Abstract
Age-related loss of muscle mass and function greatly affects quality of life in the elderly population. Several hypotheses have been proposed but accumulating evidence point to alterations in neuromuscular system during aging as a key event that leads to functional denervation, muscle wasting, and weakness. Over the past few decades, age-associated degeneration of the neuromuscular junction (NMJ) and its components have been well documented. With advancing age, pre-terminal portions of motor axons exhibit regions of abnormal thinning, distension, and sprouting whereas postsynaptic endplates decrease in size, reduce in number, length, and density of postsynaptic folds. Although the exact underlying mechanisms are still lacking, recent studies provided direct evidence that age-associated increase in oxidative stress plays a crucial role in NMJ degeneration and progression of sarcopenia. Homozygous deletion of an important antioxidant enzyme, Cu,Zn superoxide dismutase (CuZnSOD, SOD1) leads to acceleration of age-dependent muscle atrophy, with a significant NMJ degeneration similar to that seen in old wild type sarcopenic animals. In this short review, we briefly summarize the current understanding of some of the cellular and molecular changes in the NMJ during aging and suggest a role for oxidative stress and mitochondrial dysfunction in age-related changes in the maintenance of neuromuscular innervation.
1. Introduction
Aging skeletal muscle is characterized by a progressive loss of muscle mass as well as a decrease in function (Narici and Maffulli, 2010). This age-acquired deficit known as sarcopenia, contributes profoundly to quality of life in the elderly and predisposes them to an increased risk of morbidity, disability, and mortality (Janssen et al., 2004). The etiology of sarcopenia is a multi-factorial process that involves both intrinsic and extrinsic factors. However, mounting evidence from both animal and human studies suggests that degeneration of motor neurons, followed by changes in structural and functional integrity of the neuromuscular junction (NMJ), functional denervation, and loss of motor units contribute significantly to the progression of skeletal muscle aging. Aging muscle fibers are known to undergo denervation and re-innervation cycles that lead to remodeling of the motor units. A preferential denervation of the fast-twitch fibers and re-innervation by axonal sprouting from slow motor neurons results in a conversion of type II fast fibers to type I slow fibers. When denervation outpaces re-innervation, a population of muscle fibers degenerates and atrophy occurs in the remaining fibers. Loss of muscle mass ultimately contributes to a compromised contractile function. Although the contribution of neuronal-induced alterations in aging skeletal muscle has been recognized for more than 40 years, the precise molecular mechanisms of age-associated deterioration in neuromuscular system have remained elusive. Recently, technical advances in imaging in combination with molecular genetics have identified key molecular players in development of age-associated alterations in the neuromuscular system and development of sarcopenia. In this short review, we will briefly summarize the recent literature on proposed mechanisms contributing to age-related changes in the neuromuscular innervation. We will also discuss the potential role of age-related changes in trophic factors, oxidative stress, and mitochondrial dysfunction during aging that might influence the maintenance of neuromuscular innervation.
2. Age-related changes in neuromuscular system
Age-associated degeneration of the neuromuscular junction (NMJ) is well documented in several animal model systems as well as in humans (Balice-Gordon, 1997; Courtney and Steinbach, 1981; Deschenes et al., 2010; Luff, 1998; McMullen and Andrade, 2009; Smith and Chapman, 1987; Wernig and Herrera, 1986). Early studies found that aging was associated with morphological changes that were confined primarily to nerve endings with little or no degeneration or loss of primary axons, suggesting that aging was associated with a functional denervation (Fujisawa, 1976; Gutmann and Hanzlikova, 1973). Subsequent studies revealed that the age-related changes in NMJ morphology vary among different muscle types and could potentially be related to muscle activity levels. For example, the number of nerve terminal branches per endplate and the number of terminal sprouts decrease in the extensor digitorum longus muscle (EDL) between 10 and 25 months of age in Fischer-344 rats (Rosenheimer and Smith, 1985) while this same group reported an increase in the number of endplates with ultra-terminal sprouts in the EDL (but not soleus or diaphragm) muscle in the old rats (Rosenheimer, 1990). These ultra-terminal sprouts originate from the endplate region and terminate on their own or in an adjacent muscle fiber and are often found associated with terminals that have characteristics similar to denervated fibers, such as an increase in endplate area per fiber and fewer nerve terminal branches. Another study in rat diaphragm from older rats (28 months) found no change in endplate size, but there were an increased number of nerve terminals per endplate, decreased sprouting, and an increased number of synaptic vesicles (Prakash and Sieck, 1998; Smith and Rosenheimer, 1982). Interestingly, while the nerve terminal area, endplate branching, and size were all increased in Type IIb and IIx muscle fibers of diaphragm in the 24-month-old rats, there was essentially no change in the NMJs on Type I and IIa muscle fibers with age in these rats, demonstrating that differences can also occur among fiber types within a single muscle. While there are considerable variations in age-related changes in postsynaptic endplate characteristics in different muscle types, changes in endplates also vary depending on the age of the animal examined, i.e., the relative percentage of the lifespan examined. For example, a study of endplate morphology in rat soleus muscle showed that endplates increased in size and complexity up to 18 months of age, but later (at 28 months of age and close to mean lifespan), size and complexity were dramatically decreased (Pestronk et al., 1980). Similar changes in NMJ morphology have been reported in mice during aging. In C57BL6 mice, it was reported that by 27 months only 40% of the NMJs appear normal compared to 85% in young mice (Ludatscher et al., 1985).
In the presynaptic terminal, age-related declines in synaptic vesicles, mitochondrial content, and nerve terminal area were reported in both soleus and EDL muscles from 29 month CBF-1 male mice compared to 7-month-old mice (Fahim and Robbins, 1982). In contrast, these investigators found an increased content of smooth endoplasmic reticulum (SER), an increase in the number of coated vesicles and increased cisternae in the old presynaptic terminals, which could be part of a compensatory response to increased vesicle recycling and to maintain adequate neurotransmitter levels. Alterations in these structures (i.e., mitochondria, SER and cisternae, and coated vesicles) could also potentially alter calcium sequestration and affect transmitter release (Fahim and Robbins, 1982). On the postsynaptic side, there was an increase in the number of branched subsynaptic folds, an increased number of subsarcolemmal vesicles, and increased lipofuscin deposits in the older mice. In the mouse EDL muscle, a comparison of 6- and 24-month-old C57BL6 mice showed an increase in the area, perimeter, and length of the nerve terminals, along with increased number of branches and increased sprouting (Fahim, 1993).
Along with changes in the morphology of the NMJ, age-related changes in the quantal content of neurotransmitter release have also been reported in mouse EDL and soleus muscle between 10 and 32 months of age in CBF-1 mice (Kelly and Robbins, 1983, 1986). These changes in quantal release, measured as increases in amplitude of evoked endplate potentials (EPP), were correlated with a decrease in the number of synaptic vesicles, a decrease in nerve terminal area, and a decrease in the number of post-synaptic folds lacking nerve terminals. The increase in transmitter release was associated with an increased turnover of neurotransmitter in muscle from old (28–30 month) compared to young (7–8 month) old mice and a 70% decrease in synaptic vesicle density in old CBF-1 mice (Fahim and Robbins, 1982; Kelly and Robbins, 1986).
It has been postulated that the age-related changes in the NMJ are also associated with alterations in axonal transport (Gutmann and Hanzlikova, 1973). A decline in axonal transport during aging could affect parameters such as availability of trophic factors as well as movement of organelles such as mitochondria that are critical for neuronal survival. Axonal transport was compared in young (7–8 month), middle -aged (19–20 month), and old (31–32 month) male Wistar rats by monitoring cholinesterase accumulation in sciatic nerve that was ligated upstream of the terminal. Cholinesterase accumulation was dramatically decreased in the older rats suggesting transport from the cell body was significantly reduced. The reduction in transport was not due to a reduction in axon number as determined by histological evaluation of axon numbers. Interestingly, the reduction in transport appeared to correlate with the extent of muscle atrophy seen in these rats (Gutmann and Hanzlikova, 1973; McMartin and O’Connor, 1979).
Changes in endplate morphology and NMJ remodeling are likely related to the changes in motor units that occur during aging. A study in soleus of 4- and 24-month-old rats found a significant age-related degeneration of endplates leading to a loss of muscle fibers and decrease in the size of motor units (Gutmann and Hanzlikova, 1966). A loss of muscle fibers and an increase in innervation ratio leading to a 30–40% loss in motor units and preferential loss of fast motor units were demonstrated in old rats as well as in humans (Edstrom and Larsson, 1987; Einsiedel and Luff, 1992a, b).
As imaging techniques have advanced over the last few decades, our understanding of neuromuscular synapse formation and molecular players that regulate its function have been greatly enhanced. Lichtman and Sanes have elegantly demonstrated the feasibility of in vivo live animal imaging, as opposed to imaging in fixed tissue, to monitor the neuromuscular junction in mouse sternomastoid muscle of over several months (Lichtman and Sanes, 2003; Rich and Lichtman, 1989). Balice-Gordon from same group applied these techniques to monitor age-related changes in the same NMJs over time in C57BL6 mice (Balice-Gordon, 1997). They reported that following the developmental period of synapse elimination, NMJs were surprisingly stable over long period of time. However, when the animals reached the age of 12–18 months, a small percentage of NMJs started to display some loss of motor terminal branches and underlying acetylcholine receptors (AChR). In addition, the reported age-associated changes in NMJ morphology were remarkably similar to that of naturally occurring synapse elimination during development and synapse elimination following reinnervation (Balice-Gordon, 1997). Furthermore, these investigators found that motor axons appear to compensate for the loss of synaptic site by sprouting and adding new junctions. However this compensatory effect diminished dramatically as animals reached the age of 18 months and older. The newly formed junctions were also more vulnerable to degenerative changes and some of the junctions disappeared within weeks. By 24–36 months of age, the majority of NMJs were dissociated from presynaptic terminals and postsynaptic endplates were severely fragmented. In a single time-point study, our laboratory observed similar deteriorations in senescent (33 months) C57BL6 mice compared to younger (11 months) counterparts (Figure 1). By 33 months we found that over two-thirds of NMJs are either partially innervated or completely denervated with extensive sprouting of motor neurons. Moreover, we found that muscle atrophy and synaptic changes in aged muscles occur on a fiber-by-fiber basis, suggesting that denervation and alterations of NMJs are key factors in sarcopenia.
Figure 1.
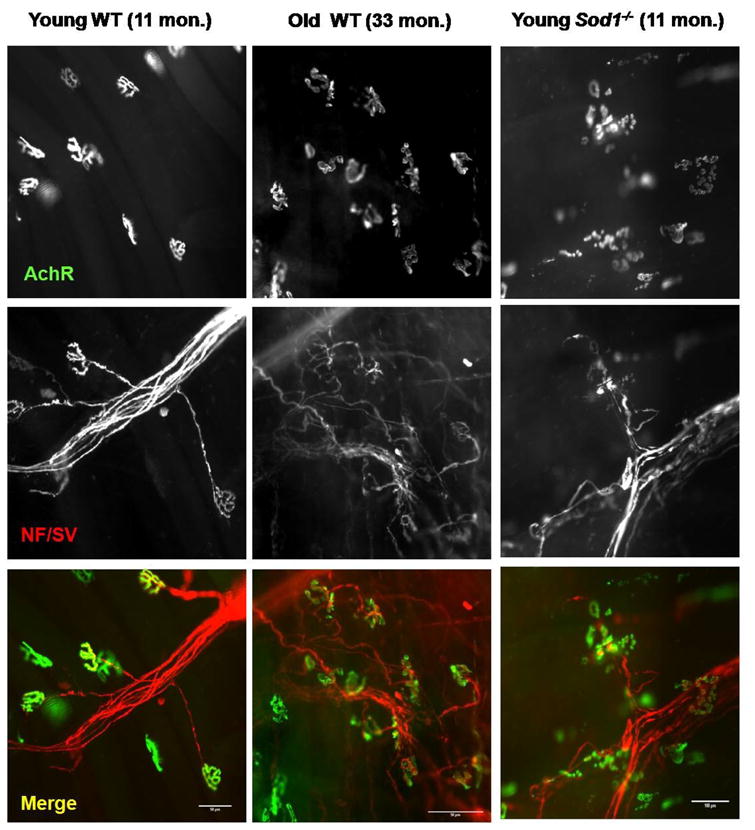
A comparison of wild-type young (11 months), wild-type old (33 months), and young Sod1−/− (11 months) mouse neuromuscular junctions (NMJ) from gastrocnemius muscle. Motor axons and presynaptic terminals (NF/SV: pseudo-colored in red) were stained with anti-neurofilament and anti-synaptophysin then alexa-555 conjugated secondary antibodies. Acetylcholine receptors (AChR: pseudo-colored in green) were stained with alexa-488 conjugated α-bugarotoxin (scale bar: 50μm).
While progressive deterioration in both presynaptic motor neurons and postsynaptic muscle fibers likely contributes to denervation, other cell types such as terminal Schwann cells, which play an important role in axonal guidance and synaptic repair following denervation, may also contribute to a functional decline of the neuromuscular system in aging. Although there have been scarce reports suggesting degeneration of terminal Schwann cell during aging (Ludatscher et al., 1985), the exact mechanism of how terminal Schwann cells modulate denervation-reinnervation cycle in respect to aging have not been thoroughly investigated. It will be interesting to see if alterations in terminal Schwann cell and/or the trophic/growth factors secreted by Schwann cells contribute to functional decline in NMJ and skeletal muscle with age. In contrast to terminal Schwann cells, age-related changes in myelinated Schwann cells have been reported by several investigators. For example, collagen, lipid droplets, and oxidative damage in myelinated peripheral nerves have been reported to accumulate (Ceballos et al., 1999). Schwann cells associated with myelinated fibers decrease in number along with widening of the nodes of Ranvier during aging (Ceballos et al., 1999). Furthermore, two independent investigators have reported that the protein expression of major myelin proteins, P0, PMP22, and MBP significantly declined with age (Ceballos et al., 1999; Rangaraju et al., 2009). Interestingly, calorie restriction (CR), which has been shown to retard the aging process in multiple experimental model organisms, significantly attenuated age-related deterioration of peripheral nerve by up-regulating chaperones and lysosomal autophagic machinery (Rangaraju et al., 2009).
3. The role of trophic factors in maintenance of neuromuscular junction (NMJ) with age
Interactions among presynaptic motor neuron, postsynaptic muscle, and Schwann cells play critical role in growth, maintenance, and survival of synapses. The exchange of trophic factors has been implicated in pre- and postsynaptic development as well as preserving neuronal and synaptic plasticity at the NMJ. Neurotrophins are known to participate in activity-induced modification of synaptic transmission (Schinder et al., 2000; Schinder and Poo, 2000). The exact role or the identity of neurotrophic and/or myotrophic factors that promotes survival and maintenance of presynaptic and postsynaptic apparatus at the NMJ in the context of aging have not been fully determined. However, recent studies indicate that a variety of trophic factors such as brain derived neurotrophic factor (BDNF), neutrophin-3 (NT-3), neutrophin-4 (NT-4), cytokines such as glial-derived neutrophic factor (GDNF) and ciliary neutrophin factor (CNTF), and other growth factors such as insulin-like growth factor (IGF-1 and IGF-II) and fibroblast growth factors (FGF) all play a modulatory role in neuromuscular system to a different extent during aging. For instance, BDNF has been shown to facilitate synaptic efficacy by increasing presynaptic depolarization at the NMJ (Huang and Reichardt, 2001). Furthermore, BDNF, NT-3, and NT-4 have been shown to be important factors for maintenance of AChR clustering in the NMJ (Belluardo et al., 2001; Gonzalez et al., 1999; Loeb et al., 2002). If neurotrophin signaling is interrupted or neurotrophin expression is suppressed, AChR is dispersed and NMJ is fragmented and affected muscles show increased fatigue (Gonzalez et al., 1999). Recently, Delbono’s group using motor neuron-targeted IGF-1 tetanus toxin fusion proteins demonstrated that IGF-1 is a potent growth factor that can reverse age-related decline in specific force as well as improving morphological abnormalities at the NMJs (Payne et al., 2006). As mentioned earlier, loss of synaptic sites during aging remarkably resembles synapse elimination during development. Motor neurons are known to organize postsynaptic endplates by secreting two factors: a large proteoglycan called agrin and an epidermal growth factor (EGF) family protein, neuregulin. These proteins bind to their receptors on postsynaptic muscle fibers and induce clustering of acetylcholine receptor and gene transcription of specialized proteins that maintains synaptic transmission and structures (Sanes and Lichtman, 1999). Loss-of-function and gain-of-function studies using transgenic and knockout mouse in developmental synapse elimination have been characterized over the last two decades (Kummer et al., 2006; Sanes and Lichtman, 2001). However the exact functional role of the synapse regulatory proteins agrin and neuregulin in aging has been largely unknown. Interestingly, in recent years, neuregulin has been reported to be a myokine that exerts relevant effects on myogenesis and the regulation of muscle metabolism (Guma et al., 2010). The rapid and chronic metabolic effects of neuregulin appear to be related to muscle contraction and resemble those of exercise. Chronic exercise training has been shown to slow the aging process in muscle (Kim et al., 2008; Vandervoort, 2002). Therefore, it may be worthwhile to investigate neuregulin as therapeutic target against sarcopenia.
4. Oxidative stress and mitochondrial dysfunction in neuromuscular innervation
The oxidative stress theory of aging predicts that the imbalance between oxidant generation and antioxidant defense mechanisms results in a steady-state accumulation of oxidative damage in a variety of macromolecules. Oxidative damage increases during aging, which results in a progressive loss in the functional efficiency of various cellular processes (Harman, 1956). Several laboratories, including ours, have tested the impact of oxidative stress on age-related muscle wasting (Jang et al., 2010; Mansouri et al., 2006; Weindruch, 1995). In order to test the direct cause and effect relation between oxidative stress and sarcopenia in vivo, we have utilized antioxidant enzyme deficient mouse models. In a recent report from our laboratory, we demonstrated that homozygous deletion of Sod1 (Cu/Zn superoxide dismutase; CuZnSOD) leads to age-dependent muscle atrophy with alterations in NMJs that are similar to normal aging muscle but with a more frequent and earlier occurrence (Jang et al., 2010; Muller et al., 2006). The morphology of the NMJ at 11 months in Sod1−/− mice is comparable to NMJ in 33-month-old, wild-type mice (Figure 1), showing extensive sprouting, thinning of axon terminals, and fragmentation of postsynaptic AChRs. These data suggest that maintenance of NMJ during aging may be critically influenced by oxidative stress.
Sod1−/− mice exhibit several neuromuscular phenotypes that are similar to that of sarcopenia. For example, Sod1−/− mice exhibit a shift from fast to slow fiber type and a grouping of slow fibers that may be the result of motor unit remodeling (Kostrominova et al., 2007). In addition, skeletal muscle of Sod1−/− mice shows age-associated mitochondrial dysfunction, which is also prevalent in senescent animals. Mitochondrial ROS generation is significantly increased compared to wild-type and further exacerbated with age in Sod1 knockout muscle. H2O2, a stable membrane permeable ROS, has been shown to inhibit the quantal release of acetylcholine and the activity vesicle fusion protein SNAP25 at the NMJ is also inhibited by H2O2 (Giniatullin et al., 2006). Moreover, both presynaptic terminal and postsynaptic endplate regions contain high concentrations of mitochondria. In presynaptic motor neuron, mitochondria travel along the microtubules using axonal transport to reach nerve terminals, whereas in postsynaptic muscle fiber, a subpopulation of mitochondria called subsarcolemma mitochondria reside around the NMJ and along the sarcolemma membrane. These mitochondria serve multiple functions such as energy support, regulation of calcium, synaptic transmission, and apoptosis. Not surprisingly, disruptions of mitochondria and their transport have been implicated in a wide variety of neurological diseases (Chan, 2006). Thus, it is plausible to hypothesize that mitochondrial dysfunction in the population of mitochondria associated with the NMJ may lead to altered calcium buffering and oxidative modification of key molecules in the NMJ and contribute to age-associated declines in neuromuscular innervation. In support of this, Zhou et al demonstrated that mitochondria adjacent to the AChR are selectively depolarized when muscle fibers are challenged by calcium in a mouse model of ALS, which exhibits significant neuromuscular degeneration (Zhou et al., 2010). In addition, other investigators have also shown that muscle-specific overexpression of uncoupling proteins (UCP1) significantly disrupted NMJ integrity. Furthermore, our laboratory has reported that isolated subsarcolemma mitochondria have significant deficits in ATP generation, oxygen consumption, and also generate more mitochondrial ROS compared to wild-type (Jang et al., 2010).
Mitochondria play a central role in buffering cytosolic Ca2+ released by sarcoplasmic reticulum. Aging is shown to cause disruption in excitation-contraction coupling and a population of fast muscle fibers that become dependent on extracellular Ca2+ to maintain tetanic force (Payne et al., 2004). Furthermore, increased cytosolic Ca2+ may activate Ca2+ dependent proteases such as calpain. Calpain has been shown to interact with the AChR clustering protein rapsyn and disrupt the AChR complex. Thus, the age-associated dispersal of AChR clusters maybe in part caused by Ca2+ dysregulation and increased activation of calpain.
Oxidative stress may also exert its effect on peripheral motor axons. Indeed in a recent study, myelinated peripheral nerves were found to be oxidatively damaged during aging, and as a consequence, inflammation, increased accumulation of aggregated proteins, and lipofuscin adducts were found in the sciatic nerve of old animals. However, these age-related detrimental changes were significantly curtailed when rats were calorie restricted (Opalach et al., 2010). In our preliminary studies, we found similar protective effect against accelerated NMJ degeneration and muscle atrophy with lower levels of oxidative damage when Sod1−/− mice were maintained on a calorie restricted diet suggesting high oxidative stress in vivo directly affects neuromuscular maintenance.
5. Concluding remarks
Alterations in the neural control of skeletal muscle during aging are a crucial modifier and promoter of the muscle weakness and fatigue that occur in sarcopenia. Aging is complicated process involving multiple systems, but several lines of evidence, including our studies in the Sod1−/− mice mentioned above, point to an important role for oxidative stress in maintenance of NMJ during skeletal muscle aging. However, one needs to be cautious in interpreting these data. In our study, we used conventional knockout mice that were created using homologous recombination. Therefore, the Sod1 gene is deleted in every tissue and we cannot rule out the developmental defects due to high oxidative stress in these animals. In addition, it is unclear which cell type (motor neuron, muscle fibers, or Schwann cell) in the NMJ is most vulnerable to oxidative damage caused by Sod1 deficiency. Currently, studies are underway in our laboratory to address these questions using tissue-specific and/or inducible Cre-loxP system that will allow us to direct the deletion to occur after development or in a tissue specific manner. Furthermore, studies aimed at determining whether oxidative stress in vivo alters the level of neurotrophic factors (i.e., BDNF, NT-4, and NT-3) or their receptors (p75 and Trk receptors) during aging will help us understand the mechanism of age-associated NMJ degeneration. Currently, very little is known about whether these trophic factors and their receptors vary with age at the NMJ. Interestingly, in the central nervous system (CNS) oxidative damage to neurotrophic factors have been implicated in age-related neurodegenerative disease such as Alzheimer and Parkinson’s disease (Mattson, 2008). In summary, future studies addressing age-related changes in the regulation and maintenance of motor neuron terminals and neuromuscular junction components will allow us to develop and evaluate therapeutic strategies to attenuate, prevent, or ultimately reverse age-related muscle wasting and weakness.
Acknowledgments
Supported by P01AG020591 (HVR) and a Julie Martin Mid-Career grant from the American Federation for Aging Research (HVR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–87. doi: 10.1002/(sici)1097-4598(1997)5+<83::aid-mus20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, Caniglia G, Pastoris O, Grassi F, Ibanez CF. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci. 2001;18:56–67. doi: 10.1006/mcne.2001.1001. [DOI] [PubMed] [Google Scholar]
- Ceballos D, Cuadras J, Verdu E, Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat. 1999;195(Pt 4):563–576. doi: 10.1046/j.1469-7580.1999.19540563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Courtney J, Steinbach JH. Age changes in neuromuscular junction morphology and acetylcholine receptor distribution on rat skeletal muscle fibres. J Physiol. 1981;320:435–447. doi: 10.1113/jphysiol.1981.sp013960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom L, Larsson L. Effects of age on contractile and enzyme-histochemical properties of fast- and slow-twitch single motor units in the rat. J Physiol. 1987;392:129–145. doi: 10.1113/jphysiol.1987.sp016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsiedel LJ, Luff AR. Alterations in the contractile properties of motor units within the ageing rat medial gastrocnemius. J Neurol Sci. 1992a;112:170–177. doi: 10.1016/0022-510x(92)90147-d. [DOI] [PubMed] [Google Scholar]
- Einsiedel LJ, Luff AR. Effect of partial denervation on motor units in the ageing rat medial gastrocnemius. J Neurol Sci. 1992b;112:178–184. doi: 10.1016/0022-510x(92)90148-e. [DOI] [PubMed] [Google Scholar]
- Fahim MA. Morphological correlates of physiological responses in partially denervated mouse muscle during aging. Int J Dev Neurosci. 1993;11:303–310. doi: 10.1016/0736-5748(93)90002-u. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Fujisawa K. Some observations on the skeletal musculature of aged rats-III. Abnormalities of terminal axons found in motor end-plates. Exp Gerontol. 1976;11:43–47. doi: 10.1016/0531-5565(76)90010-3. [DOI] [PubMed] [Google Scholar]
- Giniatullin AR, Darios F, Shakirzyanova A, Davletov B, Giniatullin R. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. J Neurochem. 2006;98:1789–1797. doi: 10.1111/j.1471-4159.2006.03997.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Guma A, Martinez-Redondo V, Lopez-Soldado I, Canto C, Zorzano A. Emerging role of neuregulin as a modulator of muscle metabolism. Am J Physiol Endocrinol Metab. 2010;298:E742–750. doi: 10.1152/ajpendo.00541.2009. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V. Motor unit in old age. Nature. 1966;209:921–922. doi: 10.1038/209921b0. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V. Basic mechanisms of aging in the neuromuscular system. Mech Ageing Dev. 1973;1:327–349. doi: 10.1016/0047-6374(73)90040-7. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Kelly SS, Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. J Physiol. 1983;343:375–383. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SS, Robbins N. Sustained transmitter output by increased transmitter turnover in limb muscles of old mice. J Neurosci. 1986;6:2900–2907. doi: 10.1523/JNEUROSCI.06-10-02900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrominova TY, Pasyk KA, Van Remmen H, Richardson AG, Faulkner JA. Adaptive changes in structure of skeletal muscles from adult Sod1 homozygous knockout mice. Cell Tissue Res. 2007;327:595–605. doi: 10.1007/s00441-006-0297-y. [DOI] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Sanes JR. Watching the neuromuscular junction. J Neurocytol. 2003;32:767–775. doi: 10.1023/B:NEUR.0000020622.58471.37. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludatscher RM, Silbermann M, Gershon D, Reznick A. Evidence of Schwann cell degeneration in the aging mouse motor end-plate region. Exp Gerontol. 1985;20:81–91. doi: 10.1016/0531-5565(85)90043-9. [DOI] [PubMed] [Google Scholar]
- Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMartin DN, O’Connor JA., Jr Effect of age on axoplasmic transport of cholinesterase in rat sciatic nerves. Mech Ageing Dev. 1979;10:241–248. doi: 10.1016/0047-6374(79)90038-1. [DOI] [PubMed] [Google Scholar]
- McMullen CA, Andrade FH. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J Gerontol A Biol Sci Med Sci. 2009;64:435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010 doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 2010;13:65–74. doi: 10.1089/rej.2009.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation--contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol. 2004;560:137–155. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E, Delbono O. Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. J Physiol. 2006;570:283–294. doi: 10.1113/jphysiol.2005.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestronk A, Drachman DB, Griffin JW. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980;70:65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rangaraju S, Hankins D, Madorsky I, Madorsky E, Lee WH, Carter CS, Leeuwenburgh C, Notterpek L. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8:178–191. doi: 10.1111/j.1474-9726.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Lichtman JW. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci. 1989;9:1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheimer JL. Ultraterminal sprouting in innervated and partially denervated adult and aged rat muscle. Neuroscience. 1990;38:763–770. doi: 10.1016/0306-4522(90)90069-g. [DOI] [PubMed] [Google Scholar]
- Rosenheimer JL, Smith DO. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J Neurophysiol. 1985;53:1567–1581. doi: 10.1152/jn.1985.53.6.1567. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron. 2000;25:151–163. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Smith DO, Chapman MR. Acetylcholine receptor binding properties at the rat neuromuscular junction during aging. J Neurochem. 1987;48:1834–1841. doi: 10.1111/j.1471-4159.1987.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Smith DO, Rosenheimer JL. Decreased sprouting and degeneration of nerve terminals of active muscles in aged rats. J Neurophysiol. 1982;48:100–109. doi: 10.1152/jn.1982.48.1.100. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Interventions based on the possibility that oxidative stress contributes to sarcopenia. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):157–161. doi: 10.1093/gerona/50a.special_issue.157. [DOI] [PubMed] [Google Scholar]
- Wernig A, Herrera AA. Sprouting and remodelling at the nerve-muscle junction. Prog Neurobiol. 1986;27:251–291. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi J, Fu R, Liu E, Siddique T, Rios E, Deng HX. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem. 2010;285:705–712. doi: 10.1074/jbc.M109.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]


