64 |
| Hypertension* | 42% | 66% | 0.078 | 0.49 |
| Systolic BP (mmHg) | 114±16 | 123±19 | 0.84 | 0.48 |
| Diastolic BP (mmHg) | 69±20 | 75±10 | 0.97 | 0.47 |
| Glucose (mmol/l) | 82±18 | 118±51 | <0.0001 a | 0.81 |
| Cholesterol | 188±33 | 178±41 | 0.364 | 0.25 |
| Triglycerides (mg/dl) | 98±42 | 125±90 | 0.228 | 0.33 |
| HbA1 C (%) | 5.83±1.5 | 6.92±1.80 | 0.021 a | 0.65 |
| Insulin (pmol/l) | 6.0±4.6 | 13.8±10.5 | <0.0001 a | 0.85 |
| Periventricular WMH | 0.40±0.89 | 0.70±0.70 | 0.40 | 0.39 |
| Deep WMH | 1.0±0.71 | 0.67±0.66 | 0.31 | 0.49 |
Data are given as mean ± standard deviation except
data in percentage
Pearson Chi-Square test for data in percentage
Significant T-Test (P value<0.05)
2.1. Association between Gray Matter Volume and Fibrinogen
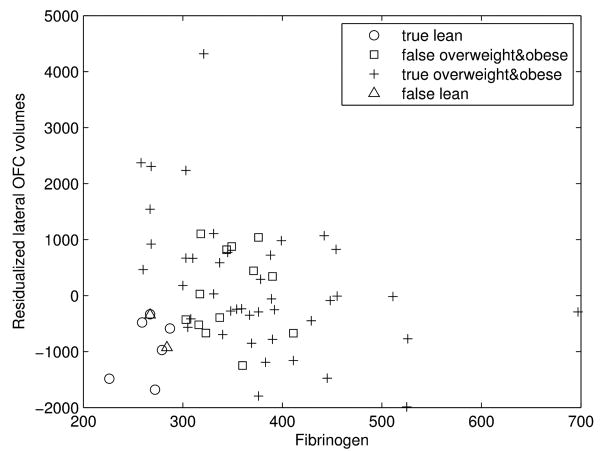
Binary logistic regressions revealed that fibrinogen was associated with the residualized lateral OFC volumes significantly classifies subjects with an excess of weight (76.2% of accuracy with a P value < 0.05). However, the association of fibrinogen and the residualized hippocampal volumes (61,9% of accuracy with P value >0.1), as well as the residualized medial OFC volumes (66,7% of accuracy with P value >0.08), did not significantly predict a weight excess (see Table 2). The model involving fibrinogen and residualized lateral OFC is quite powerful at predicting overweight and obese individuals, with a sensitivity of 95.5% (see Fig. 1), but fails to predict lean individuals (specificity of 31.6%).
Table 2. Binary logistic regression.
| Hippocampus | Medial OFC | Lateral OFC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | Std. Er. | z | P | Coef. | Std. Er. | z | P | Coef. | Std. Er. | z | P | |
| Intercept | -2.7215 | 1.7322 | -1.57 | 0.116 | -3.0220 | 1.7452 | -1.73 | 0.083 | -3.6656 | 1.8199 | -2.01 | 0.044 |
| Fibrinogen | 0.0105 | 0.0051 | 2.04 | 0.041 | 0.0113 | 0.0052 | 2.18 | 0.029 | 0.0132 | 0.0054 | 2.45 | 0.014 |
| Volumes* | -1.0451 | 1.2332 | -0.85 | 0.397 | -0.0003 | 0.0003 | -0.80 | 0.424 | 0.0006 | 0.0003 | 1.79 | 0.073 |
Residualized volumes
Fig. 1. Prediction of the BMI by the association of inflammation and intracranial vault-adjusted brain region.
Each group represents the results of the classification by the logistic regression. The true lean are the lean individuals that are predicted as lean, the false overweight and obese are the lean people that are predicted as overweight and obese, the true overweight and obese are the overweight and obese individuals that are predicated as overweight and obeses and the false lean are the overweight and obese people that are predicted as lean.
Given the sensitivity of the association between firbrinogen and residualized lateral OFC volumes to predict excess of weight, we wanted to ascertain whether fibrinogen predicted the lateral OFC volumes of the overweight and obese individuals. Correlational analyses, accounting for age and hypertension, showed that fibrinogen was significantly associated with the residualized-FreeSurfer-derived lateral OFC volume (r=-0.37, P=0.016) in the overweight and obese group but was only a statistical trend among lean participants (r=0.47, P=0.056).
In order to ascertain the unique contribution of fibrinogen in explaining lateral OFC we ran hierarchical regression analyses among overweight and obese individuals and first controlled for factors that could also affect those volumes. In these analyses the normally distributed lateral OFC volumes were treated as the dependent variable to determine how much of their variance was explained by fibrinogen. After accounting for age and hypertension (ΔR2=0.064, ΔF P=0.285), waist/hip ratio (ΔR2=0.024, ΔF P=0.330), and lipid and sugar levels (ΔR2=0.104, ΔF P=0.242), fibrinogen explained an additional and significant 9% of the variance (β=-0.348, ΔR2=0.093, ΔF P=0.046) of the lateral OFC volume.
2.2. Association between GM ADC and Fibrinogen
The voxelwise correlational analyses yielded different results for each of the two groups. Among lean individuals, and after adjusting for age and hypertension, we indentified a total of 3 significant (P< 0.001) cerebral clusters with an inverse correlation between GM ADC and fibrinogen. The largest cluster was located in the left prefrontal cortex and the other two were in the right parietal and left occipital lobes (see Table 3 and Fig.2). In contrast, among overweight and obese individuals, after correcting for age and hypertension, we found 3 clusters of positive associations (P<0.001) between GM ADC and fibrinogen. These 3 significant clusters were located in the left and right amygdala, and the right parietal lobe (see Table 4 and Fig. 3).
Table 3. Significant clusters of correlation between GM ADC and fibrinogen among lean individuals (P<0.001).
| *Talairach Coordinates | Size (#voxels) |
|||
|---|---|---|---|---|
| x | y | z | ||
| Left prefrontal | 56 | 8 | -3 | 146 |
| Right parietal | -30 | 97 | 12 | 145 |
| Left occipital | 30 | 113 | -19 | 132 |
Represents the centroid of the cluster
Fig. 2. Brain regions showing clusters of association between GM ADC and fibrinogen among lean individuals.
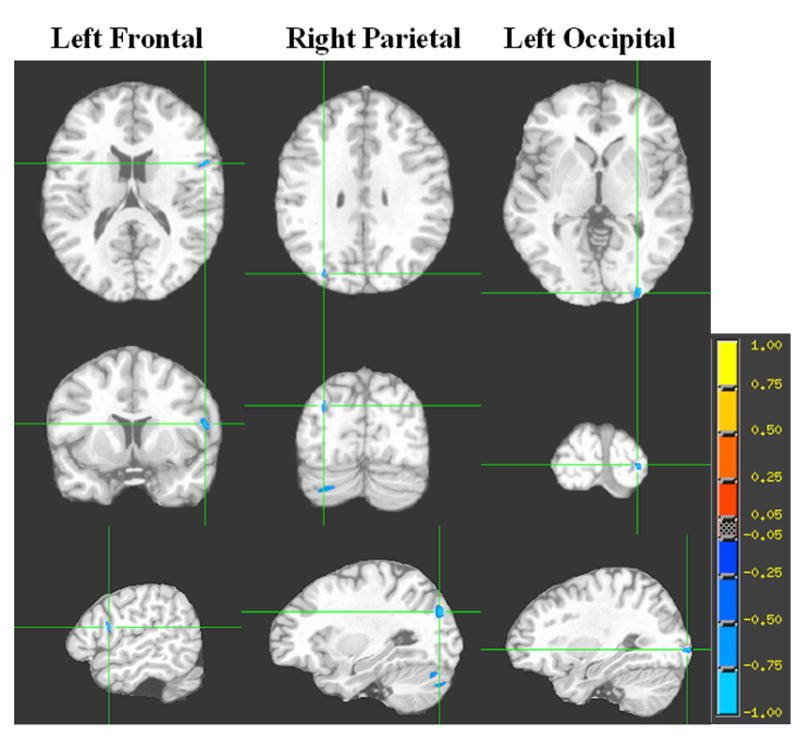
Each column represents the 3 orthogonal orientations (axial, coronal, sagittal) for the significant inverse correlation clusters (analysis controlling for age; minimum cluster size 100 voxels; p<0.001) overlaid on the T1 target image. The color bar represents the strength of the correlation.
Table 4. Significant clusters of correlation between GM ADC and fibrinogen among overweight individuals (P<0.001).
| *Talairach Coordinates | Size (#voxels) |
|||
|---|---|---|---|---|
| x | y | z | ||
| Left amygdala | 24 | 23 | -37 | 182 |
| Right amygdala | -23 | 24 | -37 | 155 |
| Right parietal | -52 | 54 | 19 | 124 |
Represents the centroid of the cluster
Fig. 3. Brain regions showing clusters of association between GM ADC and fibrinogen among overweight and obese individuals.
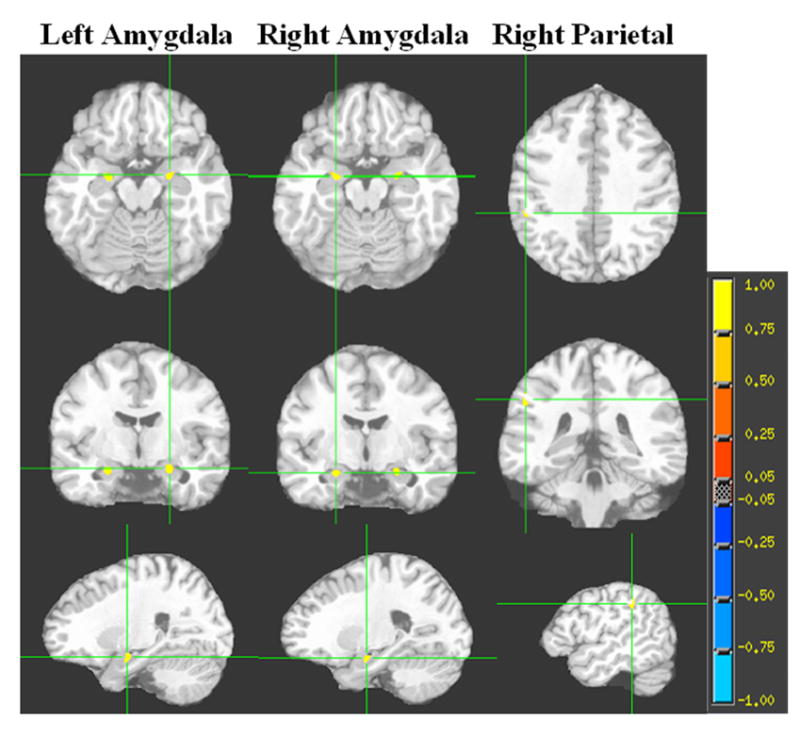
Each column represents the 3 orthogonal orientations (axial, coronal, sagittal) for the significant positive correlation clusters (analysis controlling for age; minimum cluster size 100 voxels; p<0.001) overlaid on the T1 target image. The color bar represents the strength of the correlation.
3. Discussion
This study is the first to report an association between excess weight-associated inflammation and changes in some of the structures of the brain network thought to be involved in reward and eating behaviors. We demonstrated that among overweight and obese individuals, but not their lean counterparts, and after controlling for age, hypertension, waist/hip ratio and lipid and sugar levels, increased levels of fibrinogen were related to smaller lateral orbitofrontal volumes. Furthermore, we found a different anatomic pattern in the relationship between fibrinogen levels and microstructural integrity of GM regions (independent of age and hypertension) for the lean and overweight/obese individuals. Among lean individuals, higher fibrinogen levels were associated with lower ADC (less interstitial fluid) in left prefrontal, right parietal and left occipital regions. However, among individuals with excess weight, elevations in fibrinogen concentration were associated with increased ADC (greater interstitial fluid) in both amygdala and right parietal cortex. We did not expect to find an anatomical overlap in the areas detected by the different image analysis methods given the very different resolution and precision of the voxelwise and volumetric methods. To the best of our knowledge, this is the first report demonstrating that individuals with excess weight had reductions in microstructural integrity in some of the GM regions involved in eating behavior.
We provided evidence that the fibrinogen concentrations differed significantly between lean subjects and individuals carrying excess weight. This is consistent with many previous studies that describe obesity as a state of chronic low grade inflammation. In obesity there is an infiltration of the adipose tissue by macrophages, leading to increases in inflammatory cytokine production (Vachharajani and Granger, 2009). Although the source of inflammation in obesity is known, the direction of causality of these associations requires further investigation. For instance, it is still unclear whether excess weight (increased adiposity) leads to inflammation in brain and peripheral tissues, or whether genetic factors give rise to hypothalamic inflammation, which in turn leads to loss of control of eating behaviors and obesity (Wisse and Schwartz, 2009). However, a series of experimental studies proves that fat-rich diet induces an inflammatory status in the hypothalamus and trigger the resistance to anorexigenic signals (De Souza et al., 2005) (Milanski et al., 2009) and inflammation, in combination with obesity, may result in the dysregulation of insulin signaling (Uysal et al., 1997). Furthermore, impairment in signaling can have deleterious effects on neurons in the hypothalamus leading to disruptions in normal eating behavior (Levin et al., 2004) (Sherwin, 2008). Additionally, inflammation can directly damage brain tissue by impairing blood vessel integrity leading to an increase in inflammatory cells in the cerebrospinal fluid and perivascular spaces in the brain (Man et al., 2007).
The goal of this study was to ascertain whether excess weight and its associated inflammation affect some of the limbic and paralimbic structures, structures involved in feeding behavior. We determined that the association of fibrinogen and the lateral OFC volumes was a good model to predict an excess of weight. However, the fibrinogen and medial OFC volume and hippocampal volume were not strong predictor of excess weight. Moreover, there was an association between increased inflammation and reductions in the volume of the lateral OFC. The lateral OFC plays a key role in the reward system by integrating information regarding reward outcome, and damage to this area may result in impaired decision making (Wallis, 2007). It is important to note that the OFC neurons receive gustatory and olfactory stimuli and respond to specific tastes and odors. In fact, the OFC contains the major cortical representation of tastes including sweet, salty, bitter and sour. These taste representations can act as positive or negative reinforcers for the reward system (Rolls, 2000); therefore, the ability of the OFC to code rewards is likely to impact food selection (Zald, 2009). Support for the role of the OFC in food selection comes from an fMRI study reporting differential activation of this region during imagined intake of palatable versus unpalatable food (Stice et al., 2010). It is possible that inflammation, by affecting the integrity of the OFC, impacts the reward system, and thus loosens the control on feeding behavior resulting in weight gain.
Contrary to our expectations, we found that age and hypertension did not significantly account for much variance in the orbitofrontal volume, which is in contrast with previous positive associations reported by Raz et al. (Raz et al., 1997). They found a substantial age-related decline in the volume of the prefrontal gray matter in 148 healthy volunteers ranging in age from 18 to 77 years. Our narrower age-range and smaller sample size may be the reason why we did not observe an association.
Our DTI correlational analyses detected increased water diffusivity (an indication of increased water in GM possibly indicating loss of microstructural integrity) associated with increased fibrinogen concentration in the brains of overweight and obese individuals after accounting for age and hypertension. Increases in GM diffusion may be considered an increase of tissue water movement representing very subtle “atrophy” of the tissue. The mechanism(s) underlying this increase in diffusivity still needs to be clarified. ADC represents an average value of the diffusivity inside a voxel and we cannot determine if the ADC mainly reflects intracellular or extracellular diffusion. What is clear is that GM ADC, which increases in patients with multiple sclerosis (Sijens et al., 2006) and patients with chronic liver disease (Lodi et al., 2004), reveals an increase in brain tissue water. ADC is, therefore, associated with tissue degeneration in chronic pathological states and higher ADC values may be interpreted as a reduction in the ordered anisotropic tissue structure. This suggests a loss of cell membrane integrity leading to higher net displacement of water molecules (Sotak, 2004). Specific cells in the brain, such as microglia, are particularly susceptible to increases in inflammation and loss of membrane integrity. Once they undergo changes in morphology, microglia increase their production of cytokines and chemokines leading to more inflammation (Man et al., 2007).
One of the most striking findings of this study was the strong positive correlation between inflammation and ADC in both the right and left amygdala among overweight and obese individuals. Both animal and human studies highlight key roles for the amygdala in feeding behavior and the reward system. Specifically, the amygdala is involved in the motivational control of appetite (Grundmann et al, 2005). Lesion studies in rats show that the amygdala and hypothalamus are part of the same pathway that regulates weight balance and feeding behavior (Hinton et al., 2004), and the activation of the amygdala by stress may be reduced by consuming high caloric foods (Dallman et al., 2003). In addition, the amygdala plays a role in food preference and selection; studies in primates demonstrate that animals with amygdala lesions are less discriminating and increase their selection of food that normal animals refuse. These data suggest that the amygdala performs a role in avoidance of unpalatable foods (Machado and Bachevalier, 2007). Moreover, disconnecting the OFC from the amygdala in primates demonstrates a direct functional interaction between these two structures and suggests that they compose a neural system controlling adaptive response selection and decision making (Baxter et al., 2000). Furthermore, human data utilizing positron emission tomography provides evidence for the dissociable contribution of the amygdala and OFC in motivation and decision making (Arana et al., 2003). The amygdala is activated by high incentive information regardless of whether a choice is required, whereas, the medial OFC and the lateral OFC are both recruited during incentive judgment and goal selection.
Our results in overweight and obese individuals (but not lean) showed smaller OFC volumes and increased ADC in the amygdala was associated with elevations in fibrinogen level, which may suggest structural and functional impairments in the circuitry controlling feeding behavior. The negative correlation between ADC and fibrinogen in some prefrontal, parietal and occipital brain regions of the lean group may be explained by the varying biological functions of fibrinogen depending on its levels. For instance, a high level of fibrinogen may be indicative of an inflammatory process, but at lower concentrations fibrinogen in the blood contributes to cell proliferation, adhesion and migration and even myelination (Adams et al., 2004). In addition to the amygdala, we also found a positive correlation between fibrinogen and parietal and occipital areas in the overweight and obese participants. We postulate that these additional regions could be related to the chronic state of inflammation, which might impair the blood brain barrier more broadly, and that these were the regions that crossed statistical significance.
A significant strength of this report is that we used well-established automatic and unbiased methods for quantifying brain volumes. Further strengthening this report, we used operator determined volumes for the hippocampus, a structure with poor contrast differentiation from other adjoining gray matter regions such as the amygdala and the entorhinal cortex and for which automated methods such as FreeSurfer are not valid (Morey et al., 2009a, 2009b). Another strength of this report is that our participants were well matched on years of education, which is highly associated with socioeconomic status, thus removing this as a possible confound. Fibrinogen is an appropriate marker of neuroinflammation but we plan to assess other markers of systemic inflammation in future studies.
One of the potential weaknesses of this study is that even though sophisticated techniques were used to correct for the spatial distortions that occur in the echo-planar acquisition of the DTI data, we cannot completely rule out that these distortions did not influence our amygdala findings. However, this is highly unlikely given that both hemispheres were affected. Although direct measurement of the hypothalamus would be of great interest in a study such as this, currently there are no reliable and/or valid methods for obtaining hypothalamic volumes or even for the placement of regions of interest in this area on MRI images in humans. Better measurement methods need to be developed before this key structure is included in future studies.
Our study further affirms the suggestion that excess weight should be regarded as a disease that may have anatomical and physiological cerebral abnormalities associated with the phenotype.
4. Conclusion
This is the first report utilizing DTI-based MRI assessments of water diffusivity as well as structural volume measurements to ascertain the associations between obesity-mediated inflammation and abnormalities in brain structure involved in the control of feeding behavior. This report provides evidence for a connection between inflammation, amygdala integrity and the volume of lateral OFC.
5. Experimental Procedure
5.1. Participants
Sixty-three middle aged and elderly volunteers were recruited as part of a study of normal aging and metabolic dysregulation associated with obesity. The protocol was approved by the Institutional Research Board of the NYU School of Medicine. All participants signed written informed consent and were compensated for their time and inconvenience. Subjects were screened to rule out medical (other than hypertension, dyslipidemia, insulin resistance or type-2 diabetes) and psychiatric (such as alcohol or other substances abuse and depression) conditions. Significant head trauma, stroke, hydrocephalus, lacunar infarcts, seizures, mental retardation, or any neurological disorder excluded subjects from participation. All subjects were screened for cerebrovascular disease by inspection of white matter hyperintensity (WMH): score above 2 WHMs on the modified Fazekas Scale. A comprehensive panel of blood tests was performed after a 10-12 hour overnight fast for the assessment of blood count, liver and lipid profiles, thyroid function, blood chemistries, glucose and insulin values.
Plasma fibrinogen concentration was measured by the prothrombin-time derived method with reference to the Clauss fibrinogen assay using ACL TOP 500 CTS coagulation analyzer with closed tube sampling (Instrumentation Laboratory, Beckman Coulter Inc.).
Body mass index (BMI), computed as the weight in kilograms divided by height in meters squared, was used to identify overweight and obese individuals. Another measure of adiposity, waist/hip ratio, was used to account for differences in muscle mass. Participants were considered lean if they had a BMI between 18.0 and 24.9 kg/m2, those with BMI≥25 kg/m2 were in the overweight/obese category. We had nineteen lean and forty four overweight and obese individuals.
Subjects were considered hypertensive if they received an antihypertensive medication or had a systolic blood pressure of 140mmHg or higher, or a diastolic blood pressure of 90 mmHg or more.
5.2. Magnetic Resonance Imaging
5.2.1. MRI acquisition
Magnetic resonance imaging was performed utilizing a 1.5 T Siemens Avanto MRI System. A set of structural T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (TR 1300 ms, TE 4.38 ms; TI 800 ms; NEX 1; FOV 250×250; 196 coronal slices; slice thickness 1.2 mm; Flip angle 15°) was collected. A fast fluid-attenuated inversion recovery (FLAIR; TR 9000 ms; TE 97 ms; FOV 210×210; 1 average and 2 concatenations; Flip angle 145°) was used to quantify WMHs. The DTI echo planar sequence (TR 6100 ms; TE 75 ms; delay in TR=0; b values 0, 1000; FOV 210×210; 4 average and 1 concatenation; 50 axial slices; voxel size 1.64×1.64×3 mm3) was obtained in 6 directions. The sequence was standardized at a scan angle parallel to a plane drawn through the inferior aspect of the occipital lobe and frontal lobes on the mid-sagittal plane. To correct the spatial distortion inherent in the echo planar DTI image acquisition, a T2-weighted sequence (TR 9000 ms; TE 94 ms; TI 2000ms; FOV 210×210; 50 slices; slice thickness 3 mm) was also acquired. The T2-weighted and DTI images were collected in the same orientation, thickness and number of slices in order to optimize their coregistration.
5.2.2. DTI voxelwise image processing and correlational analyses
Images were processed using an automated toolbox ART2 (Automated Registration Toolkit 2) as described previously (Yau et al., 2009). Briefly, individual structural native space images were first manually skull-stripped and normalized to Talairach space in order to get a 3D warp field containing the MPRAGE to target transformation parameters. Next, we applied a rigid body registration of the T2-weighted volume to the MPRAGE volume to generate the T2-weighted to MPRAGE transformation matrix. Third, the native b0 image was iteratively warped with T2 as anatomical grid to correct for spatial distortion based on the skull stripped image which yields the 2D warp field deformation information. The ADC maps were generated from the native DTI scans. We then combined the transformation parameters, the transformation matrix and the deformation field in a single transformation to correct for distortions and to spatially normalize the ADC maps. As a result ADC maps end up with a voxel size of 1×1×1 mm3. A GM mask created from the average MPRAGE image of all the subjects was applied to restrain the ADC within the GM region (GM ADC) and to exclude white matter or cerebrospinal fluid voxels which could confound the correlations.
5.2.3. Gray matter volumetric data
Cortical reconstruction and volumetric segmentation of the MPRAGE images were performed by FreeSurfer image analysis suite, version 4.0.2, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). Briefly, the procedure includes removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical white matter and construction of a model boundary of the cortical gray and white matter. Once the cortical models were complete, we computed the parcellation of the cerebral cortex into units based on gyral and sulcal structures using the Desikan parcellation scheme (Desikan et al., 2006). The final stage computed the volumetric labeling of two of our hypothesized regions of interest for our hypotheses, namely the lateral and medial OFC.
The hippocampus volume and the superior temporal gyrus, which serves as a control region for the hippocampus, were manually outlined on coronal image as described in (Gold et al., 2007) using the Multimodal Image Data Analysis System (MIDAS).
The MPRAGE scans were also used to determine the intracranial vault size, which is obtained by manually outlining the supratentorial compartment. This was done by underlining the margins of the dura and the tentorium on the sagital images as described in detail elsewhere (Gold et al., 2007). To account for individual variability in brain size, both the operator-derived and the FreeSurfer estimates of the GM volumes were residualized to intracranial volume. All the operator-derived volumes were conducted blind to identity and BMI of participants.
5.3. Statistical Analysis
We checked the normality of our data using the Shapiro-Wilk test. We performed two-tailed independent sample t-tests and chi-square tests to examine the group differences in demographic, endocrine and MRI brain volumetric data. The effect sizes are reported as Cohen'd coefficient. Data were analyzed using SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL). We used Matlab® version 2008a to carry out the binary logistic regressions that predict the BMI, defined as a categorical dependent variable, from the continuous predictor variables, fibrinogen and residualized volumes. Given the association between fibrinogen and residualized volumes, we predicted whether a particular subject belongs to the lean or the overweight and obese groups. Our model was constructed using maximum likelihood estimation by iteratively reweighted least squares and the goodness of the fit was assessed by the likelihood ratio test. Furthermore, using SPSS, we assessed the strength of the significant association using two-tailed partial correlation and accounted for age and hypertension and a hierarchical regression approach and accounted for potential confounders, such as age (step 1), and waist/hip ratio (step 2). In step 3, we controlled for hypertension, lipid levels and blood sugar, all of which may be associated with microvascular disease, especially in an older population. Fibrinogen acted as a surrogate marker for inflammation; therefore, fibrinogen was included as the last step in the regression to demonstrate the additional power of inflammation on structural changes and to determine if obesity-induced inflammation (fibrinogen values) were associated with the integrity of brain areas involved in the control of feeding behavior. To ascertain the possible associations between fibrinogen levels and the GM tissue density measured on the DTI images (ADC), we computed correlations between ADC and fibrinogen using voxelwise correlational analysis (Hoptman et al., 2004). To reduce the risk of the escalation of type 1 error due to multiple comparisons, only cluters of at least 100 contiguous significant voxels (0.1 cc) were defined as a significant. We chose a false discovery rate (FDR) less than 0.01 according to the original Benjamini-Hochberg (1995) procedure. We selected a significance (p-value) threshold of 0.001 to ensure that the FDR would be kept below 0.01. Because of the wide age distribution (aged 42 to 74 years) we controlled for age and hypertension to ensure that the correlations were not driven by either age or hypertension-related changes in brain structure. The correlation map was registered to the standard Montreal Neurological Institute (MNI) T1 MRI template and visualized with AFNI (Analysis of Functional NeuroImages).
Acknowledgments
Financial Disclosure(s): This study was supported by a grant from the National Institutes of Health DK064087 and supported in part by grant1UL1RR029893 from the National Center for Research Resources.
Footnotes
None of the authors have any financial/conflicting interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fanny Cazettes, Email: cazetf01@nuymc.org.
Jessica I. Cohen, Email: cohenj25@nyumc.org.
Po Lai Yau, Email: ply2001@nyu.edu.
Antonio Convit, Email: antonio.convit@med.nyu.edu.
References
- Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4:163–76. doi: 10.1124/mi.4.3.6. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults. Obes Res. 2000;8:279–86. doi: 10.1038/oby.2000.33. [DOI] [PubMed] [Google Scholar]
- Grundmann SJ, Pankey EA, Cook MM, Wood AL, Rollins BL, King BM. Combination unilateral amygdaloid and ventromedial hypothalamic lesions: evidence for a feeding pathway. Am J Physiol Regul Integr Comp Physiol. 2005;288:R702–707. doi: 10.1152/ajpregu.00460.2004. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118:1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20:1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- Hirai S, Takahashi N, Goto T, Lin S, Uemura T, Yu R, Kawada T. Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm. 2010;2010:367838. doi: 10.1155/2010/367838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeoka D, Mader JK, Pieber TR. Adipose tissue, inflammation and cardiovascular disease. Rev Assoc Med Bras. 2010;56:116–121. doi: 10.1590/s0104-42302010000100026. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Lodi R, Tonon C, Stracciari A, Weiger M, Camaggi V, Iotti S, Donati G, Guarino M, Bolondi L, Barbiroli B. Diffusion MRI shows increased water apparent diffusion coefficient in the brains of cirrhotics. Neurology. 2004;62:762–766. doi: 10.1212/01.wnl.0000113796.30989.74. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: the effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland LA, Gianaros JP, Abramowitch MS, Manuck BS, Hariri RA. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JBC, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, Mc Carthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009a;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, Mc Carthy G. Rebuttal to Hasan and Pedraza in comments and controversies: “Improving the reliability of manual and automated methods for hippocampal and amygdala volume measurements”. Neuroimage. 2009b;48:499–500. doi: 10.1016/j.neuroimage.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rizvi AA. Hypertension, Obesity, and Inflammation: The Complex Designs of a Deadly Trio. Metab Syndr Relat Disord. 2010 doi: 10.1089/met.2009.0116. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost. 2009;7(Suppl. 1):151–4. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin RS. Bringing to light the dark side of insulin: a journey across the blood-brain barrier. Diabetes. 2008;57:2259–2268. doi: 10.2337/db08-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijens PE, Irwan R, Potze JH, Mostert JP, De Keyser J, Oudkerk M. Relationships between brain water content and diffusion tensor imaging parameters (apparent diffusion coefficient and fractional anisotropy) in multiple sclerosis. Eur Radiol. 2006;16:898–904. doi: 10.1007/s00330-005-0033-0. [DOI] [PubMed] [Google Scholar]
- Soreca I, Rosano C, Jennings JR, Sheu LK, Kuller LH, Matthews KA, Aizenstein HJ, Gianaros PJ. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–490. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak CH. Nuclear magnetic resonance (NMR) measurement of the apparent diffusion coefficient (ADC) of tissue water and its relationship to cell volume changes in pathological states. Neurochem Int. 2004;45:569–582. doi: 10.1016/j.neuint.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe DA, Pratley ER, Lawson M, Reiman ME, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotmisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso LA. The brain is the conductor: diet-induced inflammation overlapping physiological control of body mass and metabolism. Arq Bras Endocrinol Metabol. 2009;53:151–158. doi: 10.1590/s0004-27302009000200006. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Schwartz MW. Does hypothalamic inflammation cause obesity? Cell Metab. 2009;10:241–242. doi: 10.1016/j.cmet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Yau PL, Javier D, Tsui W, Sweat V, Bruehl H, Borod JC, Convit A. Emotional and neutral declarative memory impairments and associated white matter microstructural abnormalities in adults with type 2 diabetes. Psychiatry Res. 2009;174:223–230. doi: 10.1016/j.pscychresns.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. Orbitofrontal cortex contributions to food selection and decision making. Ann Behav Med. 2009;38 1:S18–24. doi: 10.1007/s12160-009-9117-4. [DOI] [PubMed] [Google Scholar]