Abstract
The purpose of this review is to examine the literature in an attempt to elucidate a biomechanical basis for glaucomatous cupping. In particular, this work focuses on the role of biomechanics in driving connective tissue remodeling in the progression of laminar morphology from a normal state to that of an excavated glaucomatous state. While there are multiple contributing factors to the pathogenesis of glaucoma, we focus on laminar extracellular matrix (ECM) remodeling in glaucoma and the feedback mechanisms and signals that may guide progressive laminar cupping. We review the literature on the potential mechanisms of glaucomatous changes in the laminar ECM at the anatomic, structural, cellular and subcellular levels in the context of the biomechanical paradigm of glaucomatous onset and progression. From this review several conclusions can be drawn. First, extensive remodeling of the lamina cribrosa ECM occurs in primary open angle glaucoma. Second, there is surprisingly little evidence to support acute mechanical damage to the lamina as the principal mechanism of cupping. Third, ONH astrocytes and lamina cribrosa cells can sense their mechanical environment and respond to mechanical stimuli by remodeling the ECM. Fourth, there is evidence suggesting that chronic remodeling of the lamina results in a progressive posterior migration of the laminar insertion into the canal wall, which eventually results in the posterior lamina inserting into the pia mater. Finally, modeling studies suggest that laminar remodeling may be a biomechanical feedback mechanism through which cells modify their environment in an attempt to return to a homeostatic mechanical environment. It is plausible that biomechanics-driven connective tissue remodeling is a mechanism in the progression of laminar morphology from a normal state to that of a cupped, excavated glaucomatous state.
Keywords: glaucoma, optic nerve head, intraocular pressure, lamina cribrosa, biomechanics, remodeling, extracellular matrix
Introduction and Scope
Lowering intraocular pressure (IOP) remains the only proven method of preventing the onset and progression of glaucoma. The role of IOP in the disease, however, remains controversial. This largely arises from the wide spectrum of individual susceptibility to IOP wherein a significant number of patients with normal IOPs develop glaucoma (e.g. normotensive glaucoma), and other individuals with elevated IOP show no signs of the disease. It is therefore important to understand the relationship between glaucomatous optic neuropathy and IOP, an inherently mechanical phenomenon.
One common clinical feature of glaucoma is ONH cupping. This cupping can be described as having two components: prelaminar and laminar (Yang, Downs et al., 2007; Burgoyne and Downs, 2008). Prelaminar cupping of the ONH surface is characterized by progressive loss of the prelaminar neural tissues which serves to increase both the depth and width of the cup, thereby increasing the cup-to-disk ratio. Laminar cupping is connective tissue-based, with the lamina cribrosa progressively moving posteriorly and excavating beneath the anterior scleral canal. In most cases, glaucomatous cupping is a combination of these two components, reflecting both damage to and remodeling of the laminar connective tissues and progressive loss of retinal ganglion cell (RGC) axons.
The purpose of this review is to examine the literature in an attempt to elucidate a biomechanical basis for glaucomatous cupping. In particular, this work will focus on the role of biomechanics in driving cell-mediated connective tissue remodeling in the progression of laminar morphology from a normal state to that of an excavated glaucomatous state (Figure 1) While we acknowledge that there are multiple contributing factors to the pathogenesis of glaucoma (Figure 2) we will focus on laminar extracellular matrix (ECM) remodeling in glaucoma and the feedback mechanisms and signals that may guide progressive laminar cupping. We review the literature on the potential mechanisms of glaucomatous changes in the laminar ECM at the anatomic, structural, cellular and subcellular levels in the context of the biomechanical paradigm of glaucomatous onset and progression.
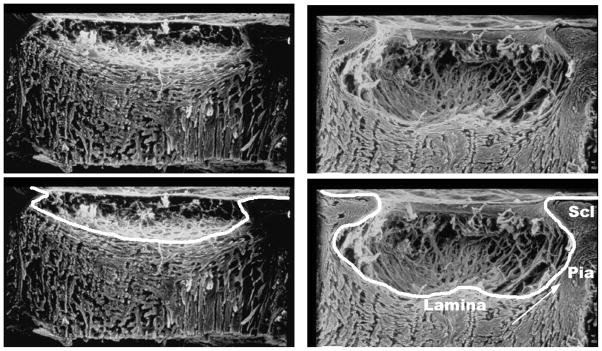
Figure 1.
Scanning electron micrographs of trypsin-digested optic nerve heads from normal (left) and advanced glaucoma (right) human eyes. The lamina cribrosa is cupped and excavated beneath the scleral canal rim in the glaucomatous eye. The connective tissue cups of both eyes are delineated in the lower panels. Note the thick pia mater, the laminar insertion into the pia (arrow), and the difficulty in distinguishing between the lamina and the retrolaminar septa in the glaucomatous eye. Scl, sclera. Images courtesy of Harry A. Quigley, MD.
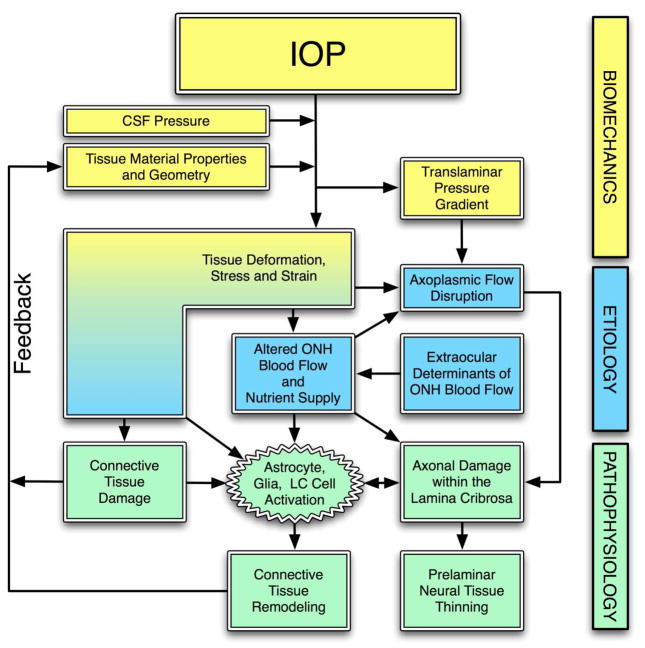
Figure 2.
The biomechanical paradigm of glaucomatous pathophysiology. IOP acts mechanically on the tissues of the eye, producing deformations, strain and stress within the tissues. These deformations depend on the particular tissue geometry and material properties of an individual eye. IOP-induced stress and strain could acutely alter blood flow in the laminar region, and/or delivery of nutrients (secondarily) through chronic alterations in connective tissue. IOP-related stress and strain could also induce alterations in the connective tissues directly (collagen or elastin fiber yield or failure), or indirectly. Indirect effects could include cell-mediated ECM remodeling or non-cell mediated alterations in laminar collagen (Ruberti and Hallab, 2005). These changes in the ONH connective tissues alter their geometries and mechanical responses to loading, which feeds back directly into the mechanical effects of IOP on the ONH. Adapted from Figure 1 in (Sigal, Roberts et al., 2010).
A Biomechanical Perspective of Glaucoma
It is generally accepted that the laminar region of the ONH is the principal site of RGC axonal insult in glaucoma, and therefore a natural site of interest when studying glaucoma (Quigley, 2005). In addition, the region is also interesting from a biomechanical perspective because it is a discontinuity in the corneoscleral shell. Such discontinuities are often considered weak spots in mechanically loaded systems as they can sometimes be the site of substantial stress concentrations.
The biomechanics of the tissues includes both the acute mechanical response of tissues to mechanical load (i.e., how they deform under load) and the longer term changes in morphology, microstructure, and material properties that are driven by the mechanical environment. In a biomechanical paradigm of glaucomatous optic neuropathy, an ONH’s susceptibility to IOP insult is a function of both the acute and long-term response of the constituent tissues to elevated IOP (Burgoyne, Downs et al., 2005). Tissue biomechanics can modulate ischemic, cellular, and other events in the ONH. Eyes with a particular combination of connective tissue geometry and stiffness, blood supply, and cellular reactivity may be more susceptible to damage at normal levels of IOP, whereas others may have a combination of these factors that can withstand prolonged periods of relatively high levels of IOP without clinically significant deleterious effects.
Our group (Burgoyne, Downs et al., 2005; Downs, Roberts et al., 2008; Sigal and Ethier, 2009) and others (Levy and Crapps, 1984; Radius, 1987; Zeimer, 1995; Albon, Purslow et al., 2000; Edwards and Good, 2001; Jonas, Berenshtein et al., 2004; Quigley, 2005; Wells, Garway-Heath et al., 2008; Ren, Wang et al., 2009) have hypothesized that IOP-related deformations cause acute yield and/or failure of the anterior laminar beams, thereby transferring load to adjacent beams in a cascade of damage that results in glaucomatous connective tissue cupping. While this seems plausible, there is surprisingly little evidence to support acute mechanical damage to the lamina as the principal mechanism of cupping. We are not aware of any studies that have reported IOP-induced yield or failure of the laminar beams, although this may be due to the lack of appropriate experimental assessment methods. Several studies have indicated that optic disk hemorrhages is a risk factor for focal glaucomatous progression(Leske, Heijl et al., 2003). These hemorrhages could arise from rupture of prelaminar capillaries or failure of capillary-containing laminar beams, although this association is speculative.
Recent experiments in monkeys have shown that the lamina cribrosa did not deform posteriorly (cup) in response to acute IOP increases (or deformed minimally), while the scleral canal expanded in most eyes (Yang, Downs et al., 2009) (Figure 3). In vivo OCT imaging in the human has yielded similar laminar displacement results, but scleral canal expansion data was not reported (Agoumi, Artes et al., 2009). In a separate study, Poostchi et al showed that optic disc diameter is significantly increased in humans after acute IOP elevations (Poostchi, Wong et al., 2010). Modeling studies have shown that even if the lamina does not displace posteriorly, laminar stress and strain are significantly elevated compared to that at normal IOP (Sigal, Flanagan et al., 2009; Roberts, Liang et al., 2010; Roberts, Sigal et al., In-Press (May 2010)). This arises from the tensile stretch imposed on the lamina by the acutely expanding scleral canal.
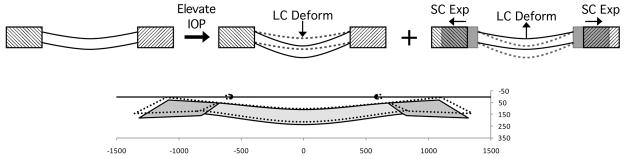
Figure 3.
From a mechanical perspective it is useful to recognize two components of IOP-induced deformation of the lamina cribrosa (top row). One component is the effect of IOP on the anterior laminar surface, which deforms the lamina posteriorly (top middle). Another component is the effect of IOP on the sclera, which causes an expansion of the canal (top right). The deformations are transmitted to the lamina through its insertion into the canal wall, resulting in a lamina that pulls “taut” displacing anteriorly. As IOP increases, both components act simultaneously. The magnitudes of the components of deformation depend on both the material stiffnesses of the lamina and sclera (Sigal, Flanagan et al., 2005; Roberts, Liang et al., 2010; Roberts, Sigal et al., In-Press (May 2010)). Interestingly, models (Roberts, Hart et al., 2007; Sigal, Flanagan et al., 2007; Sigal, Flanagan et al., 2009; Sigal, Yang et al., In revision) and recent experimental evidence in both monkeys (Yang, Downs et al., 2009) and humans (Agoumi, Artes et al., 2009) suggests that often the two components of laminar deformation combine to produce a very small (under 10 um) net anterior-posterior laminar displacement (bottom). The bottom panel shows a schematic representation of the lamina cribrosa and peripapillary sclera of contralateral eyes of a monkey, fixed at low (10 mmHg, solid colors) or high (45 mmHg, dotted lines) IOP (adapted from (Yang, Downs et al., 2009)). It is important to note that even under a very small net anterior-posterior laminar displacement, the IOP-related strains and stresses within the lamina and peripapillary sclera may be substantial.
If elevated IOP does not cause appreciable acute laminar cupping, then it is reasonable to regard the connective tissue deformation and cupping typically seen in human and experimental glaucoma as a chronic phenomenon. Early glaucomatous damage has not been rigorously studied in humans because human cadaver eyes with well-characterized early damage are rare. In monkey eyes exposed to chronically elevated IOP, the lamina cribrosa thickens and cups (Yang, Downs et al., 2007; Roberts, Grau et al., 2009) (Figure 4). This change in laminar morphology has occurred at the onset of confocal scanning laser tomography-detected ONH surface change, the earliest detectable stage of glaucomatous damage in the monkey.
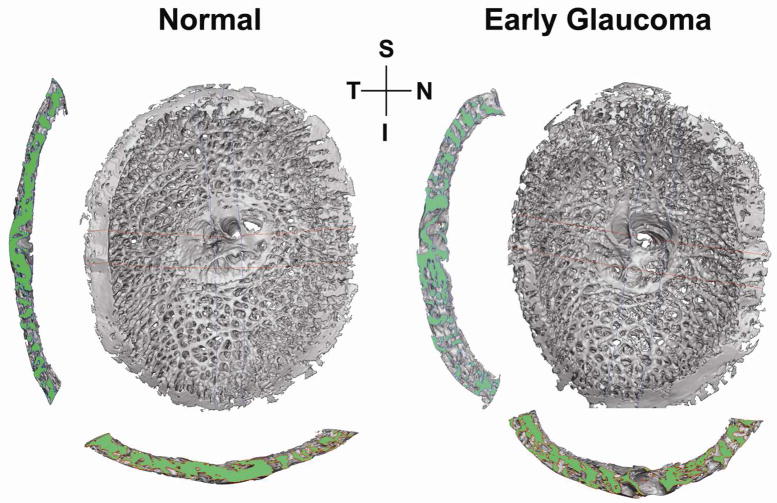
Figure 4.
3D reconstructions of the laminar connective tissues of a monkey, with one eye having early experimental glaucoma. Shown are en face views of the laminar reconstructions, as well as views of the central vertical (left) and horizontal (below) sections. Note the thicker and deeper (cupped) lamina in the early glaucoma eye (Roberts, Grau et al., 2009). Both eyes are shown in OD configuration. S, superior; I, inferior; N, Nasal; T, temporal.
Laminar Connective Tissue Remodeling
Roberts and coworkers have shown that in a monkey model of early experimental glaucoma, the volume of the laminar connective tissues is approximately 80% larger in glaucoma eyes compared to their contralateral controls (Roberts, Grau et al., 2009), but that the relative proportion of connective to neural tissue within the laminar region changed minimally. This study also showed that the early glaucoma eyes had an average of 27% more horizontally-oriented laminar beams through the thickness of the lamina than their contralateral controls. One interpretation of these results is that in the experimental glaucoma eyes, the immediate retrolaminar septa synthesized connective tissue and were essentially recruited into the 3D load-bearing structure of the lamina. These changes may be driven by responses to the altered biomechanical environment (see below), and we propose that the lamina cribrosa be viewed as a portion of the larger 3D glial structure of the ONH (Oyama, Abe et al., 2006) that has synthesized the additional connective tissue components necessary to bear the forces of IOP.
There is also evidence suggesting that remodeling of the lamina results in a progressive posterior movement of the laminar insertion into the canal wall. Such a migration eventually results in the posterior lamina inserting into the pia mater. This has been observed in ostensibly healthy human eyes (Sigal, Flanagan et al., 2010)(Figure 5), and more recently in monkey eyes with experimental glaucoma (Yang, Williams et al., 2010). Careful inspection of the glaucoma eye in Figure 1 suggests that the lamina may partially insert into the pia.
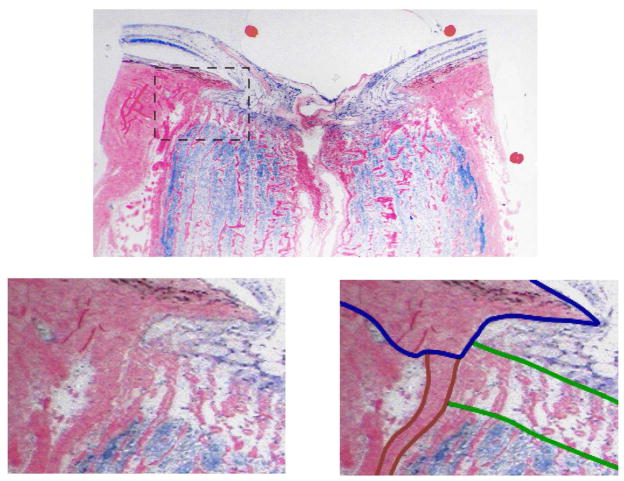
Figure 5.
Example of lamina cribrosa partially inserting into the pia mater (from (Sigal, Flanagan et al., 2010)). Shown is a superior-inferior section of an ostensibly healthy (i.e. not glaucomatous) eye from a 79-year-old male donor fixed at 5 mmHg. The bottom panel is a zoomed view of the rectangle marked in the top panel. The bottom right panel shows outlines of the regions delineated as lamina cribrosa (green), sclera (blue) and pia mater (red). It has been traditionally thought that the lamina inserted solely into the sclera, but a laminar insertion into the pia has now been reported in humans (Sigal, Flanagan et al., 2010) and monkeys (Yang, Williams et al., 2010).
Extensive remodeling of the lamina cribrosa extracellular matrix (ECM) occurs in primary open angle glaucoma. For example, in human eyes with a history of glaucoma, there was a significantly greater percentage of area occupied by elastin in the lamina cribrosa compared with age-matched control eyes (Pena, Netland et al., 1998). Hernandez and colleagues observed loss and fragmentation of elastin fibers at the bottom of the glaucomatous cup and disorganization in the peripheral walls of the cup. They described a thick dense collagenous matrix separating the remnant nerve bundles at the posterior laminar boundary (where the axons become myelinated) that was not present in normal eyes (Hernandez, 1992).
It has been proposed that ONH astrocytes and lamina cribrosa cells play a central role in mediating the laminar ECM remodeling response and the resulting axonal insult (Hernandez, 2000; Morgan, 2000). Cell activity associated with ECM remodeling has been observed in response to glaucoma in humans and exposure to chronically elevated IOP in animal models. Agapova and colleagues showed that matrix metalloproteinases (MMPs) are elevated in the lamina cribrosa of monkeys with experimental glaucoma, but not those with optic nerve transaction(Agapova, Kaufman et al., 2003). These compounds are known to break down the ECM and allow cells to migrate and rebuild the matrix(Hernandez, 2000). This result supports the hypothesis that elevated IOP, and presumably mechanical insult to the laminar cells and/or reduced blood flow in the laminar region, underlie the significant ECM remodeling observed in glaucomatous eyes. Interestingly, these potential mechanisms were both driven by exposure to chronically elevated IOP, a biomechanical insult, and are not simply a secondary effect of axonal damage and death.
Mechanotransduction in the Lamina Cribrosa
Several studies have investigated the mechanisms of cell mechanotransduction in astrocyte and LC cells in vitro (O’Brien, Butt et al., 1997) (Kirwan, Fenerty et al., 2005) (Kirwan, Crean et al., 2004; Rogers, Ackloo et al., 2010). Kirwan and colleagues (Kirwan, Crean et al., 2004) have shown that cyclical mechanical stretch of the substrate on which cells were grown induced significant increases in TGF-β 1 mRNA synthesis after 12 hours and TGF-β 1 protein secretion after 24 hours. Both applied cyclical stretch and exogenously delivered TGF-β 1 significantly increased MMP-2 activity in cell media.
Integrins are proteins that span the laminar astrocyte basement membranes and bind the cell cytoskeleton to the surrounding the ECM. Thus, integrins are particularly well-suited to act as mechanosensory elements in the lamina. Morrison has described the location and alteration of integrin subunits in normal and glaucomatous human and monkey eyes and proposed them as an important link between laminar deformation, IOP-induced cell stretch and damage, laminar connective tissue remodeling and laminar astrocyte mediated axonal insult in glaucoma. In an in vitro study, O’Brien and colleague have shown that hypotonic membrane stress activates stretch activated channels and Ca2+-dependent maxi-K+ channels in LC cells(Irnaten, Barry et al., 2009), which could act as another potential mechanotransduction mechanism in the lamina.
It has been established in other systems that the biologic response of tissues and cells depends strongly on the mode of the strain stimulus (tension, compression or shear), as well as on their magnitudes and temporal profiles (Edwards, Wang et al., 2001; LaPlaca, Cullen et al., 2005; Pedersen and Swartz, 2005). It is therefore of interest to determine which modes of strain and stress the tissues of the ONH are exposed to as IOP is elevated. Note that strains are generally not homogenous (Sigal, Flanagan et al., 2007). When the LC deforms, some regions could be highly strained in different modes, while others remain largely unaffected. This is important because the biological effects on cells are likely to be more dependent on the local levels of strain or stress than on global levels (Tan, Kalapesi et al., 2006; Wang and Thampatty, 2006).
Developing a comprehensive mechanistic understanding of the role of biomechanics in glaucoma using in vitro studies has been hampered by the lack of data on the true stresses and strains that the cells in the lamina experience. This is principally due to the technical challenges of measuring these quantities in vivo. Advances in imaging, such as second harmonic imaging (Brown, Morishige et al., 2007), or deep-scanning SD-OCT (Kagemann, Ishikawa et al., 2008; Agoumi, Artes et al., 2009) may soon enable the reliable measurement of IOP-induced laminar deformation in vivo.
Modeling of Laminar Biomechanics
Numerical modeling has therefore become a common approach to study ONH biomechanics and evaluate hypothetical scenarios (Edwards and Good, 2001; Sigal, Flanagan et al., 2005; Sander, Downs et al., 2006; Downs, Roberts et al., 2009; Grytz and Meschke, 2009; Sigal, 2009; Sigal, Flanagan et al., 2009; Roberts, Liang et al., 2010; Roberts, Sigal et al., In-Press (May 2010)). Figure 6 shows an example of the predictions made with a finite element model of the ONH. Although the magnitude and distribution of the strains depends on the assumed shape and mechanical properties of the tissues, there has been remarkable consistency in the predictions made with generic (Bellezza, Hart et al., 2000; Sigal, Flanagan et al., 2005; Grytz and Meschke, 2009; Sigal, 2009), eye-specific (Sigal, Flanagan et al., 2007; Sigal, Flanagan et al., 2009; Roberts, Liang et al., 2010; Roberts, Sigal et al., In-Press (May 2010)), and analytic (Edwards and Good, 2001; Sander, Downs et al., 2006) models. All models consistently predict regions of relatively large strain in the peripheral lamina.
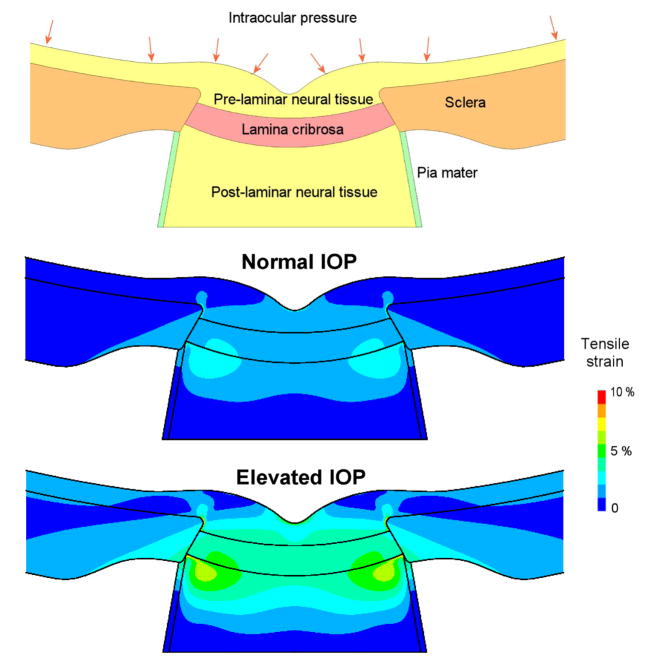
Figure 6.
Generic, axisymmetric finite element model of the ONH with five tissue regions: sclera, lamina cribrosa, pre- and post-laminar neural tissues and the pia mater (top). The model was used to predict the IOP-related strains at normal (12.5 mmHg, middle) and elevated (25 mmHg, bottom) IOP. Note the regions of high strain in the post-laminar neural tissues near the laminar insertion into the sclera, and in the pre-laminar neural tissues near the scleral canal opening.
Models have also been used to study how ONH biomechanics are affected by the changes in the lamina and sclera associated with early experimental glaucoma. Eye-specific models created from 3D reconstructions of monkey ONHs show that for an identical IOP increase, laminar stresses were lower in the early experimental glaucoma eye than in the contralateral normal eye (Roberts, Sigal et al., In-Press (May 2010)). The stress-lowering effects of laminar thickening and cupping have also been reported in sensitivity studies based on axisymmetric generic models of the human ONH (Sigal, Flanagan et al., 2005; Sigal, 2009) and in parameterized models of the monkey eye (Sigal, Yang et al., In revision). When considered together, these studies suggest that connective tissue remodeling may be triggered within an ONH exposed to levels of stress that exceed the physiologic tolerance of the resident cells. This suggests a biomechanical feedback mechanism through which cells modify their local environment in an attempt to return to a homeostatic mechanical environment. Note that this cascade of events could occur at IOPs in the normal range in eyes that are particularly susceptible to IOP-related stress, strain, ischemia, or cellular activation because of their individual geometric, material, vascular, and other properties.
Conclusion
When viewed in the context of ONH biomechanics and the glaucomatous changes in the laminar ECM at the anatomic, structural, cellular and subcellular levels, it seems plausible that connective tissue remodeling is a mechanism in the progression of laminar morphology from a normal state to that of a cupped, excavated glaucomatous state (Figure 7). This remodeling is adaptive, at least for the load-bearing connective tissue, although it may not prevent irreparable harm to the retinal ganglion cells.
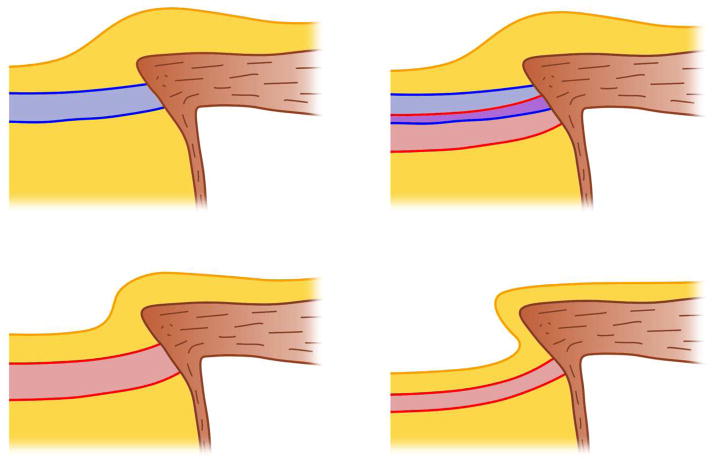
Figure 7.
Diagram illustrating our proposed progression of an ONH from a normal morphology, through early IOP-induced laminar remodeling, to end stage glaucomatous cupping and excavation. The top left pane shows a normal, healthy ONH (the lamina cribrosa is blue, pre-and retrolaminar tissues are yellow, and the sclera and pia mater are brown). Following chronic exposure to stress and/or strain that are beyond the physiologic tolerance of the resident cells, the lamina is remodeled to a thickened and cupped shape, and the laminar insertion begins to move posteriorly into the pia (top right; normal lamina in blue and remodeled lamina in red). As glaucomatous damage progresses with continued exposure to a biomechanically driven insult, the prelaminar neural tissues begin to thin (bottom left). Eventually, most of the RGC axons are lost and the lamina scars and thins to a classic cupped, excavated glaucomatous morphology (bottom right).
Acknowledgments
This work was supported by USPHS grants R01-EY18926 (JCD) and R01-EY19333 (JCD) from the National Eye Institute, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapova OA, Kaufman PL, Lucarelli MJ, Gabelt BT, Hernandez MR. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967(1–2):132–43. doi: 10.1016/s0006-8993(02)04234-8. [DOI] [PubMed] [Google Scholar]
- Agoumi Y, Artes PH, Nicolela MT, Chauhan BC. Spectral Domain Optical Coherence Tomography Measurements of Laminar and Prelaminar Tissue Movement After Intraocular Pressure Elevation. ARVO; Ft. Lauderdale: 2009. E-Abstract 4898–A295. [Google Scholar]
- Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84(3):318–23. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000;41(10):2991–3000. [PubMed] [Google Scholar]
- Brown DJ, Morishige N, Neekhra A, Minckler DS, Jester JV. Application of second harmonic imaging microscopy to assess structural changes in optic nerve head structure ex vivo. J Biomed Opt. 2007;12(2):024029. doi: 10.1117/1.2717540. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008;17(4):318–28. doi: 10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85(6):425–35. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF, Hart RT. Multiscale finite element modeling of the lamina cribrosa microarchitecture in the eye. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4277–80. doi: 10.1109/IEMBS.2009.5332755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards ME, Good TA. Use of a mathematical model to estimate stress and strain during elevated pressure induced lamina cribrosa deformation. Curr Eye Res. 2001;23(3):215–225. doi: 10.1076/ceyr.23.3.215.5460. [DOI] [PubMed] [Google Scholar]
- Edwards ME, Wang SS, Good TA. Role of viscoelastic properties of differentiated SH-SY5Y human neuroblastoma cells in cyclic shear stress injury. Biotechnol Prog. 2001;17(4):760–7. doi: 10.1021/bp010040m. [DOI] [PubMed] [Google Scholar]
- Grytz R, Meschke G. A computational remodeling approach to predict the physiological architecture of the collagen fibril network in corneoscleral shells. Biomech Model Mechanobiol. 2009 doi: 10.1007/s10237-009-0173-2. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. Ultrastructural immunocytochemical analysis of elastin in the human lamina cribrosa. Changes in elastic fibers in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1992;33(10):2891–903. [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19(3):297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Irnaten M, Barry RC, Quill B, Clark AF, Harvey BJ, O’Brien CJ. Activation of stretch-activated channels and maxi-K+ channels by membrane stress of human lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2009;50(1):194–202. doi: 10.1167/iovs.08-1937. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004;45(8):2660–5. doi: 10.1167/iovs.03-1363. [DOI] [PubMed] [Google Scholar]
- Kagemann L, Ishikawa H, Wollstein G, Brennen PM, Townsend KA, Gabriele ML, Schuman JS. Ultrahigh-resolution spectral domain optical coherence tomography imaging of the lamina cribrosa. Ophthalmic Surg Lasers Imaging. 2008;39(4 Suppl):S126–131. doi: 10.3928/15428877-20080715-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan RP, Crean JK, Fenerty CH, Clark AF, O’Brien CJ. Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head. Journal of Glaucoma. 2004;13(4):327. doi: 10.1097/00061198-200408000-00011. [DOI] [PubMed] [Google Scholar]
- Kirwan RP, Fenerty CH, Crean JK, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;11:798–810. [PubMed] [Google Scholar]
- LaPlaca MC, Cullen DK, McLoughlin JJ, Cargill RS., 2nd High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J Biomech. 2005;38(5):1093–105. doi: 10.1016/j.jbiomech.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Levy NS, Crapps EE. Displacement of optic nerve head in response to short-term intraocular pressure elevation in human eyes. Arch Ophthalmol. 1984;102(5):782–786. doi: 10.1001/archopht.1984.01040030630037. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 2000;14 ( Pt 3B):437–44. doi: 10.1038/eye.2000.128. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Butt Z, Ludlam C, Detkova P. Activation of the coagulation cascade in untreated primary open-angle glaucoma. Ophthalmology. 1997;104(4):725–9. doi: 10.1016/s0161-6420(97)30245-0. discussion 729–30. [DOI] [PubMed] [Google Scholar]
- Oyama T, Abe H, Ushiki T. The connective tissue and glial framework in the optic nerve head of the normal human eye: light and scanning electron microscopic studies. Arch Histol Cytol. 2006;69(5):341–56. doi: 10.1679/aohc.69.341. [DOI] [PubMed] [Google Scholar]
- Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33(11):1469–90. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- Pena JD, Netland PA, Vidal I, Dorr DA, Rasky A, Hernandez MR. Elastosis of the lamina cribrosa in glaucomatous optic neuropathy. Exp Eye Res. 1998;67(5):517–24. doi: 10.1006/exer.1998.0539. [DOI] [PubMed] [Google Scholar]
- Poostchi A, Wong T, Chan KC, Kedzlie L, Sachdev N, Nicholas S, Garway-Heath DF, Wells AP. Optic disc diameter increases during acute elevations of intraocular pressure. Invest Ophthalmol Vis Sci. 2010;51(5):2313–6. doi: 10.1167/iovs.09-3756. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005;46(8):2662–70. doi: 10.1167/iovs.04-1070. [DOI] [PubMed] [Google Scholar]
- Radius RL. Anatomy of the optic nerve head and glaucomatous optic neuropathy. Surv Ophthalmol. 1987;32(1):35–44. doi: 10.1016/0039-6257(87)90072-5. [DOI] [PubMed] [Google Scholar]
- Ren R, Wang N, Li B, Li L, Gao F, Xu X, Jonas JB. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length. Invest Ophthalmol Vis Sci. 2009;50(5):2175–84. doi: 10.1167/iovs.07-1429. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, Downs JC. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(2):681–90. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Hart RT, Liang Y, Bellezza A, Burgoyne CF, Downs J. Continuum-level finite element modeling of the optic nerve head using a fabric tensor based description of the lamina cribrosa. ASME Summer Bioengineering Conference; Keystone, Colorado. 2007. [Google Scholar]
- Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 2010;51(1):295–307. doi: 10.1167/iovs.09-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Sigal IA, Liang Y, Burgoyne CF, Downs JC. Changes in the biomechanical response of the optic nerve head in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2010 May; doi: 10.1167/iovs.10-5411. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Ackloo S, Dharsee M, Flanagan JG. Biomarkers in Response to Biomechanical Stress From Human Optic Nerve Head Astrocytes. ARVO; Ft. Lauderdale: 2010. E-Abstract 2184. [Google Scholar]
- Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336(2):483–9. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- Sander EA, Downs JC, Hart RT, Burgoyne CF, Nauman EA. In-plane mechanics of the optic nerve head with cellular solids models. World Congress of Biomechanics; Munich, Germany. 2006. [Google Scholar]
- Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009;50(6):2785–95. doi: 10.1167/iovs.08-3095. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88(4):799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46(11):4189–99. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Predicted extension, compression and shearing of optic nerve head tissues. Exp Eye Res. 2007;85(3):312–322. doi: 10.1016/j.exer.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2009;8(2):85–98. doi: 10.1007/s10237-008-0120-7. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2009;8(2):99–109. doi: 10.1007/s10237-008-0119-0. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. 3D morphometry of the human optic nerve head. Exp Eye Res. 2010;90(1):70–80. doi: 10.1016/j.exer.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Roberts MD, Girard M, Burgoyne CF, Downs JC. Biomechanical Changes of the Optic Disc. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. Elsevier; New York: 2010. [Google Scholar]
- Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90(3):383–8. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5(1):1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wells AP, Garway-Heath DF, Poostchi A, Wong T, Chan KC, Sachdev N. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci. 2008;49(8):3262–8. doi: 10.1167/iovs.07-1556. [DOI] [PubMed] [Google Scholar]
- Yang H, Downs JC, Bellezza A, Thompson H, Burgoyne CF. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Invest Ophthalmol Vis Sci. 2007;48(11):5068–84. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Lamina Cribrosa and Peripapillary Scleral Position and Thickness. Invest Ophthalmol Vis Sci. 2007;48(10):4597–607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF. Deformation of the normal monkey optic nerve head connective tissue after acute IOP elevation within 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2009;50(12):5785–99. doi: 10.1167/iovs.09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Williams G, Downs J, Sigal IA, Roberts MD, Grimm J, Thompson H, Burgoyne C. Optic Nerve Head (ONH) Lamina Cribrosa Insertion Migration and Pialization in Early Non-Human Primate (NHP) Experimental Glaucoma. ARVO; Ft. Lauderdale: 2010. E-Abstract 1631. [Google Scholar]
- Zeimer RC. Biomechanical properties of the optic nerve head. In: Drance SM, Anderson DR, editors. Optic nerve in glaucoma. Kugler Publications; Amsterdam: 1995. pp. 107–121. [Google Scholar]