Abstract
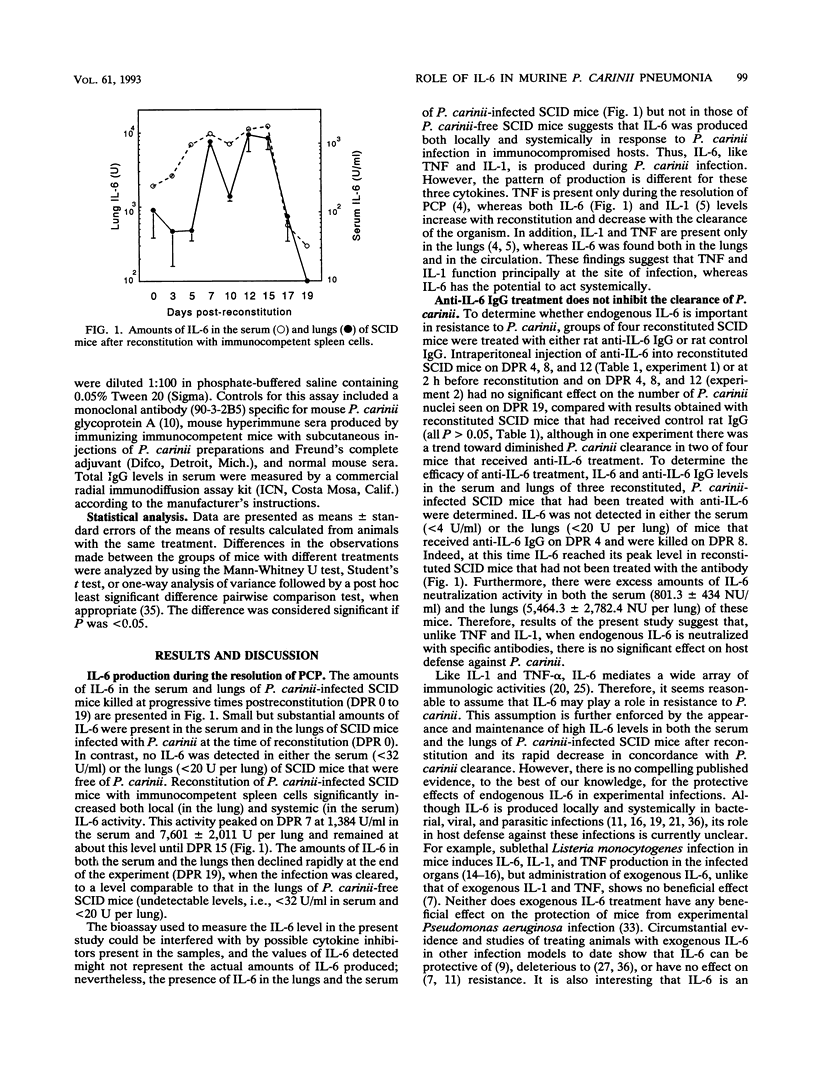
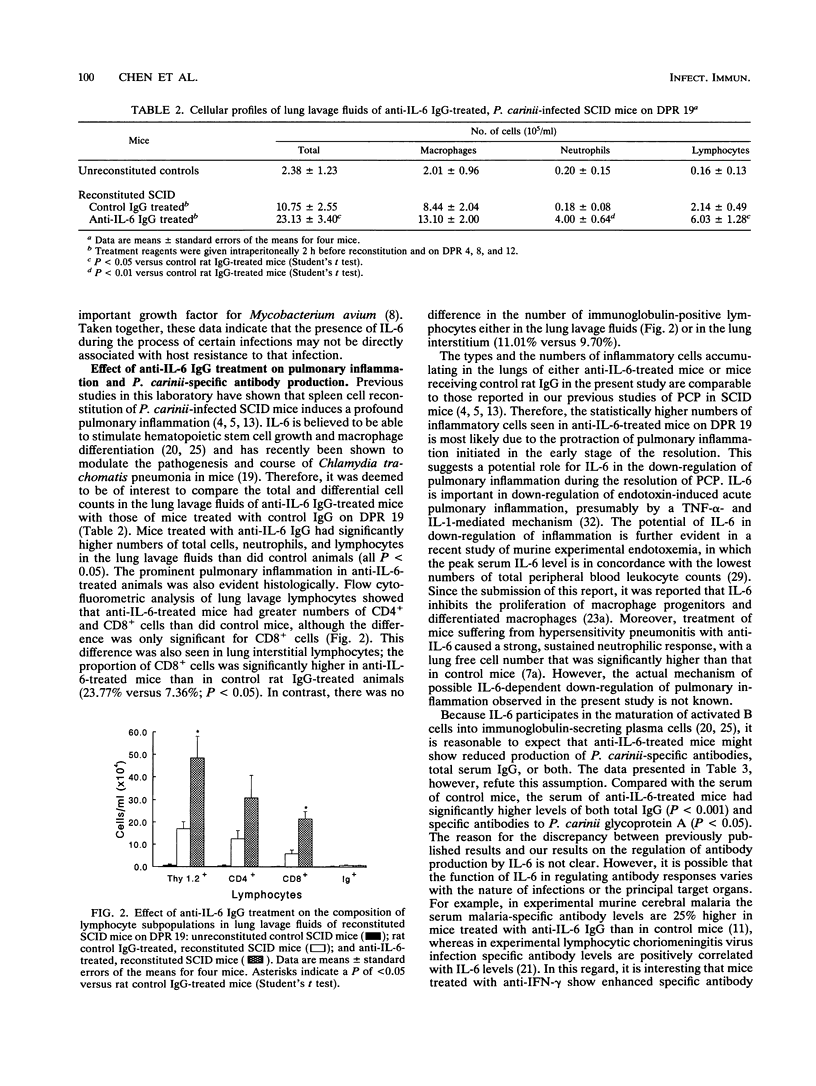
The production of interleukin-6 (IL-6) and its possible relationship to host resistance and inflammatory response to Pneumocystis carinii infection were examined in mice with severe combined immunodeficiency (SCID mice). IL-6 activity was detected in the serum and lungs of P. carinii-infected mice but not in mice free of P. carinii. Moreover, the IL-6 levels in P. carinii-infected mice increased markedly after spleen cell reconstitution but then decreased to an undetectable level after the clearance of P. carinii. However, neutralization of IL-6 activity in spleen cell-reconstituted SCID mice by treatment with anti-IL-6 immunoglobulin G (IgG) resulted in no significant effect on the clearance of P. carinii (P > 0.05). Both the serum and lungs of treated mice contained an excess amount of anti-IL-6 IgG and lacked detectable IL-6. These results suggest that failure to inhibit the P. carinii clearance by anti-IL-6 treatment was not due to insufficient administration of antibody or incomplete neutralization of IL-6 activity. However, compared with mice receiving rat control IgG, mice treated with anti-IL-6 IgG had significantly higher numbers of neutrophils and lymphocytes (particularly CD8+ cells) in the lung lavage fluids (P < 0.05 for both) at day 19 after reconstitution. In addition, the levels of both total IgG (P < 0.001) and P. carinii-specific antibodies (P < 0.05) in the serum of mice treated with anti-IL-6 were significantly higher than those in control mice. These results indicate that although P. carinii infection causes both local and systemic production of IL-6 in SCID mice, IL-6 does not appear to play a crucial role in the clearance of P. carinii. However, it appears that during resolution of P. carinii pneumonia, IL-6 plays a role in the regulation of pulmonary inflammation and antibody responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Brakenhoff J. P., de Groot E. R., Evers R. F., Pannekoek H., Aarden L. A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987 Dec 15;139(12):4116–4121. [PubMed] [Google Scholar]
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Chen W., Havell E. A., Harmsen A. G. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect Immun. 1992 Apr;60(4):1279–1284. doi: 10.1128/iai.60.4.1279-1284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Havell E. A., Moldawer L. L., McIntyre K. W., Chizzonite R. A., Harmsen A. G. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J Exp Med. 1992 Sep 1;176(3):713–718. doi: 10.1084/jem.176.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Ruffolo J. J., Walzer P. D. Analysis of the developmental stages of Pneumocystis carinii, in vitro. Lab Invest. 1988 Mar;58(3):324–331. [PubMed] [Google Scholar]
- Czuprynski C. J., Haak-Frendscho M., Maroushek N., Brown J. F. Effects of recombinant human interleukin-6 alone and in combination with recombinant interleukin-1 alpha and tumor necrosis factor alpha on antibacterial resistance in mice. Antimicrob Agents Chemother. 1992 Jan;36(1):68–70. doi: 10.1128/aac.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J Leukoc Biol. 1992 Aug;52(2):197–201. doi: 10.1002/jlb.52.2.197. [DOI] [PubMed] [Google Scholar]
- Denis M. Interleukin-6 is used as a growth factor by virulent Mycobacterium avium: presence of specific receptors. Cell Immunol. 1992 Apr 15;141(1):182–188. doi: 10.1016/0008-8749(92)90137-e. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6). Infect Immun. 1990 Jan;58(1):269–271. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992 Feb;165(2):329–336. doi: 10.1093/infdis/165.2.329. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988 Aug;56(8):1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Moldawer L. L., Helfgott D., Kilian P. L., Sehgal P. B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992 Mar 1;148(5):1486–1492. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Havell E. A., Sehgal P. B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991 Jan 15;146(2):756–761. [PubMed] [Google Scholar]
- Magee D. M., Smith J. G., Bleicker C. A., Carter C. J., Bonewald L. F., Schachter J., Williams D. M. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992 Mar;60(3):1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Hirano T. Interleukin 6 (IL-6). Biotherapy. 1990;2(4):363–373. doi: 10.1007/BF02170085. [DOI] [PubMed] [Google Scholar]
- McKinney M. M., Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987 Feb 11;96(2):271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- Moskophidis D., Frei K., Löhler J., Fontana A., Zinkernagel R. M. Production of random classes of immunoglobulins in brain tissue during persistent viral infection paralleled by secretion of interleukin-6 (IL-6) but not IL-4, IL-5, and gamma interferon. J Virol. 1991 Mar;65(3):1364–1369. doi: 10.1128/jvi.65.3.1364-1369.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. F., Garay S. M., Hopewell P. C., Mills J., Snider G. L., Stover D. E. NHLBI workshop summary. Pulmonary complications of the acquired immunodeficiency syndrome: an update. Report of the second National Heart, Lung and Blood Institute workshop. Am Rev Respir Dis. 1987 Feb;135(2):504–509. doi: 10.1164/arrd.1987.135.2.504. [DOI] [PubMed] [Google Scholar]
- Neta R., Perlstein R., Vogel S. N., Ledney G. D., Abrams J. Role of interleukin 6 (IL-6) in protection from lethal irradiation and in endocrine responses to IL-1 and tumor necrosis factor. J Exp Med. 1992 Mar 1;175(3):689–694. doi: 10.1084/jem.175.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedy M. C., Stewart C. C. Inhibitory role of interleukin-6 in macrophage proliferation. J Leukoc Biol. 1992 Jul;52(1):125–127. doi: 10.1002/jlb.52.1.125. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Marshall J. D., Allen R. D., Carlson G. A., Sidman C. L. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990 May;136(5):1173–1186. [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med. 1990 Nov;195(2):183–191. doi: 10.3181/00379727-195-43129d. [DOI] [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Terebuh P. D., Otterness I. G., Strieter R. M., Lincoln P. M., Danforth J. M., Kunkel S. L., Chensue S. W. Biologic and immunohistochemical analysis of interleukin-6 expression in vivo. Constitutive and induced expression in murine polymorphonuclear and mononuclear phagocytes. Am J Pathol. 1992 Mar;140(3):649–657. [PMC free article] [PubMed] [Google Scholar]
- Torrico F., Heremans H., Rivera M. T., Van Marck E., Billiau A., Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991 May 15;146(10):3626–3632. [PubMed] [Google Scholar]
- Tsai W. P., Hirose K., Nara P. L., Kuang Y. D., Conley S., Li B. Q., Kung H. F., Matsushima K. Decrease in cytokine production by HIV-infected macrophages in response to LPS-mediated activation. Lymphokine Cytokine Res. 1991 Dec;10(6):421–429. [PubMed] [Google Scholar]
- Ulich T. R., Yin S., Guo K., Yi E. S., Remick D., del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991 May;138(5):1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Van der Meer J. W., Helle M., Aarden L. Comparison of the effects of recombinant interleukin 6 and recombinant interleukin 1 on nonspecific resistance to infection. Eur J Immunol. 1989 Feb;19(2):413–416. doi: 10.1002/eji.1830190229. [DOI] [PubMed] [Google Scholar]
- Walzer P. D. Immunopathogenesis of Pneumocystis carinii infection. J Lab Clin Med. 1991 Sep;118(3):206–216. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Passlick B., Käfferlein E., Coulie P. G., Izbicki J. R. Protection against lethal pneumococcal septicemia in pigs is associated with decreased levels of interleukin-6 in blood. Infect Immun. 1992 Apr;60(4):1692–1694. doi: 10.1128/iai.60.4.1692-1694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


