Abstract
Background and Purpose
Hyperbaric oxygen preconditioning (HBO-PC) affords brain protection against transient global ischemia. In the present study we hypothesize that the mechanism of HBO-PC involves the induction of cyclooxygenase-2 (COX-2) in cerebral tissues prior to ischemia, which leads to a suppression of COX-2 and its downstream targets after global ischemic insult.
Methods
One hundred twenty nine male Sprague Dawley (SD) rats (280–300g body weight) were allocated to the naïve control group, sham operation group, three groups of animals subjected to 15 min four-vessel occlusion (4VO): untreated, preconditioned with HBO 2.5 atmospheres absolutes (ATA) for 1 hour daily for 5 days, preconditioned as above and administered with COX-2 inhibitor, NS-398 (1 mg/kg bw i.p.) prior to each preconditioning session, as well as normal rats preconditioned with HBO without ischemia. The mortality, the incidence of seizures and T-maze scores were recorded. The quantitative cell count in Nissl stain and TUNEL was conducted on day 7 after ischemia. The brain expression of COX-2 was analyzed with Western blotting and immunofluorescence staining.
Results
HBO-PC increased the number of surviving neurons in the CA1, which was associated with the reduced COX-2 expression in the hippocampus and in the cerebral cortex at 1 and 3 days post-ischemia. HBO-PC improved functional performance and tended to decrease mortality and the frequency of seizures. These beneficial effects of HBO-PC were abolished by the COX-2 selective inhibitor, NS-398.
Conclusions
HBO-PC reduced COX-2 expression and provided brain protection after global ischemia. Administration of COX-2 inhibitor with HBO prior to ischemia, abolished preconditioning effect, thereby implicating COX-2 as a mediator of HBO-PC in the ischemic brain.
Keywords: HBO preconditioning, ischemic tolerance, global cerebral ischemia, COX-2, NS-398
Introduction
Hyperbaric oxygen preconditioning (HBO-PC) has been shown to reduce neuronal injuries in animal models of neurological diseases. HBO-PC afforded neuroprotection against focal and global cerebral ischemia 1, 2, spinal cord ischemia 3, traumatic and surgical brain injury 4, 5 and neonatal hypoxia ischemia. 6 It has been postulated that HBO-PC alleviates ischemic brain injury by the upregulation of HIF-1α and its downstream adaptive genes 7, 8, inhibition of neuronal apoptotic pathways (blockage of caspase 3 and caspase-9 activity) 6, reduction of early apoptosis 9 or the upregulation of antioxidant enzymes. 10 However, so far conducted investigations have not determined whether HBO-PC can provide brain protection through anti-inflammatory mechanisms in the setting of severe global cerebral ischemia.
Mounting evidence indicates that neuroinflammation contributes to brain injury developing after cerebral ischemia. 11 The formation of arachidonic acid end products, catalyzed by cyclooxygenase-2 (COX-2) is a critical component of postischemic neuroinflammation. 12, 13 Pharmacological blockade of COX-2 with highly selective inhibitors (e.g., rofecoxib) or genetic ablation of COX-2 confers robust neuroprotection in laboratory animals subjected to focal or global cerebral ischemia; whereas neuronal overexpression of COX-2 in transgenic mice potentiates neuronal injury after global ischemic insult. 14–16
We have, therefore, conceptualized that the brain level of COX-2 expression may determine the outcome after transient global cerebral ischemia as either ischemic cell death or tolerance. We hypothesized that the mechanism of HBO-PC is mediated by a preischemic increase of COX-2 expression/activity followed by a suppression of COX-2 and its targets post ischemia thereby producing brain-protective effect.
Materials and Methods
Animal Groups and the Model of Global Cerebral Ischemia
One hundred twenty nine male SD rats weighing 280~300g were randomly divided into six groups of rats: normal (Norm; n=6), normal with HBO preconditioning (Norm + HBO-PC; n=8), a sham-operated (Sham; n=20); global ischemic (GI, n=37), global ischemic preconditioned with HBO (HBO-PC; n=31) and global ischemic preconditioned with HBO and pretreated with NS-398 i.p. (HBO-PCI; n=27).
To maintain blind fashion of the study, we marked each animal with ID number and kept the experimenters performing behavioral tests and cell count unaware of group assignment. After the data were collected, another researcher classed the rats and computed original data, then compared the results amongst groups. All surgical and euthanasia procedures were performed under anesthesia with ketamine (100 mg/kg; i.p.) and xylazine (10 mg/kg; i.p.) following atropine premedication (0.05 mg/kg; s.c.). The animals were intubated and mechanically ventilated during surgery. The four-vessel occlusion rat model of 15-min global brain ischemia 17 with our modifications was performed as described. 9, 18
Laser Doppler flowmetry performed in our previous study showed that one-stage, anterior approach 4VO produces a profound decrease of cortical CBF, down to 12% of baseline level18.
Rectal temperature was maintained at 36.8–37.2°C during and 2 hours after surgery. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Loma Linda University.
HBO Preconditioning Regimen
Rats were pressurized in a research hyperbaric chamber (1300B, Sechrist, CA) at 2.5 ATA with 100% oxygen (flow of 22 L/min). Compression and decompression was maintained at a rate of 5 psi/min. One hour-long HBO session was administered daily for 5 consecutive days; the last dive was carried out 24 hours before ischemia.
NS-398 Treatment
NS-398 at a dose of 1 mg/kg suspended in 10% dimethyl sulfoxide (DMSO) in PBS (n=31) was intraperitoneally injected ten minutes prior to each HBO session. The rats in HBO-PC group received vehicle by itself (10% DMSO/PBS) according to the same injection regimen.
Neurobehavioral Testing
Short term memory deficits were tested in the T-maze. The evaluation was conducted at 3 days or 7 days after ischemia, as described. 19 The results were expressed as percent of spontaneous alternations with respect to 50% reference. 20
Nissl Staining and TUNEL
On days 1, 3 and 7 after global ischemia, rats were perfused transcardially with 200 mL of ice-cold phosphate buffered saline, followed by 300 ml of phosphate buffered 10% formalin. Brains were postfixed and cryoprotected as described. 21 Brain tissue blocks were cryosectioned into 10 micrometer thick sections. A total of fourteen tissue sections per brain were selected for CA1 neuronal count. In order to ascertain that the CA1 neurons were counted at the same rostrocaudal level, we first dissected the same regions from each brain, using bregma as a reference point. Then we collected 10 μm thick coronal slices of the dorsal hippocampus (−3.3 mm to −4.5 mm from Bregma) at the same levels of sectioned specimens. To this end we determined the distance between each analyzed brain section and the anterior face of the tissue block.
The frozen sections were dried at room temperature, rehydrated and immersed in 0.5% cresyl violet (2 min) for Nissl staining. 9 TUNEL was done with an In Situ Cell Death Detection Kit (Roche, IN). 19
Tripple Immunofluorescence Staining
In order to determine neuron specific colocalization of cleaved caspase 3 and COX-2 in CA1 and in the cerebral cortex 3 days after ischemia we conducted triple fluorescence staining as described. 22 Briefly, primary antibodies were rabbit anti cleaved caspase 3 (Cell Signaling), goat anti COX-2 (Santa Cruz Inc.) and mouse anti NeuN (Millipore), all diluted 1:100. After blocking with donkey serum 5% for 2 hrs at room temperature and incubation at 4oC overnight the sections were treated with donkey secondary antibodies (1:200; RT for 2 hours) raised against rabbit, goat and mouse IgG, conjugated with Texas Red, FITC and AMCA, respectively.
Western Blot Analysis
The animals were euthanized under general anesthesia on day 1 and 3 after ischemia or at 24 hours after the last HBO session. The brains were collected and processed for Western blotting as described. 23 Equal amounts (30 μg) of total protein were separated in 10% SDS–polyacrylamide gel electrophoresis (PAGE) and blotted onto cellulose membranes. The probing antibodies included rabbit anti COX-2 polyclonal antibody (1:500; Cayman Chemical Company, MI), rabbit anti heme oxygenase 1 (HO-1) polyclonal antibody (1:500; Enzo Life Sciences, Inc., PA), rabbit anti hypoxia-inducible factor 1α (HIF-1α) polyclonal antibody (1:500, Santa Cruz, Inc,CA), goat anti beta actin (1:2000, Santa Cruz, Inc.), and the respective HPx-conjugated secondary antibodies diluted 1:2000 (Santa Cruz, Inc.). Bands were detected by the chemiluminescence kit (Amersham Bioscience, NJ), and recorded on X-ray film (Kodak, NY). Bands were quantified by optical density method (Image J), and densities were expressed relative to beta actin and sham (sham n=6, GI n=6, HBO-PC n=6, HBO-PCI n=5).
Quantitative Cell Count
The brains of animals from day 7 were collected and processed for quantitative analysis of Nissl staining and TUNEL (sham n=4, GI n=4, HBO-PC n=4, HBO-PCI n=4). A total of 24 Nissl photomicrographs from the dorsal CA1 per brain were taken at 100x magnification for cell counts. Eight photomicrographs of CA1 were taken from each animal for counting TUNEL positive cells.
Statistical Analysis
Data are expressed as mean ± S.E.M. Analysis of variance (ANOVA) supported by Sigma Stat (Systat Software, Richmond, CA) was used to verify the significance of intergroup differences for Western blot, T-maze results, and cell counts. The repeated measures ANOVA was applied to analyze differences in body weight. A chi-squared test was used for analyzing mortality and the incidence of seizures.
Results
Mortality, Neurological Scores and the Incidence of Seizures
The body weight measured daily significantly decreased on day 1 through 3 and went back to the basal level on day 7 in all groups except HBO-PCI (Figure 1A). The body weight decrease was statistically equivalent regardless the type of treatment. In the HBO-PC group, however, it showed a mild tendency toward earlier recovery. The mortality at 7 days was 0% (0 out of 20 rats) in the sham group, 24.32% (9 out of 37 rats) in the GI group, 12.90% (4 out of 31 rats) in the HBO-PC group and 25.92% (7 out of 27 rats) in the group pretreated with COX-2 inhibitor and HBO (Figure 1B). Most of deaths occurred within the first 48 hours after the ischemic insult. 3 rats died within 24 hours and 6 rats died between 24 and 48 hours in GI group. In HBO-PC group 1 rat died within first 24 hours and 3 rats died between 24 and 48 hours. In the HBO-PC group treated with COX-2 inhibitor, 3 rats died within 24 hours, 3 rats died between 24 and 48 hours and 1 rat died between 48 and 72 hours. Although the mortality in HBO-PC group tends to be lower than in the no treatment group the differences between groups did not qualify as statistically significant in chi-square analysis.
Figure 1.
(A) Body weight of rats measured daily and expressed as percent of preoperative weight. * p<0.05 vs. day 0 for all groups; # p<0.05 vs. day 0 for HBO-PCI group. (B) Seven-day mortality rates calculated by dividing the number of deceased animals by the number of total animals used in each group. (C) T-maze testing revealed functional deterioration in GI rats on day 3 after 4VO. * p<0.05 vs. sham; # p<0.05 vs. GI group. (D). The incidence of seizures tended to be lower in HBO preconditioned rats as compared to untreated ischemia or inhibitor-pretreated HBO-PC rats.
T-maze testing for spontaneous alternation demonstrated worse performance of the GI animals and HBO preconditioned animals with COX-2 inhibitor pretreatment on day 3 and 7 compared to the HBO preconditioned animals (Figure 1C).
The incidence of seizure was 0% (0/25) in the sham group, 21.62% (8/37) in the GI group, 6.45% (2/31) in the HBO-PC group and 25.92% (7/27) in the HBO-PC group with NS-398 pretreatment. The seizure incidence in each group was statistically equivalent in chi-square analysis (Figure 1D).
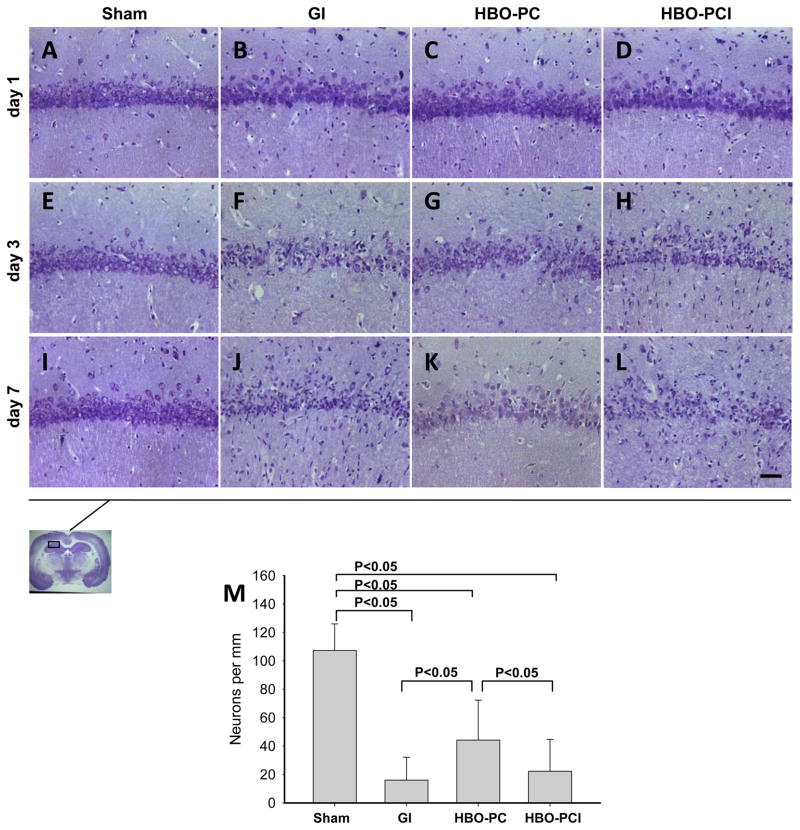
Nissl Stain
Nissl histology revealed delayed cell death characteristic for this model (Figure 2). The sham group did not exhibit dead cells in CA-1 (2A,E,I). Cell loss and the presence of injured neurons with twisted axonal processes were noted in the CA1 at days 3 and 7 after ischemia (2F,J). In the HBO–PC group (2G,K) relatively less dead cells were observed compared to the GI group and HBO-PC plus inhibitor group (2H,L) on day 3 and 7. Dark neurons were seen throughout CA1, however. Quantitative cell count revealed that 14.94% of CA1 neurons survived global ischemia till day 7 in GI group (Sham: 107.31± 18.66; GI: 16.04 ± 16.07 [neurons/mm]; Figure 2M). The number of surviving neurons increased 2.75-fold in the HBO preconditioned rats, to 44.22 ± 28.07. However, pretreatment with COX-2 inhibitor prior to HBO-PC reduced the level of surviving neurons in CA1 after ischemia (22.25 ± 22.24).
Figure 2.
(A–L) Nissl stain shows cell loss in the sector CA1 of the hippocampus on days 3 and 7 after global cerebral ischemia. HBO-PC markedly decreased the loss of the pyramidal cells in this hippocampal zone. Scale bar represent 100μm (M) Bar graph presents cell counts of surviving neurons after ischemia.
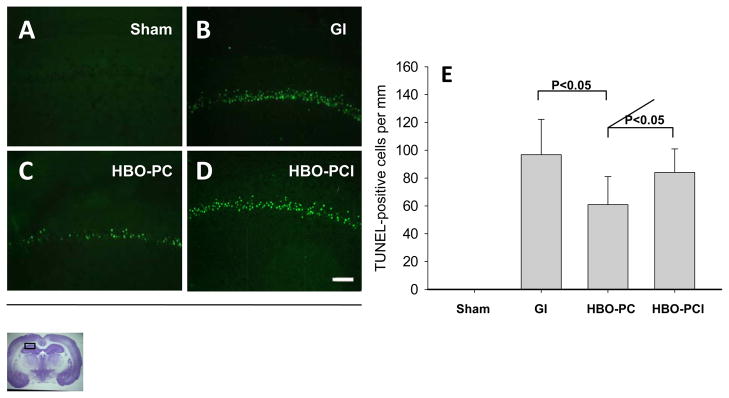
TUNEL
There were no TUNEL positive cells in the sham group (Figure 3A). The CA1 cells in the non preconditioned rats were almost entirely TUNEL positive at 7 days after global ischemia (3B). A remarkable decrease of TUNEL was observed in the HBO-preconditioned group (3C) compared to GI group. An increase of TUNEL positivity was observed in HBO-PCI group (3D) compared to HBO-PC group. Cell counting (3E) showed that HBO-PC reduced number of TUNEL positive cells by 37.10%. Pretreatment with COX-2 inhibitor increased the number of TUNEL positive cells, to 86.77% of the level in GI group.
Figure 3.
(A–D) Photomicrographs of TUNEL preparations indicate the presence of DNA strand breaks in CA1 at 7 days after ischemia. Scale bar represent 100μm. (E) Bar graph shows that HBO-PC decreased the number of apoptotic neurons in the CA1 and that this effect could be reversed with COX-2 inhibitor pretreatment.
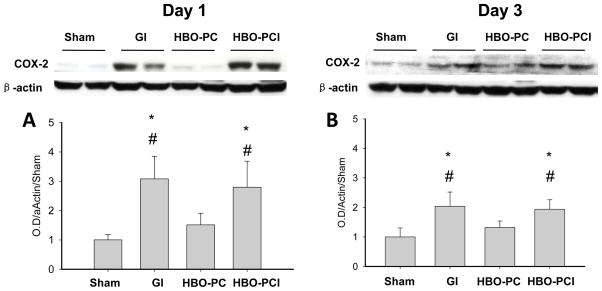
COX-2 Protein Expression in the Brain after Global Ischemia
Western blot analysis demonstrated an increase in COX-2 protein on day 1 and 3 after non-preconditioned global ischemia (Figure 4A,B). There was a statistically significant decrease in COX-2 in the HBO-PC group as compared to GI and HBO-PC plus inhibitor groups at both time points.
Figure 4.
Western blot analysis of COX-2 levels in the hippocampus on day 1 (A) and day 3 (B) after global cerebral ischemia. *p<0.05 vs. sham #p<0.05 vs. HBO-PC.
Colocalization of COX-2, Cleaved Caspase 3 and NeuN
The results of triple immunofluorescence staining on day 3 post ischemia are shown in the Figure 5. Neither normal (5A,G,M,S), sham-operated (5B,H,N,T) or normal preconditioned with HBO (5F,L,R,Y) rats showed cleaved caspase 3 or COX-2 positivity in the CA1 and in the cerebral cortex. After non preconditioned global ischemia the majority of CA1 and cortical neurons showed cleaved caspase 3 and COX-2 colocalization (5C,I). In the preconditioned group the reduced COX-2 positivity and even more reduced cleaved caspase 3 also colocalized with the neuronal marker (5D,J,P,W). In this group the NeuN immunoreactivity was noticeably stronger than after global ischemia without preconditioning. Pretreatment with COX-2 inhibitor resulted in the reappearance of strong immunoreactivity for cleaved caspase 3 and COX-2 in predominately neuronal cells (5E,K,Q,X).
Figure 5.
(A–Y) Triple immunofluorescence staining for cleaved caspase 3 (Texas red), COX-2 (FITC, green) and the neuronal marker, NeuN (AMCA, blue) on day 3 post-ischemia, revealed a predominant colocalization of these epitopes (composite white color) in the CA1 (regular panels) and in the cerebral cortex (insets) affected by the ischemic insult. HBO-PC reduced immunoreactivity for both cleaved caspase 3 and COX-2, while it tended to preserve the immunoreactivity of neuronal marker, NeuN. Scale bars represent 30μm.
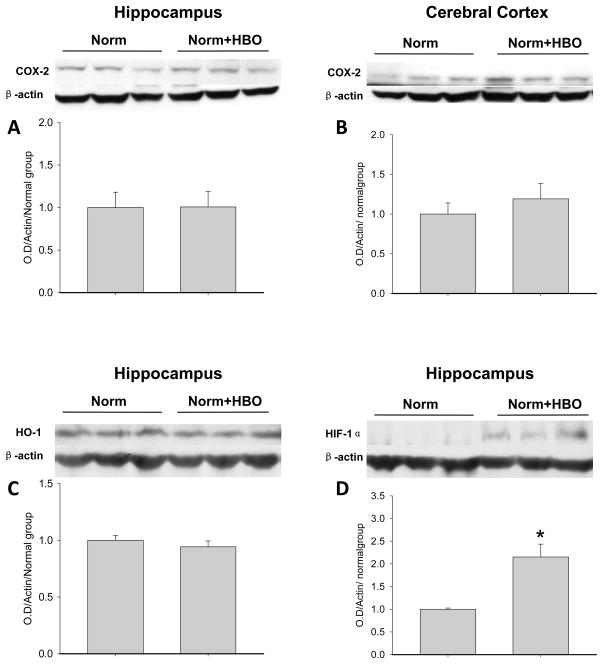
HBO Preconditioning by Itself Increases HIF-1α Brain Level Without Affecting COX-2 or HO-1
We also measured COX-2, HO-1 and HIF-1α levels in brains of normal animals preconditioned with 5 HBO sessions (Figure 6A,B). There was no significant change in COX-2 or HO-1 levels following HBO-PC alone, either in the cerebral cortex (6A) or in the hippocampus (Figure 6B, 6C). In contrast, 5 HBO sessions induced HIF-1α in the hippocampus at 24 hrs after the last session (p=0.002; Figure 6D).
Figure 6.
Western blotting of COX-2 (A,B), HO-1 (6C) and HIF-1α (6D) in brains of normal rats subjected to HBO preconditioning. HBO-PC by itself had no effect on COX-2 or HO-1 levels, however, it increased the preischemic HIF-1α expression in the hippocampus; *p=0.002 vs. Norm.
Discussion
Our study shows that HBO-PC increased the number of surviving neurons, reduced apoptosis and ameliorated cognitive deficit after global cerebral ischemia. We also observed that HBO-PC tended to decrease mortality and the incidence of seizures. In addition, HBO-PC reduced post ischemic COX-2 upregulation. All these effects were significantly reduced by COX-2 inhibitor administered prior to HBO preconditioning sessions.
Consistent with reports by earlier authors, the damage of CA1 pyramidal neurons was detectable within 3–7 days after global ischemic episode. 17, 24 Consequently, global cerebral ischemia resulted in the disorder of cognition and memory and the appearance of seizures. 25 HBO-PC proved effective in conferring CA1 protection, lasting at least till 7th day following ischemia. Even though the duration of occlusion (15 min.) was longer than in our previous study (10 min. 4VO used by Ostrowski et al.) we could still observe the beneficial effect of HBO-PC, which points towards a high efficacy of this modality.
We found a tremendous upregulation of COX-2 in the brain at 24 and 72 hours after global ischemia. On the basis of colocalization of cleaved caspase 3, COX-2 and NeuN we infer that the majority of cells displaying COX-2 positivity were neurons dying through caspase 3-depended apoptosis. More importantly, we found that the level of COX-2 in the hippocampus in HBO-PC group is relatively lower than in GI group at 2 different time points.
The large body of experimental evidence emphasizes the significance of blocking COX-2 pathway in therapeutic strategy for the global cerebral ischemia. The expression of COX-2 dramatically increased in the injured hippocampus following global cerebral ischemia in animal models 14, 15 and in autopsied brains obtained from patients who died of cardiac arrest. 26 Conversely, the degree of hippocampal neuronal injury produced by global ischemia in COX-2-deficient mice was lessened as compared to that in the wild-type mice, coincident with attenuation of DNA fragmentation in the hippocampus. 15
In the present study we used the selective COX-2 inhibitor, NS-398 before each exposure to HBO in order to verify the role of COX-2 in the HBO-PC. We found that the neuroprotective effect of HBO-PC was abolished by administration of low-dose NS-398, as reflected by the reduced number of surviving neurons and decreased percentage of spontaneous alternations. The increased mortality and incidence of seizures in the HBO-PC plus COX-2 inhibitor group further supports the notion that inhibition of COX-2 inactivates a major preconditioning mechanism.
However, we need to point out that the detailed mechanism for how blockade of COX-2 with NS-398 removes the preconditioning effect remains unclear.At first we hypothesized that NS-398 might reduce COX-2 downstream target, HO-1. In ischemic preconditioning COX-2 plays a mandatory role for induction of HO-1 that, once induced, may decrease heme-dependent enzymes, including COX-227,28. However, Western blot analysis showed that HBO-PC failed to affect HO-1 levels in the brain. Therefore, it is unlikely that the effect of NS398 on HO-1, if any, would be relevant in this setting. In contrast, our further investigation revealed that HBO-PC by itself increased the level of HIF-1α in the hippocampus. This finding opens the possibility that NS398 removes the preconditioning effect by decreasing HIF-1 activation, as HIF-1 is an established mediator of ischemic tolerance. In addition, studies in other systems have shown that COX-2 product PGE2 may directly upregulate HIF-1α at both mRNA and protein level29. Therefore, the role of COX-2 in regulating HIF-1 pathway in HBO preconditioning deserves further study.
We also hypothesized that HBO-PC may trigger an increase in COX-2 expression in the brain, and protect neurons from ischemia-induced damage by depleting COX-2 protein prior to the ischemic insult. In some experimental systems, preconditioning effects have been reported to occur via an increase in COX-2 expression. 30 But unfortunately, there was no significant difference in COX-2 protein levels in the cerebral cortex and in the hippocampus between normal preconditioned and normal non-preconditioned rats.
Overall the role of COX-2 in HBO-PC shows similarities to its involvement in the ischemic preconditioning of the brain or in the noble gas preconditioning of the heart. COX-2 was induced in rat brains subjected to preconditioning by the middle cerebral artery occlusion for 10 minutes followed by different amounts of reperfusion time (1–24 hours). Pretreatment with the COX-2-selective inhibitor, rofecoxib, increased infarct size and abolished preconditioning-induced COX-2 expression in vivo. 28 Furthermore, the study led by Weber found that Xenon-induced late myocardial preconditioning did not cause increased expression ofCOX-2 mRNA and protein although the ischemic tolerance was abolished by functional blockade of COX-2 prior to ischemia. 31 A recent study also pointed out the role of COX-2 in the glutamine-induced preconditioning of the heart. 30 Collectively, these data may indicate that COX-2 is a common component of preconditioning mechanisms in different modalities and types of injury.
Although the neuroprotective effects of HBO-PC in the global ischemia have been reported previously, the underlying mechanisms have not been to date established. Our recent study of surgical brain injury demonstrated the important role of COX-2 in HBO-PC5, however until now it was not known if this paradigm can hold true in the setting of brain ischemia. Therefore we believe that our present work is novel as it offers a new insight on the mechanism through which HBO-PC reduces brain damage in the global cerebral ischemia.
Summary
In summary, HBO-PC–induced brain protection after global ischemia is associated with a reduction of COX-2 levels in cerebral tissues. Pretreatment with COX-2 inhibitor abolished neuroprotective effect, which indirectly confirmed the role of COX-2 activation in the mechanism of HBO-PC. However, the molecular nature of COX-2 pathway in this setting remains to be elucidated.
Acknowledgments
Sources of Funding: Work was supported by grants NS43338 and HD43120 from NIH to John H. Zhang
Footnotes
Disclosures: Oumei Cheng: None
Robert Ostrowski: None
Bihua Wu: None
Wenwu Liu: None
Chunhua Chen: None
John H. Zhang: Grant support as mentioned above
References
- 1.Wada K, Miyazawa T, Nomura N, Tsuzuki N, Nawashiro H, Shima K. Preferential conditions for and possible mechanisms of induction of ischemic tolerance by repeated hyperbaric oxygenation in gerbil hippocampus. Neurosurgery. 2001;49:160–166. doi: 10.1097/00006123-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, Guan YT, Sun XJ. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience. 2009;159:1309–1315. doi: 10.1016/j.neuroscience.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Li W, Kang Z, Liu Y, Deng X, Tao H, Xu W, Li R, Sun X, Zhang JH. Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord ischemia in rats. J Neurotrauma. 2009;26:55–66. doi: 10.1089/neu.2008.0538. [DOI] [PubMed] [Google Scholar]
- 4.Hu SL, Hu R, Li F, Liu Z, Xia YZ, Cui GY, Feng H. Hyperbaric oxygen preconditioning protects against traumatic brain injury at high altitude. Acta Neurochir Suppl. 2008;105:191–196. doi: 10.1007/978-3-211-09469-3_37. [DOI] [PubMed] [Google Scholar]
- 5.Jadhav V, Ostrowski RP, Tong W, Matus B, Jesunathadas R, Zhang JH. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–3142. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Liu W, Kang Z, Lv S, Han C, Yun L, Sun X, Zhang JH. Mechanism of hyperbaric oxygen preconditioning in neonatal hypoxia-ischemia rat model. Brain Res. 2008;1196:151–156. doi: 10.1016/j.brainres.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Gu GJ, Li YP, Peng ZY, Xu JJ, Kang ZM, Xu WG, Tao HY, Ostrowski RP, Zhang JH, Sun XJ. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol. 2008;104:1185–1191. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- 8.Peng Z, Ren P, Kang Z, Du J, Lian Q, Liu Y, Zhang JH, Sun X. Up-regulated HIF-1alpha is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 2008;1212:71–78. doi: 10.1016/j.brainres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, Corleone D, Tang J, Zhang JH. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–13. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie H, Xiong L, Lao N, Chen S, Xu N, Zhu Z. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab. 2006;26:666–674. doi: 10.1038/sj.jcbfm.9600221. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–1120. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- 13.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki T, Kitagawa K, Yamagata K, Takemiya T, Tanaka S, Omura-Matsuoka E, Sugiura S, Matsumoto M, Hori M. Amelioration of hippocampal neuronal damage after transient forebrain ischemia in cyclooxygenase-2-deficient mice. J Cereb Blood Flow Metab. 2004;24:107–113. doi: 10.1097/01.WCB.0000100065.36077.4A. [DOI] [PubMed] [Google Scholar]
- 16.Xiang Z, Thomas S, Pasinetti G. Increased neuronal injury in transgenic mice with neuronal overexpression of human cyclooxygenase-2 is reversed by hypothermia and rofecoxib treatment. Curr Neurovasc Res. 2007;4:274–279. doi: 10.2174/156720207782446342. [DOI] [PubMed] [Google Scholar]
- 17.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Calvert JW, Kusaka G, Zhang JH. One-stage anterior approach for four-vessel occlusion in rat. Stroke. 2005;36:2212–2214. doi: 10.1161/01.STR.0000182238.08510.c5. [DOI] [PubMed] [Google Scholar]
- 19.Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007;1136:200–207. doi: 10.1016/j.brainres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 21.Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-kappaB. Neuroscience. 2010;166:1101–1109. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke. 2006;37:1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 25.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 26.Maslinska D, Wozniak R, Kaliszek A, Modelska I. Expression of cyclooxygenase-2 in astrocytes of human brain after global ischemia. Folia Neuropathol. 1999;37:75–79. [PubMed] [Google Scholar]
- 27.Abraham NG, Drummond G. CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 28.Park MK, Kang YJ, Lee HS, Kim HJ, Seo HG, Lee JH, Chang KC. The obligatory role of COX-2 expression for induction of HO-1 in ischemic preconditioned rat brain. Biochem Biophys Res Commun. 2008;377:1191–1194. doi: 10.1016/j.bbrc.2008.10.149. [DOI] [PubMed] [Google Scholar]
- 29.Critchley HO, Osei J, Henderson TA, Boswell L, Sales KJ, Jabbour HN, Hirani N. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2) Endocrinology. 2006;147:744–53. doi: 10.1210/en.2005-1153. [DOI] [PubMed] [Google Scholar]
- 30.McGuinness J, Neilan TG, Cummins R, Sharkasi A, Bouchier-Hayes D, Redmond JM. Intravenous glutamine enhances COX-2 activity giving cardioprotection. J Surg Res. 2009;152:140–147. doi: 10.1016/j.jss.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Weber NC, Frassdorf J, Ratajczak C, Grueber Y, Schlack W, Hollmann MW, Preckel B. Xenon induces late cardiac preconditioning in vivo: a role for cyclooxygenase 2? Anesth Analg. 2008;107:1807–1813. doi: 10.1213/ane.Ob013e31818874bf. [DOI] [PubMed] [Google Scholar]